A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Mice
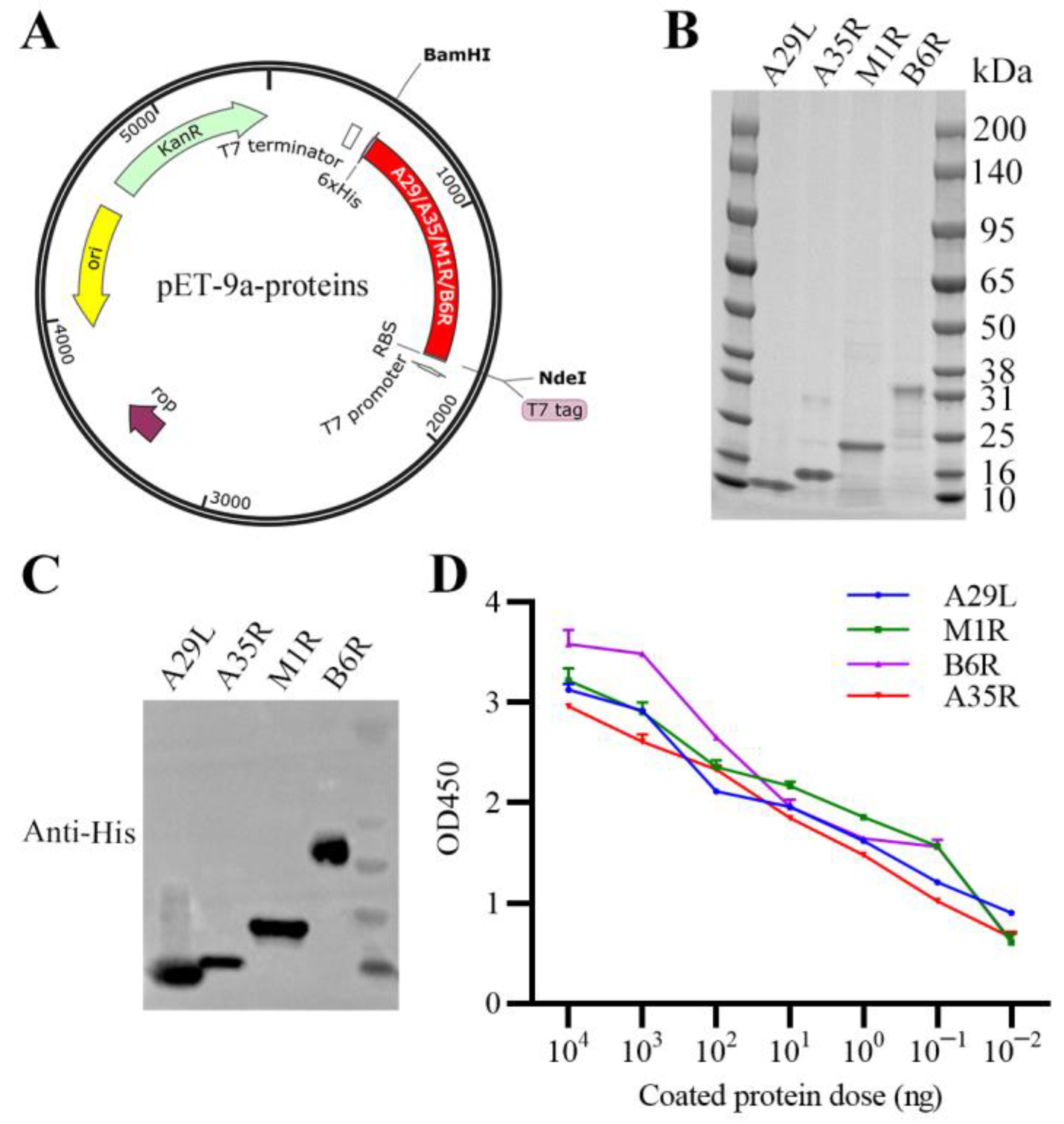
2.2. Protein Expression and Purification
2.3. Western Blotting (WB)
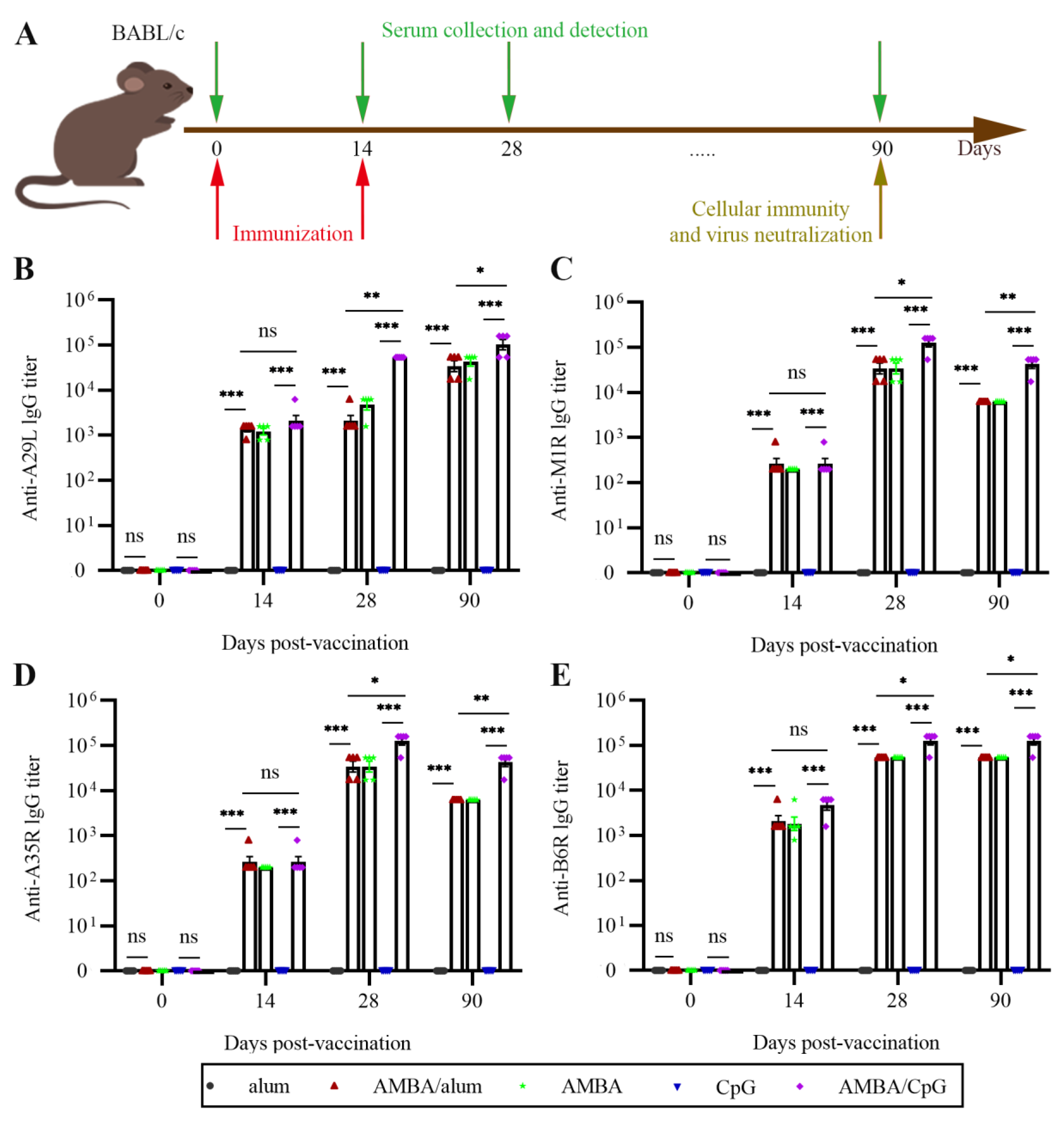
2.4. Mice Immunization
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
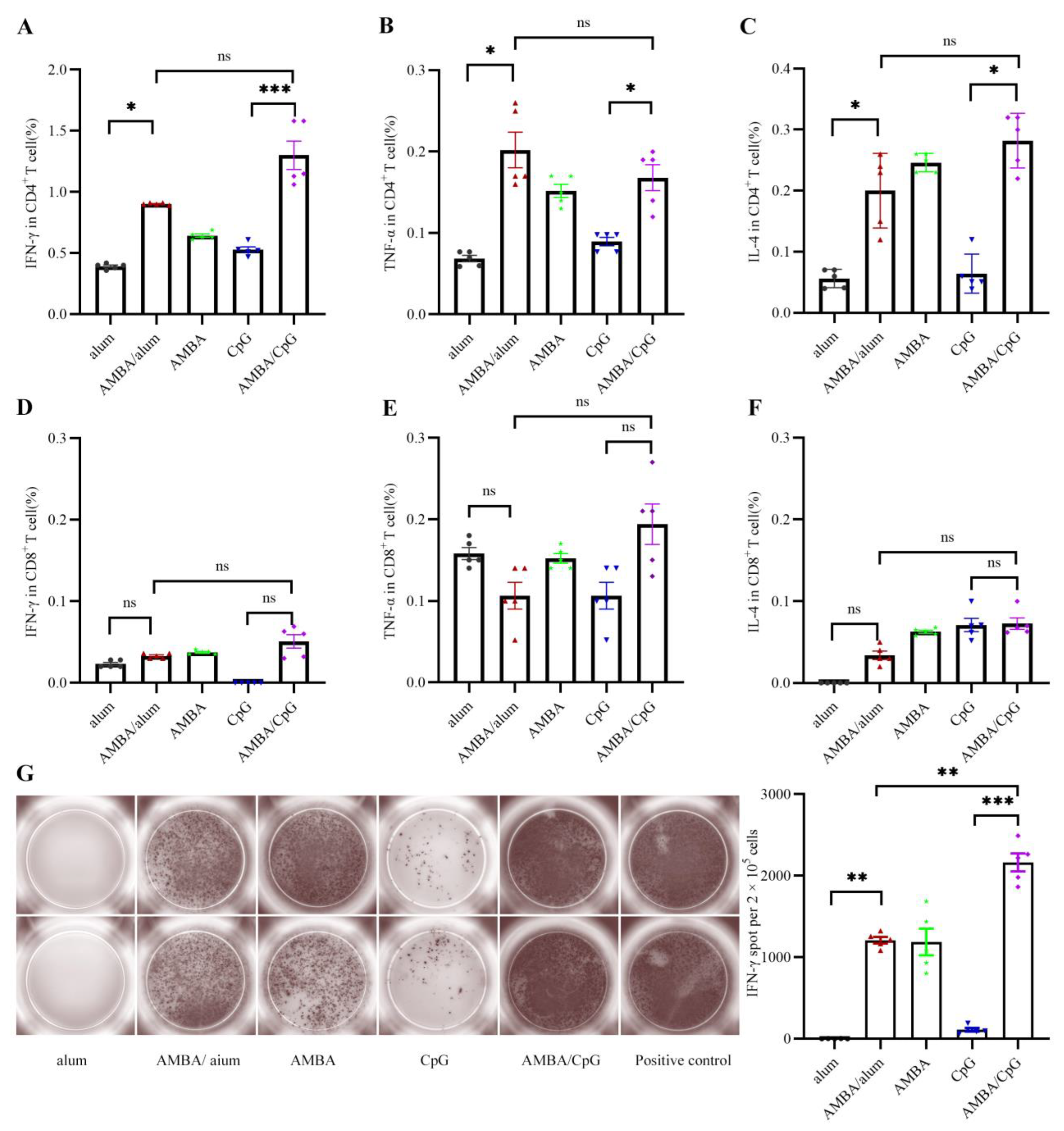
2.6. Flow Cytometry Analysis
2.7. Enzyme-Linked Immunospot (ELISPOT) Assay
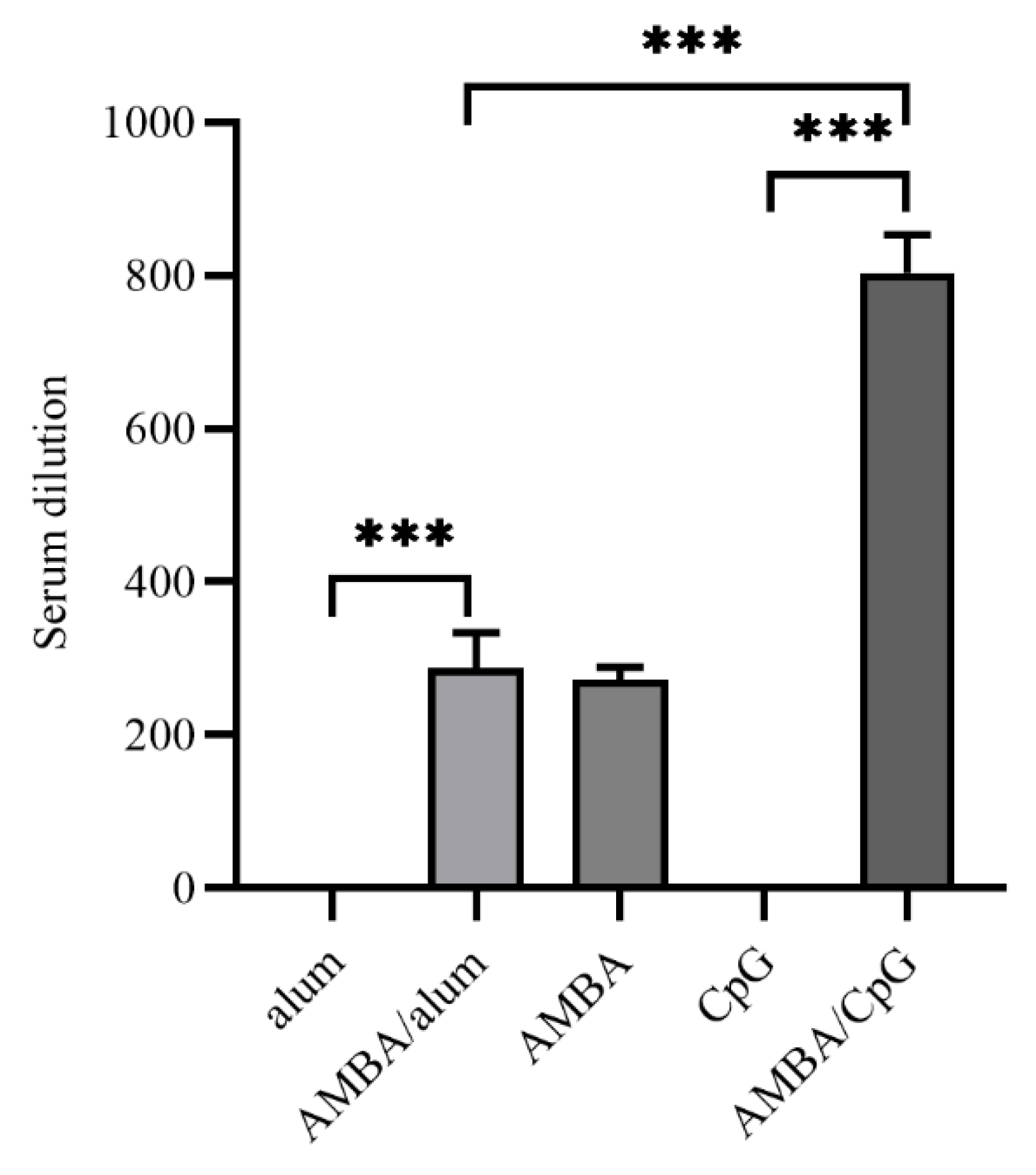
2.8. Virus Neutralization
2.9. Statistical Analysis
3. Results
3.1. Expression, Purification, and Characterization of Recombinant MPXV A29L, M1R, B6R, and A35R
3.2. Immunization with A29L, M1R, B6R, and A35R Elicited High-Level Antigen-Specific Antibodies in Mice
3.3. Immunization with A29L, M1R, B6R, and A35R Elicited Cellular Immune Response in Mice
3.4. Immunization with MPXV A29L, M1R, B6R, and A35R Elicited Neutralizing Antibodies against VACV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 4 July 2023).
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: A re-emergent threat to humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, D.B.; Eckburg, P.B. Human monkeypox: An emerging zoonosis. Lancet Infect. Dis. 2004, 4, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.W.; Harms, T.J.; Reynolds, M.G.; Harrison, L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses—Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. Mmwr-Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sanchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. Mmwr-Morb. Mortal. Wkly. Rep. 2022, 71, 886. [Google Scholar] [CrossRef]
- Álvarez-López, P.; Borras-Bermejo, B.; López Pérez, L.; Antón, A.; Piñana, M.; García-Pérez, J.; Descalzo, V.; Monforte, A.; Martínez-Gómez, X.; Falcó, V.; et al. Suspected case of monkeypox reinfection versus reactivation in a immunocompetent patient, Barcelona, 2022. Int. J. STD AIDS 2023, 34, 9564624231162426. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Canetti, D.; Mileto, D.; Tamburini, A.M.; Candela, C.; Albarello, L.; Bracchitta, F.; Mancon, A.; Micheli, V.; Gismondo, M.R.; et al. Two individuals with potential monkeypox virus reinfection. Lancet Infect. Dis. 2023, 23, 522–524. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Candela, C.; Mileto, D.; Bruzzesi, E.; Canetti, D.; Bertoni, C.; Castagna, A.; Nozza, S. Breakthrough monkeypox infection among individuals previously immunized with smallpox or monkeypox vaccination. J. Infect. 2023, 86, 154–225. [Google Scholar] [CrossRef]
- Musumeci, S.; Najjar, I.; Amari, E.B.E.; Schibler, M.; Jacquerioz, F.; Yerly, S.; Renzoni, A.; Calmy, A.; Kaiser, L. A Case of Mpox Reinfection. Clin. Infect. Dis. 2023, 77, 135–137. [Google Scholar] [CrossRef]
- Golden, J.; Harryman, L.; Crofts, M.; Muir, P.; Donati, M.; Gillett, S.; Irish, C. Case of apparent mpox reinfection. Sex. Transm. Infect. 2023, 99, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Thy, M.; Peiffer-Smadja, N.; Mailhe, M.; Kramer, L.; Ferre, V.M.; Houhou, N.; Tarhini, H.; Bertin, C.; Beaumont, A.L.; Gare, M.; et al. Breakthrough Infections after Postexposure Vaccination against Mpox. N. Engl. J. Med. 2022, 387, 2477–2479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Wang, Z.J.; Zhu, Y.L.; Tang, W.; Zhou, C.; Zhao, S.Q.; Wu, M.; Ming, T.; Deng, Y.Q.; Chen, Q.; et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg. Microbes Infect. 2023, 12, 2192815. [Google Scholar] [CrossRef] [PubMed]
- Pechenick Jowers, T.; Featherstone, R.J.; Reynolds, D.K.; Brown, H.K.; James, J.; Prescott, A.; Haga, I.R.; Beard, P.M. RAB1A promotes Vaccinia virus replication by facilitating the production of intracellular enveloped virions. Virology 2015, 475, 66–73. [Google Scholar] [CrossRef]
- Papukashvili, D.; Rcheulishvili, N.; Liu, C.; Wang, X.; He, Y.; Wang, P.G. Strategy of developing nucleic acid-based universal monkeypox vaccine candidates. Front. Immunol. 2022, 13, 1050309. [Google Scholar] [CrossRef]
- Keasey, S.; Pugh, C.; Tikhonov, A.; Chen, G.; Schweitzer, B.; Nalca, A.; Ulrich, R.G. Proteomic basis of the antibody response to monkeypox virus infection examined in cynomolgus macaques and a comparison to human smallpox vaccination. PLoS ONE 2010, 5, e15547. [Google Scholar] [CrossRef]
- Buchman, G.W.; Cohen, M.E.; Xiao, Y.; Richardson-Harman, N.; Silvera, P.; DeTolla, L.J.; Davis, H.L.; Eisenberg, R.J.; Cohen, G.H.; Isaacs, S.N. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine 2010, 28, 6627–6636. [Google Scholar] [CrossRef]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef]
- Mucker, E.M.; Golden, J.W.; Hammerbeck, C.D.; Kishimori, J.M.; Royals, M.; Joselyn, M.D.; Ballantyne, J.; Nalca, A.; Hooper, J.W. A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge. J. Virol. 2022, 96, e0150421. [Google Scholar] [CrossRef]
- Du, S.; Li, C.; Wang, Y.; Liu, C.; Ren, D.; Li, Y.; Qin, Y.; Wang, M.; Sun, D.; Zhu, N.; et al. Construction and evaluation of a new triple-gene expression cassette vaccinia virus shuttle vector. J. Virol. Methods 2012, 185, 175–183. [Google Scholar] [CrossRef]
- Kalra, A.; Edula, J.R.; Gupta, P.K.; Pandey, A.K.; Chauhan, V.S. Antigenicity of a Bacterially Expressed Triple Chimeric Antigen of Plasmodium falciparum AARP, MSP-311 and MSP-119: PfAMSP-Fu35. PLoS ONE 2016, 11, e0165720. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Zeeshan, M.; Saini, E.; Muneer, A.; Khurana, S.; Kumar Chourasia, B.; Deshmukh, A.; Kaur, I.; Dabral, S.; Singh, N.; et al. Human Cyclophilin B forms part of a multi-protein complex during erythrocyte invasion by Plasmodium falciparum. Nat. Commun. 2017, 8, 1548. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, Q.; Cheng, H.; Tan, X. The Efficacy and Pharmacological Mechanism of Zn7MT3 to Protect against Alzheimer’s Disease. Sci. Rep. 2017, 7, 13763. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Götz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Alec, W.F.; Caroline, A.; Patricia, L.E.; Jeffrey, L.A.; Gwo-Yu, C.; Harini, N.; Tiffany, F.; Jason, G.; Juan, I.M.; Ruth, H.; et al. A monkeypox mRNA-lipid nanoparticle vaccine targeting virus binding, entry, and transmission drives protection against lethal orthopoxviral challenge. BioRxiv 2022, 2022-12, 520886. [Google Scholar]
- Su, C.; Wen, Y.; Geng, X.; Yang, C.; Yin, Q.; Xiong, Y.; Liu, Z. A Quadrivalent mRNA immunization elicits potent immune responses against vaccinia and monkeypox viral antigens—A step closer to a broad orthopoxvirus vaccine. BioRxiv 2023, 2023-04, 537951. [Google Scholar]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. Novel mRNA vaccines encoding Monkeypox virus M1R and A35R protect mice from a lethal virus challenge. BioRxiv 2022, 2022-11, 517190. [Google Scholar]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Wang, X.; et al. Monkeypox virus quadrivalent mRNA vaccine induces antibody responses and cellular immunity and protects mice against Vaccinia virus. BioRxiv 2022, 2022-11, 517500. [Google Scholar]
- Fang, Z.; Paul, A.R.; Kazushi, S.; Sidi, C. Polyvalent mRNA vaccination elicited potent immune response to monkeypox surface antigens. BioRxiv 2022, 2022-11, 518427. [Google Scholar]
- Xia, H.; He, Y.R.; Zhan, X.Y.; Zha, G.F. Mpox virus mRNA-lipid nanoparticle vaccine candidates evoke antibody responses and drive protection against the Vaccinia virus challenge in mice. Antiviral Res. 2023, 216, 105668. [Google Scholar] [CrossRef]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer-Based Covid-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, M.; Yu, W.; Xu, Z.; Liu, X.; Jia, Q.; Guan, X.; Zhang, W. CpG-Based Nanovaccines for Cancer Immunotherapy. Int. J. Nanomed. 2021, 16, 5281–5299. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Artaud, C.; Bay, S.; Ganneau, C.; Campone, M.; Delaloge, S.; Gourmelon, C.; Loirat, D.; Medioni, J.; Pein, F.; et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol. Immunother. CII 2020, 69, 703–716. [Google Scholar] [CrossRef]
- Zent, C.S.; Smith, B.J.; Ballas, Z.K.; Wooldridge, J.E.; Link, B.K.; Call, T.G.; Shanafelt, T.D.; Bowen, D.A.; Kay, N.E.; Witzig, T.E.; et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk. Lymphoma 2012, 53, 211–217. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]
- Panchanathan, V.; Chaudhri, G.; Karupiah, G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 2006, 80, 6333–6338. [Google Scholar] [CrossRef]
- Lum, F.M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia virus vaccines: Past, present and future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef]
- Spriggs, M.K.; Koller, B.H.; Sato, T.; Morrissey, P.J.; Fanslow, W.C.; Smithies, O.; Voice, R.F.; Widmer, M.B.; Maliszewski, C.R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc. Natl. Acad. Sci. USA 1992, 89, 6070–6074. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yang, X.; Du, S.; Hu, C.; Yang, X.; Wang, X.; Hu, X.; Rcheulishvili, N.; Wang, P.G.; Lin, J. A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice. Vaccines 2023, 11, 1420. https://doi.org/10.3390/vaccines11091420
Yang X, Yang X, Du S, Hu C, Yang X, Wang X, Hu X, Rcheulishvili N, Wang PG, Lin J. A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice. Vaccines. 2023; 11(9):1420. https://doi.org/10.3390/vaccines11091420
Chicago/Turabian StyleYang, Xuetao, Xidan Yang, Shouwen Du, Congxia Hu, Xiu Yang, Xingyun Wang, Xing Hu, Nino Rcheulishvili, Peng George Wang, and Jihui Lin. 2023. "A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice" Vaccines 11, no. 9: 1420. https://doi.org/10.3390/vaccines11091420
APA StyleYang, X., Yang, X., Du, S., Hu, C., Yang, X., Wang, X., Hu, X., Rcheulishvili, N., Wang, P. G., & Lin, J. (2023). A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice. Vaccines, 11(9), 1420. https://doi.org/10.3390/vaccines11091420






