Population-Level Immunity for Transient Suppression of COVID-19 Waves in Japan from April 2021 to September 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Number of Reported Positive Cases per Day
2.2. Effectiveness of Individual Vaccination and Natural Immunity
2.3. Estimation of Population-Level Immunity
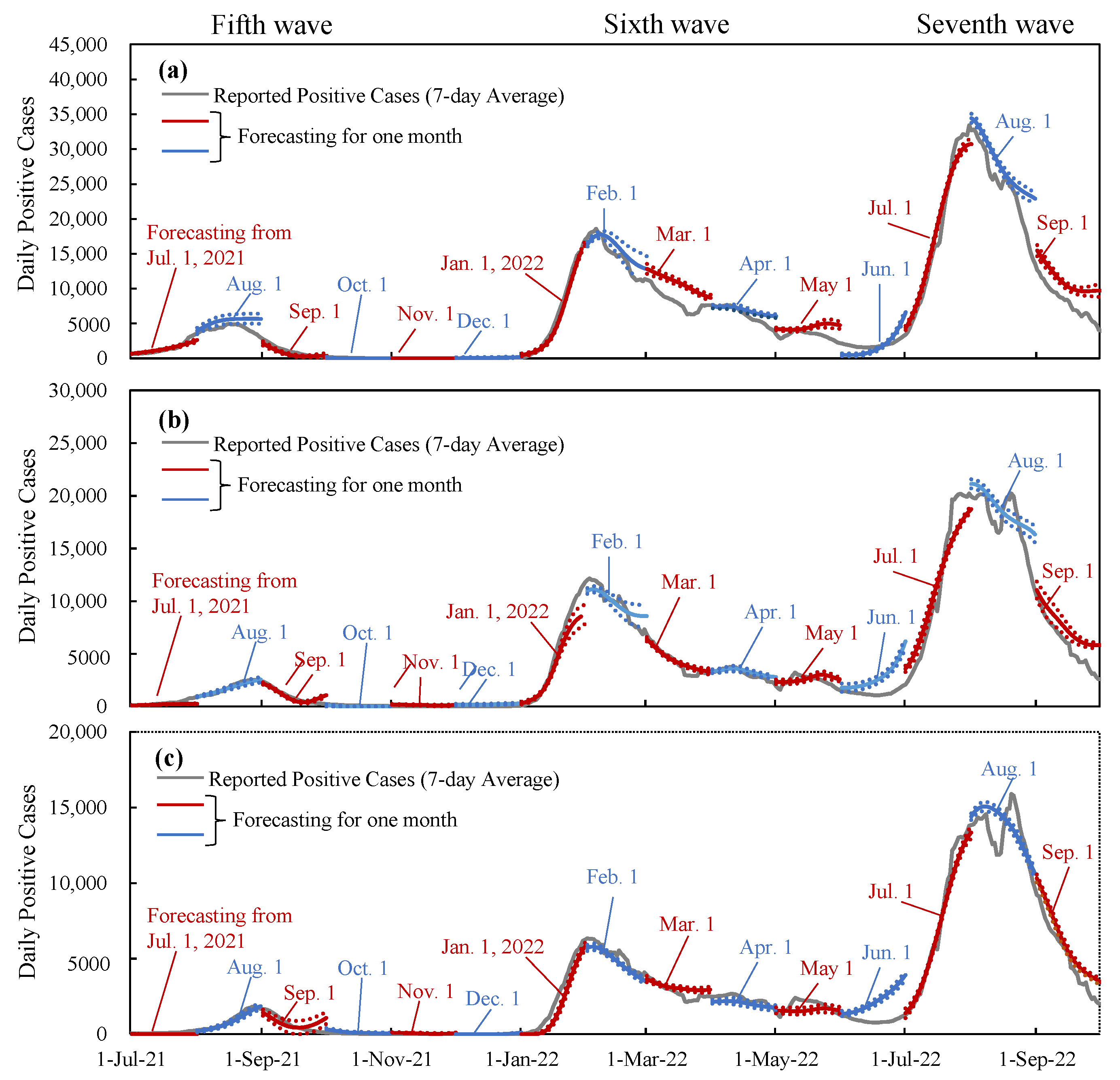
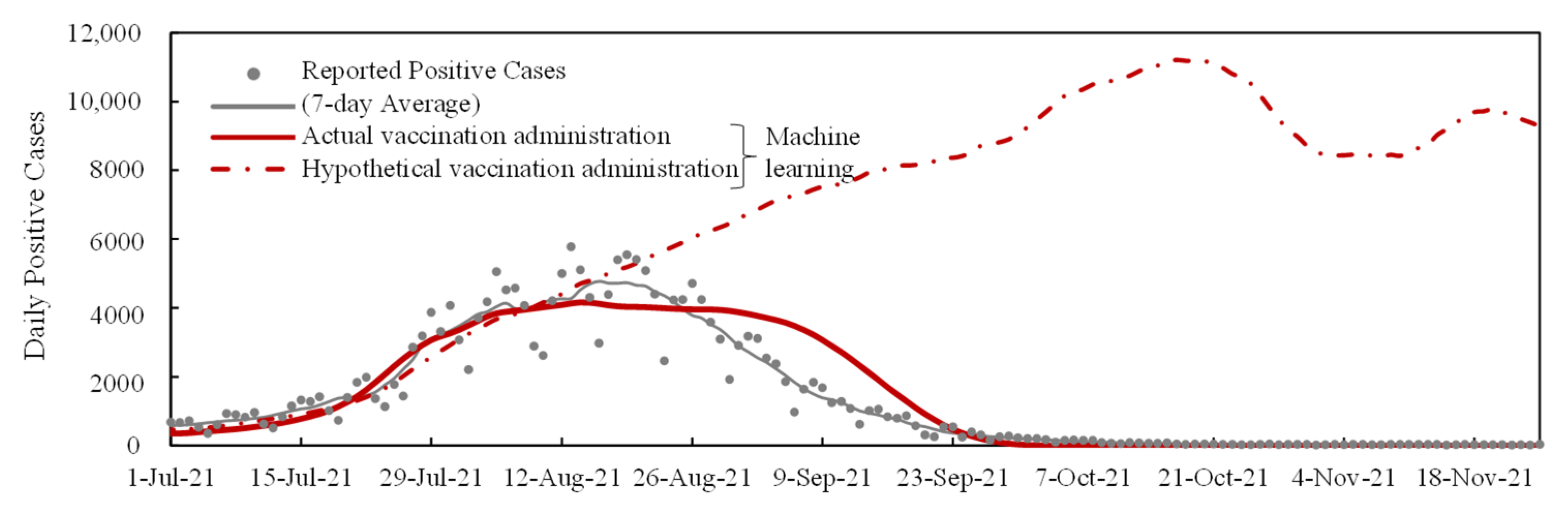
2.4. Replication with Machine Learning
3. Results
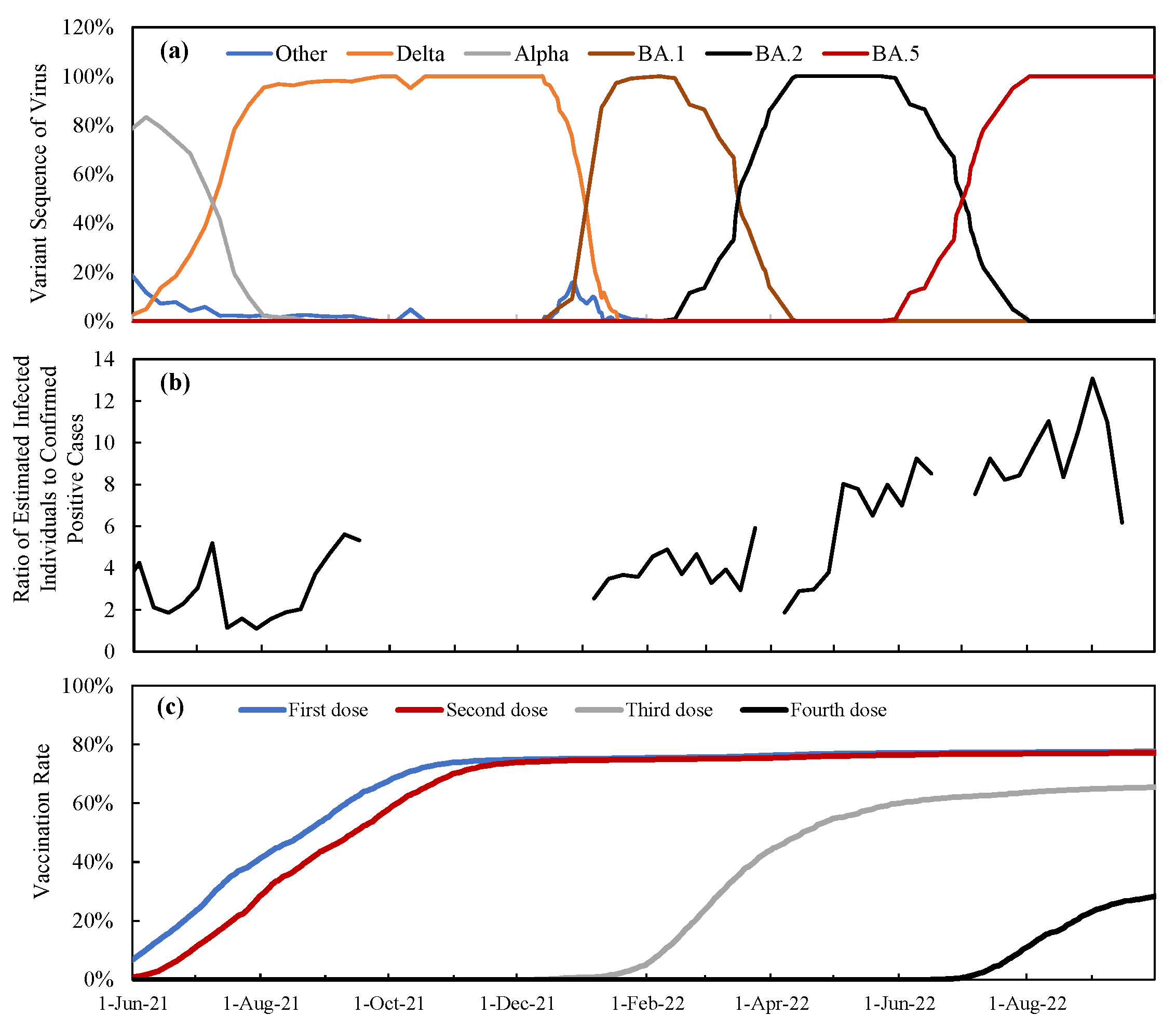
3.1. Time Course of Vaccination Rate and Varriants in Japan
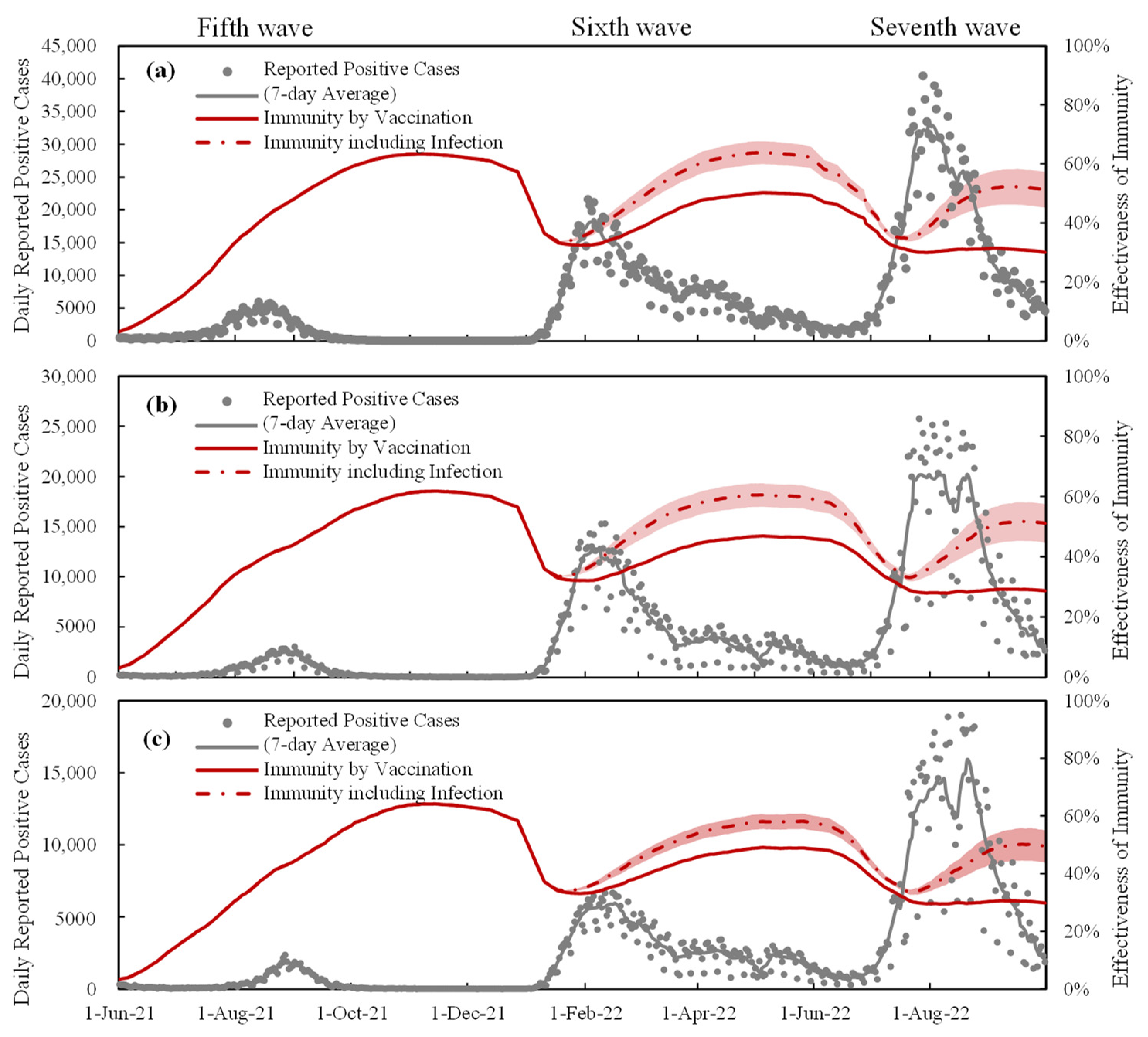
3.2. Daily Reported Positive Cases and Estimated Population-Level Immunity
3.3. Confirmed Cases and Vaccination Rates
3.4. Verification and Interpolation with Machine Learning
4. Discussion
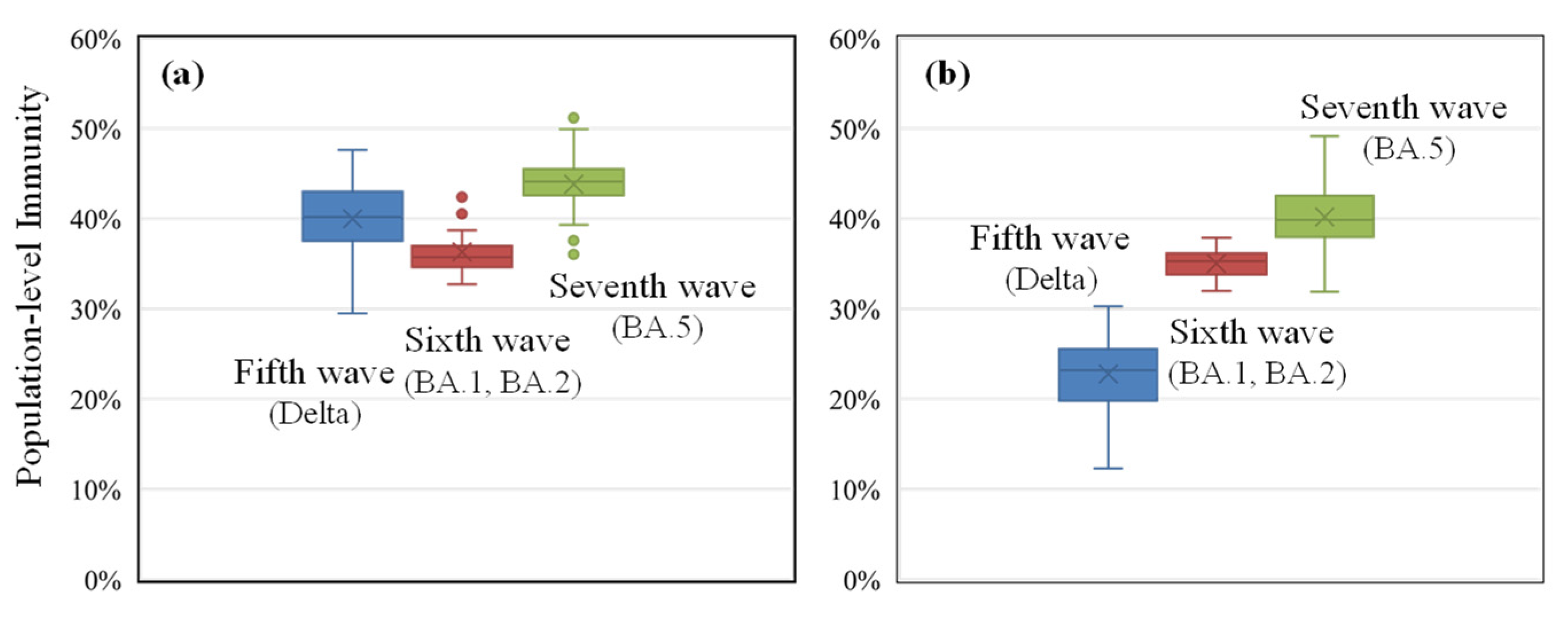
- Due to the limited number of people who were infected in Japan prior to the sixth wave, the population with asymptomatic infection is a major source of uncertainty (see Figure 2). Even when considering a factor of four to eight for the ratio of asymptomatic infected individuals to the DPC, the population-level immunity in the sixth and seventh waves varied by 8% and 12%, respectively. Consequently, the uncertainty associated with the box plots in Figure 3 would be somewhat greater.
- There is additional uncertainty in the definition of waves, and the number of DPCs per population in the fifth wave is lower than in previous waves, making the definition of the wave relatively arbitrary in certain prefectures with small populations (for example, the maximum DPC in the Tottori prefecture was 47). In the interest of simplification, it is assumed that the decay of infection-acquired immunity is identical to that of vaccination. It is known that the former is longer than the latter. This simplification does not lead to significant differences as the number of infected people in Japan was limited until the wave we focused on. Similarly, the consideration of hybrid immunity [44,45,46], defined as immunity acquired through complete vaccination and infection, was marginal.
- Note that even for the abovementioned limitations, its impact on the forecasting or the transmission was demonstrated to be marginal through machine learning replication.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
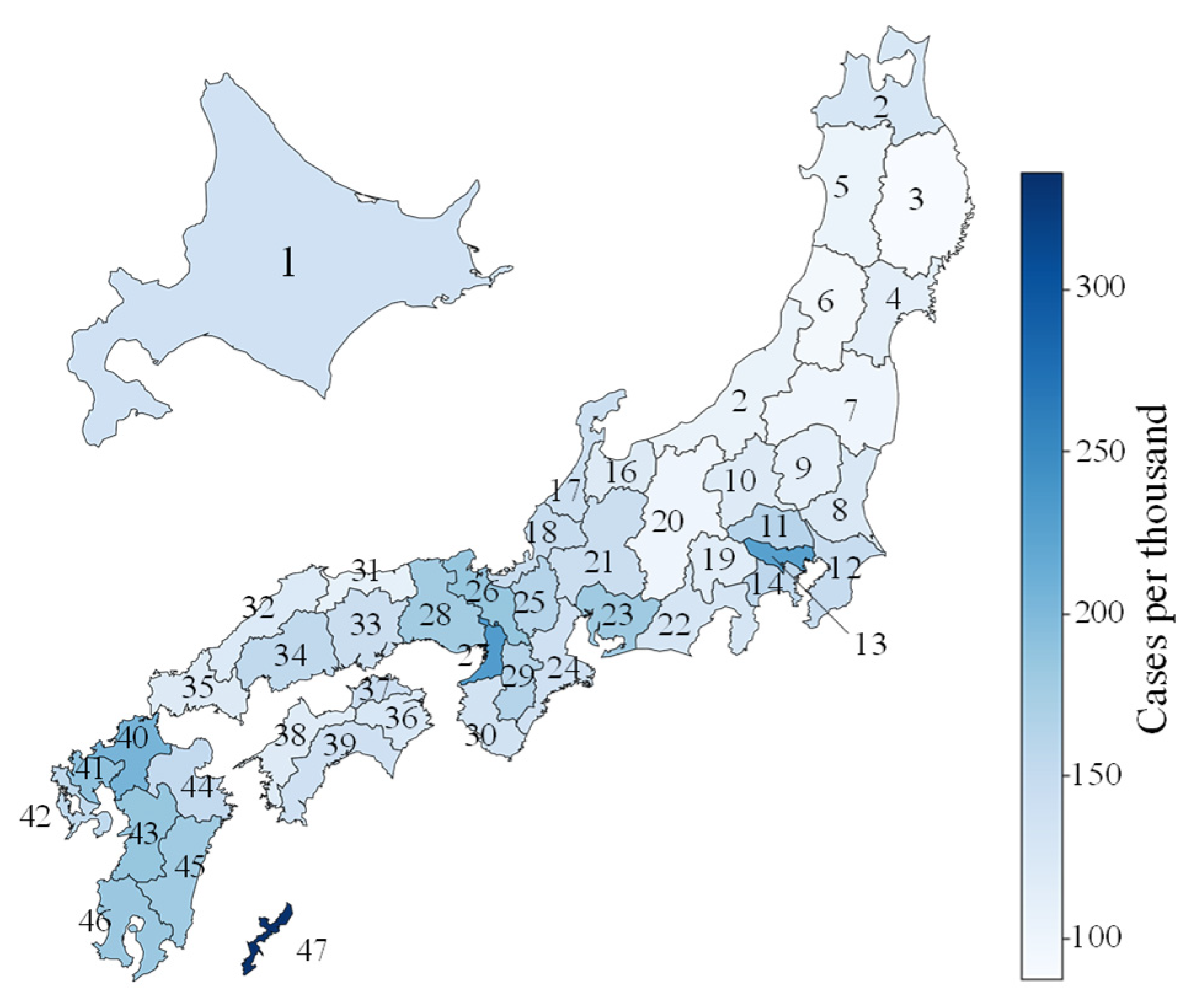


Appendix B
References
- World Health Organization (WHO) Coronavius (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 20 January 2023).
- Badr, H.S.; Du, H.; Marshall, M.; Dong, E.; Squire, M.M.; Gardner, L.M. Association between mobility patterns and COVID-19 transmission in the USA: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 1247–1254. [Google Scholar] [CrossRef]
- Kodera, S.; Hikita, K.; Rashed, E.A.; Hirata, A. The effects of time window-averaged mobility on effective reproduction number of COVID-19 viral variants in urban cities. J. Urban Health 2022, 100, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Kim, Y.; Hua, J. Governance, technology and citizen behavior in pandemic: Lessons from COVID-19 in East Asia. Prog. Disaster Sci. 2020, 6, 100090. [Google Scholar] [CrossRef]
- Han, E.; Tan, M.M.J.; Turk, E.; Sridhar, D.; Leung, G.M.; Shibuya, K.; Asgari, N.; Oh, J.; García-Basteiro, A.L.; Hanefeld, J.; et al. Lessons learnt from easing COVID-19 restrictions: An analysis of countries and regions in Asia Pacific and Europe. Lancet 2020, 396, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Unruh, L.; Allin, S.; Marchildon, G.; Burke, S.; Barry, S.; Siersbaek, R.; Thomas, S.; Rajan, S.; Koval, A.; Alexander, M.; et al. A comparison of 2020 health policy responses to the COVID-19 pandemic in Canada, Ireland, the United Kingdom and the United States of America. Health Policy 2022, 126, 427–437. [Google Scholar] [CrossRef]
- Diao, Y.; Kodera, S.; Anzai, D.; Gomez-Tames, J.; Rashed, E.A.; Hirata, A. Influence of population density, temperature, and absolute humidity on spread and decay durations of COVID-19: A comparative study of scenarios in China, England, Germany, and Japan. One Health 2021, 12, 100203. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, H.; Yang, Y.; Wan, H.; Wang, Y. Impacts of geographic factors and population density on the COVID-19 spreading under the lockdown policies of China. Sci. Total Environ. 2020, 746, 141347. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Khosrawipour, V.; Kocbach, P.; Mikolajczyk, A.; Schubert, J.; Bania, J.; Khosrawipour, T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J. Travel Med. 2020, 27, taaa037. [Google Scholar] [CrossRef]
- Odagaki, T. Analysis of the outbreak of COVID-19 in Japan by SIQR model. Infect. Dis. Model. 2020, 5, 691–698. [Google Scholar] [CrossRef]
- Tetteh, J.N.A.; Nguyen, V.K.; Hernandez-Vargas, E.A. Network models to evaluate vaccine strategies towards herd immunity in COVID-19. J. Theor. Biol. 2021, 531, 110894. [Google Scholar] [CrossRef]
- Tang, J.W.; Wu, S.; Kwok, K.O. Can Asia now learn from the experience of the West? Clin. Microbiol. Infect. 2021, 27, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.V.; Maslov, S.; Elbanna, A.; Wong, G.N.; Weiner, Z.J.; Goldenfeld, N. Time-dependent heterogeneity leads to transient suppression of the COVID-19 epidemic, not herd immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2015972118. [Google Scholar] [CrossRef] [PubMed]
- Global Change Data Lab Our World in Data. Available online: https://ourworldindata.org/ (accessed on 7 November 2022).
- Arashiro, T.; Arima, Y.; Muraoka, H.; Sato, A.; Oba, K.; Uehara, Y.; Arioka, H.; Yanai, H.; Kuramochi, J.; Ihara, G.; et al. COVID-19 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection During Delta-Dominant and Omicron-Dominant Periods in Japan: A Multicenter Prospective Case-control Study (Factors Associated with SARS-CoV-2 Infection and the Effectiveness of COVID-19 Vaccines Study). Clin. Infect. Dis. 2023, 76, e108–e115. [Google Scholar] [CrossRef]
- Buchan, S.A.; Chung, H.; Brown, K.A.; Austin, P.C.; Fell, D.B.; Gubbay, J.B.; Nasreen, S.; Schwartz, K.L.; Sundaram, M.E.; Tadrous, M.; et al. Estimated effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. JAMA Netw. Open 2022, 5, e2232760. [Google Scholar] [CrossRef]
- Reis, B.Y.; Barda, N.; Leshchinsky, M.; Kepten, E.; Hernán, M.A.; Lipsitch, M.; Dagan, N.; Balicer, R.D. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N. Engl. J. Med. 2021, 385, 2101–2103. [Google Scholar] [CrossRef]
- Kodera, S.; Rashed, E.A.; Hirata, A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against Delta and Omicron variants in Japan. Vaccines 2022, 10, 430. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Toffa, S.; Sachdeva, R.; Gallagher, E.; Groves, N.; O’Connell, A.-M.; Chand, M.; Ramsay, M.; et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 2022, 22, 931–933. [Google Scholar] [CrossRef]
- Seong, H.; Hyun, H.J.; Yun, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Song, J.Y. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: Importance of early public health intervention. Int. J. Infect. Dis. 2021, 104, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Kodera, S.; Niimi, Y.; Rashed, E.A.; Yoshinaga, N.; Toyoda, M.; Hirata, A. Estimation of mRNA COVID-19 vaccination effectiveness in Tokyo for Omicron variants BA.2 and BA.5: Effect of social behavior. Vaccines 2022, 10, 1820. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare Visualizing the Data: Information on COVID-19 Infections. Available online: https://covid19.mhlw.go.jp/en/ (accessed on 7 February 2023).
- Sanada, T.; Honda, T.; Yasui, F.; Yamaji, K.; Munakata, T.; Yamamoto, N.; Kurano, M.; Matsumoto, Y.; Kohno, R.; Toyama, S.; et al. Serologic survey of IgG against SARS-CoV-2 among hospital visitors without a history of SARS-CoV-2 Infection in Tokyo, 2020–2021. J. Epidemiol. 2022, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Zapata, S.F.; Benites-Peralta, B.; Mayta-Tristan, P.; Rodríguez-Morales, A.J. High seroprevalence for SARS-CoV-2 infection in South America, but still not enough for herd immunity! Int. J. Infect. Dis. 2021, 109, 244–246. [Google Scholar] [CrossRef]
- Rashed, E.A.; Kodera, S.; Hirata, A. COVID-19 forecasting using new viral variants and vaccination effectiveness models. Comput. Biol. Med. 2022, 149, 105986. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare Open Data (in Japanese). Available online: https://www.mhlw.go.jp/stf/covid-19/open-data.html (accessed on 7 November 2022).
- Digital Agency Vaccination Record System. Available online: https://info.vrs.digital.go.jp/opendata/ (accessed on 7 November 2022).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Kodera, S.; Takada, A.; Rashed, E.A.; Hirata, A. Projection of COVID-19 Positive Cases Considering Hybrid Immunity: Case Study in Tokyo. Vaccines 2023, 11, 633. [Google Scholar] [CrossRef]
- Tokyo Metropolitan Agent the Disaster Prevention Information, Tokyo. Available online: https://www.bousai.metro.tokyo.lg.jp/taisaku/saigai/index.html (accessed on 20 January 2023).
- Cooper, I.; Mondal, A.; Antonopoulos, C.G. A SIR model assumption for the spread of COVID-19 in different communities. Chaos Solitons Fractals 2020, 139, 110057. [Google Scholar] [CrossRef]
- Carcione, J.M.; Santos, J.E.; Bagaini, C.; Ba, J. A simulation of a COVID-19 epidemic based on a deterministic SEIR model. Front. Public Health 2020, 8, 230. [Google Scholar] [CrossRef]
- Annas, S.; Isbar Pratama, M.; Rifandi, M.; Sanusi, W.; Side, S. Stability analysis and numerical simulation of SEIR model for pandemic COVID-19 spread in Indonesia. Chaos Solitons Fractals 2020, 139, 110072. [Google Scholar] [CrossRef]
- Arora, P.; Kumar, H.; Panigrahi, B.K. Prediction and analysis of COVID-19 positive cases using deep learning models: A descriptive case study of India. Chaos Solitons Fractals 2020, 139, 110017. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.A.B.; Silal, S.P.; Li, M.W.Z.; Dushoff, J.; Bolker, B.M.; Abbott, S.; van Schalkwyk, C.; Benjamin, M. Bounding the levels of transmissibility & immune evasion of the Omicron variant in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Shi, Y.; Xiong, Q.; Liu, Z.; Guo, Q.; Zou, L.; et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Nat. Commun. 2022, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Manica, M.; De Bellis, A.; Guzzetta, G.; Mancuso, P.; Vicentini, M.; Venturelli, F.; Zerbini, A.; Bisaccia, E.; Litvinova, M.; Menegale, F.; et al. Intrinsic generation time of the SARS-CoV-2 Omicron variant: An observational study of household transmission. Lancet Reg. Health Eur. 2022, 19, 100446. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020, 323, 2249. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare Advisory Boad Meetings of COVID-19 Measures. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00294.html (accessed on 10 January 2023).
- Hirata, A.; Kodera, S.; Diao, Y.; Rashed, E.A. Did the Tokyo Olympic Games enhance the transmission of COVID-19? An interpretation with machine learning. Comput. Biol. Med. 2022, 146, 105548. [Google Scholar] [CrossRef]
- Crotty, S. Hybrid immunity. Science 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Dhama, K.; Agoramoorthy, G.; Chakraborty, C. Hybrid immunity against COVID-19 in different countries with a special emphasis on the Indian scenario during the Omicron period. Int. Immunopharmacol. 2022, 108, 108766. [Google Scholar] [CrossRef]
- Nakanishi, M.; Shibasaki, R.; Yamasaki, S.; Miyazawa, S.; Usami, S.; Nishiura, H.; Nishida, A. On-site dining in Tokyo during the COVID-19 pandemic: Time series analysis using mobile phone location data. JMIR Mhealth Uhealth 2021, 9, e27342. [Google Scholar] [CrossRef]
- Google COVID-19 Community Mobility Reports. Available online: https://www.google.com/covid19/mobility/ (accessed on 7 November 2022).

| Fifth Wave | Sixth Wave | Seventh Wave | |||||
|---|---|---|---|---|---|---|---|
| No. | Prefecture | Start | Peak | Start | Peak | Start | Peak |
| 1 | Hokkaido | 30-Jun-21 | 21-Aug-21 | 15-Jan-22 | 8-Feb-22 | 28-Jun-22 | 21-Aug-22 |
| 2 | Aomori | 1-Aug-21 | 29-Aug-21 | 16-Nov-21 | 8-Mar-22 | 10-Jun-22 | 27-Aug-22 |
| 3 | Iwate | 20-Jun-21 | 17-Aug-21 | 14-Oct-21 | 28-Feb-22 | 25-Jun-22 | 21-Aug-22 |
| 4 | Miyagi | 31-Jul-21 | 20-Aug-21 | 7-Jan-22 | 18-Feb-22 | 27-Jun-22 | 21-Aug-22 |
| 5 | Akita | 8-Aug-21 | 22-Aug-21 | 25-Jan-22 | 27-Jan-22 | 29-Jun-22 | 21-Aug-22 |
| 6 | Yamagata | 21-Jul-21 | 20-Aug-21 | 26-Jan-22 | 4-Feb-22 | 22-Jun-22 | 21-Aug-22 |
| 7 | Fukushima | 10-Jul-21 | 15-Aug-21 | 26-Jan-22 | 5-Feb-22 | 28-Jun-22 | 21-Aug-22 |
| 8 | Ibaraki | 2-Jul-21 | 20-Aug-21 | 17-Jan-22 | 9-Feb-22 | 11-Jun-22 | 22-Aug-22 |
| 9 | Tochigi | 29-Jun-21 | 22-Aug-21 | 27-Dec-21 | 17-Feb-22 | 7-Jul-22 | 2-Aug-22 |
| 10 | Gumma | 22-Jul-21 | 21-Aug-21 | 18-Jan-22 | 2-Feb-22 | 26-Jun-22 | 21-Aug-22 |
| 11 | Saitama | 8-Jul-21 | 18-Aug-21 | 17-Jan-22 | 8-Feb-22 | 2-Jul-22 | 3-Aug-22 |
| 12 | Chiba | 9-Jul-21 | 18-Aug-21 | 16-Jan-22 | 7-Feb-22 | 5-Jul-22 | 31-Jul-22 |
| 13 | Tokyo | 25-Jun-21 | 16-Aug-21 | 17-Jan-22 | 5-Feb-22 | 30-Jun-22 | 31-Jul-22 |
| 14 | Kanagawa | 24-Jun-21 | 23-Aug-21 | 21-Jan-22 | 7-Feb-22 | 1-Jul-22 | 30-Jul-22 |
| 15 | Niigata | 29-Jun-21 | 24-Aug-21 | 16-Jan-22 | 27-Jan-22 | 26-Jun-22 | 21-Aug-22 |
| 16 | Toyama | 28-Jul-21 | 19-Aug-21 | 6-Jan-22 | 1-Mar-22 | 28-Jun-22 | 21-Aug-22 |
| 17 | Ishikawa | 13-Jul-21 | 29-Jul-21 | 22-Jan-22 | 3-Feb-22 | 27-Jun-22 | 22-Aug-22 |
| 18 | Fukui | 7-Jun-21 | 27-Aug-21 | 6-Dec-21 | 10-Mar-22 | 30-Jun-22 | 22-Aug-22 |
| 19 | Yamanashi | 16-Jul-21 | 20-Aug-21 | 21-Jan-22 | 28-Jan-22 | 18-Jun-22 | 20-Aug-22 |
| 20 | Nagano | 27-Jul-21 | 21-Aug-21 | 10-Jan-22 | 3-Feb-22 | 25-Jun-22 | 21-Aug-22 |
| 21 | Gifu | 9-Aug-21 | 27-Aug-21 | 22-Dec-21 | 16-Feb-22 | 21-Jun-22 | 20-Aug-22 |
| 22 | Shizuoka | 30-Jul-21 | 22-Aug-21 | 12-Jan-22 | 7-Feb-22 | 19-Jun-22 | 21-Aug-22 |
| 23 | Aichi | 8-Aug-21 | 29-Aug-21 | 21-Dec-21 | 16-Feb-22 | 17-Jun-22 | 20-Aug-22 |
| 24 | Mie | 14-Aug-21 | 24-Aug-21 | 13-Jan-22 | 6-Feb-22 | 13-Jun-22 | 21-Aug-22 |
| 25 | Shiga | 25-Jul-21 | 22-Aug-21 | 11-Jan-22 | 7-Feb-22 | 15-Jun-22 | 22-Aug-22 |
| 26 | Kyoto | 17-Jul-21 | 23-Aug-21 | 12-Jan-22 | 7-Feb-22 | 2-Jul-22 | 2-Aug-22 |
| 27 | Osaka | 9-Jul-21 | 29-Aug-21 | 9-Jan-22 | 8-Feb-22 | 18-Jun-22 | 20-Aug-22 |
| 28 | Hyogo | 20-Jul-21 | 25-Aug-21 | 13-Jan-22 | 7-Feb-22 | 15-Jun-22 | 21-Aug-22 |
| 29 | Nara | 10-Jul-21 | 29-Aug-21 | 3-Jan-22 | 17-Feb-22 | 23-Jun-22 | 22-Aug-22 |
| 30 | Wakayama | 16-Jul-21 | 22-Aug-21 | 15-Jan-22 | 5-Feb-22 | 15-Jun-22 | 21-Aug-22 |
| 31 | Tottori | 9-Jul-21 | 2-Aug-21 | 28-Nov-22 | 24-Feb-22 | 13-Jun-22 | 20-Aug-22 |
| 32 | Shimane | 24-Jun-21 | 27-Aug-21 | 15-Jan-22 | 20-Jan-22 | 13-Jun-22 | 20-Aug-22 |
| 33 | Okayama | 26-Jul-21 | 20-Aug-21 | 15-Jan-22 | 3-Feb-22 | 26-Jun-22 | 21-Aug-22 |
| 34 | Hiroshima | 6-Aug-21 | 22-Aug-21 | 8-Jan-22 | 23-Jan-22 | 24-Jun-22 | 22-Aug-22 |
| 35 | Yamaguchi | 5-Aug-21 | 21-Aug-21 | 3-Jan-22 | 27-Jan-22 | 21-Jun-22 | 21-Aug-22 |
| 36 | Tokushima | 14-Aug-21 | 27-Aug-21 | 2-Jan-22 | 1-Mar-22 | 18-Jul-22 | 23-Aug-22 |
| 37 | Kagawa | 27-Jul-21 | 22-Aug-21 | 31-Dec-21 | 20-Feb-22 | 24-Jun-22 | 20-Aug-22 |
| 38 | Ehime | 29-Jul-21 | 19-Aug-21 | 10-Jan-22 | 31-Jan-22 | 15-Jun-22 | 20-Aug-22 |
| 39 | Kochi | 16-Aug-21 | 24-Aug-21 | 19-Jan-22 | 12-Feb-22 | 19-Jun-22 | 24-Aug-22 |
| 40 | Fukuoka | 12-Jul-21 | 21-Aug-21 | 13-Jan-22 | 5-Feb-22 | 15-Jun-22 | 20-Aug-22 |
| 41 | Saga | 9-Aug-21 | 20-Aug-21 | 14-Jan-22 | 5-Feb-22 | 5-Jun-22 | 20-Aug-22 |
| 42 | Nagasaki | 18-Jul-21 | 20-Aug-21 | 18-Jan-22 | 30-Jan-22 | 1-Jul-22 | 21-Aug-22 |
| 43 | Kumamoto | 23-Jul-21 | 20-Aug-21 | 17-Jan-22 | 30-Jan-22 | 6-Jun-22 | 20-Aug-22 |
| 44 | Oita | 12-Aug-21 | 22-Aug-21 | 19-Jan-22 | 1-Feb-22 | 23-Jun-22 | 20-Aug-22 |
| 45 | Miyazaki | 6-Aug-21 | 24-Aug-21 | 20-Jan-22 | 29-Jan-22 | 19-Jun-22 | 21-Aug-22 |
| 46 | Kagoshima | 8-Aug-21 | 19-Aug-21 | 17-Jan-22 | 4-Feb-22 | 14-Jun-22 | 21-Aug-22 |
| 47 | Okinawa | 8-Jul-21 | 17-Aug-21 | 7-Jan-22 | 15-Jan-22 | 14-May-22 | 2-Aug-22 |
| Total Positive Cases | ||||||
|---|---|---|---|---|---|---|
| No. | Prefecture | Population | Population Density (per km2) | Fifth Wave | Sixth Wave | Seventh Wave |
| 1 | Hokkaido | 5,226,603 | 62.6 | 11,297 | 55,724 | 215,433 |
| 2 | Aomori | 1,259,615 | 130.6 | 1519 | 19,016 | 75,803 |
| 3 | Iwate | 1,220,823 | 79.9 | 865 | 7268 | 41,598 |
| 4 | Miyagi | 2,281,989 | 332.6 | 2835 | 17,700 | 105,508 |
| 5 | Akita | 971,288 | 83.5 | 318 | 753 | 37,124 |
| 6 | Yamagata | 1,069,562 | 160.8 | 668 | 2358 | 38,085 |
| 7 | Fukushima | 1,862,059 | 135.1 | 2243 | 4989 | 65,197 |
| 8 | Ibaraki | 2,907,675 | 477.0 | 7073 | 22,438 | 126,973 |
| 9 | Tochigi | 1,955,401 | 305.1 | 4778 | 22,427 | 37,213 |
| 10 | Gumma | 1,958,101 | 307.8 | 4393 | 12,542 | 85,080 |
| 11 | Saitama | 7,393,799 | 1962.3 | 33,513 | 91,036 | 224,180 |
| 12 | Chiba | 6,322,892 | 1244.2 | 27,062 | 72,937 | 153,762 |
| 13 | Tokyo | 13,843,329 | 6579.5 | 113,536 | 267,383 | 571,349 |
| 14 | Kanagawa | 9,220,206 | 3816.3 | 64,261 | 114,260 | 230,852 |
| 15 | Niigata | 2,213,174 | 213.5 | 2634 | 5089 | 92,893 |
| 16 | Toyama | 1,047,674 | 512.1 | 1204 | 13,287 | 51,134 |
| 17 | Ishikawa | 1,132,656 | 270.6 | 859 | 4963 | 61,562 |
| 18 | Fukui | 774,583 | 184.9 | 1389 | 13,260 | 47,221 |
| 19 | Yamanashi | 820,997 | 195.4 | 1525 | 2195 | 40,526 |
| 20 | Nagano | 2,071,737 | 158.1 | 1826 | 10,061 | 74,963 |
| 21 | Gifu | 2,016,791 | 206.5 | 4413 | 24,353 | 110,110 |
| 22 | Shizuoka | 3,686,260 | 508.1 | 7422 | 30,258 | 200,497 |
| 23 | Aichi | 7,558,802 | 1477.5 | 24,286 | 154,567 | 515,468 |
| 24 | Mie | 1,800,557 | 312.5 | 3058 | 11,824 | 102,564 |
| 25 | Shiga | 1,418,843 | 376.7 | 3170 | 16,395 | 88,404 |
| 26 | Kyoto | 2,530,542 | 548.6 | 9177 | 45,319 | 84,156 |
| 27 | Osaka | 8,839,511 | 4649.9 | 60,432 | 235,649 | 757,601 |
| 28 | Hyogo | 5,523,625 | 657.9 | 18,102 | 88,104 | 384,344 |
| 29 | Nara | 1,344,739 | 364.3 | 4498 | 28,476 | 82,196 |
| 30 | Wakayama | 944,432 | 199.8 | 1254 | 7908 | 58,189 |
| 31 | Tottori | 556,788 | 158.8 | 319 | 4474 | 29,649 |
| 32 | Shimane | 672,815 | 100.3 | 678 | 782 | 43,418 |
| 33 | Okayama | 1,893,791 | 270.2 | 3247 | 13,098 | 97,163 |
| 34 | Hiroshima | 2,812,433 | 331.7 | 3401 | 16,441 | 152,939 |
| 35 | Yamaguchi | 1,356,110 | 221.8 | 943 | 5900 | 70,437 |
| 36 | Tokushima | 734,949 | 177.2 | 593 | 7867 | 35,718 |
| 37 | Kagawa | 973,896 | 523.0 | 1420 | 10,903 | 53,050 |
| 38 | Ehime | 1,356,219 | 238.8 | 1053 | 4793 | 72,963 |
| 39 | Kochi | 701,167 | 98.7 | 580 | 4458 | 45,237 |
| 40 | Fukuoka | 5,124,170 | 1057.2 | 19,918 | 73,264 | 443,370 |
| 41 | Saga | 818,222 | 335.3 | 1156 | 7706 | 70,347 |
| 42 | Nagasaki | 1,335,938 | 325.4 | 1306 | 6526 | 91,356 |
| 43 | Kumamoto | 1,758,645 | 242.0 | 3616 | 11,233 | 159,905 |
| 44 | Oita | 1,141,741 | 223.9 | 1585 | 4928 | 76,497 |
| 45 | Miyazaki | 1,087,241 | 160.0 | 1402 | 3537 | 90,348 |
| 46 | Kagoshima | 1,617,517 | 178.8 | 1824 | 8681 | 135,958 |
| 47 | Okinawa | 1,485,118 | 652.2 | 12,733 | 13,124 | 183,673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodera, S.; Ueta, H.; Unemi, T.; Nakata, T.; Hirata, A. Population-Level Immunity for Transient Suppression of COVID-19 Waves in Japan from April 2021 to September 2022. Vaccines 2023, 11, 1457. https://doi.org/10.3390/vaccines11091457
Kodera S, Ueta H, Unemi T, Nakata T, Hirata A. Population-Level Immunity for Transient Suppression of COVID-19 Waves in Japan from April 2021 to September 2022. Vaccines. 2023; 11(9):1457. https://doi.org/10.3390/vaccines11091457
Chicago/Turabian StyleKodera, Sachiko, Haruto Ueta, Tatsuo Unemi, Taisuke Nakata, and Akimasa Hirata. 2023. "Population-Level Immunity for Transient Suppression of COVID-19 Waves in Japan from April 2021 to September 2022" Vaccines 11, no. 9: 1457. https://doi.org/10.3390/vaccines11091457
APA StyleKodera, S., Ueta, H., Unemi, T., Nakata, T., & Hirata, A. (2023). Population-Level Immunity for Transient Suppression of COVID-19 Waves in Japan from April 2021 to September 2022. Vaccines, 11(9), 1457. https://doi.org/10.3390/vaccines11091457






