Evaluation of Hematological Profiles and Monocyte Subpopulations in Water Buffalo Calves after Immunization with Two Different IBR Marker Vaccines and Subsequent Infection with Bubaline alphaherpesvirus-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Virus
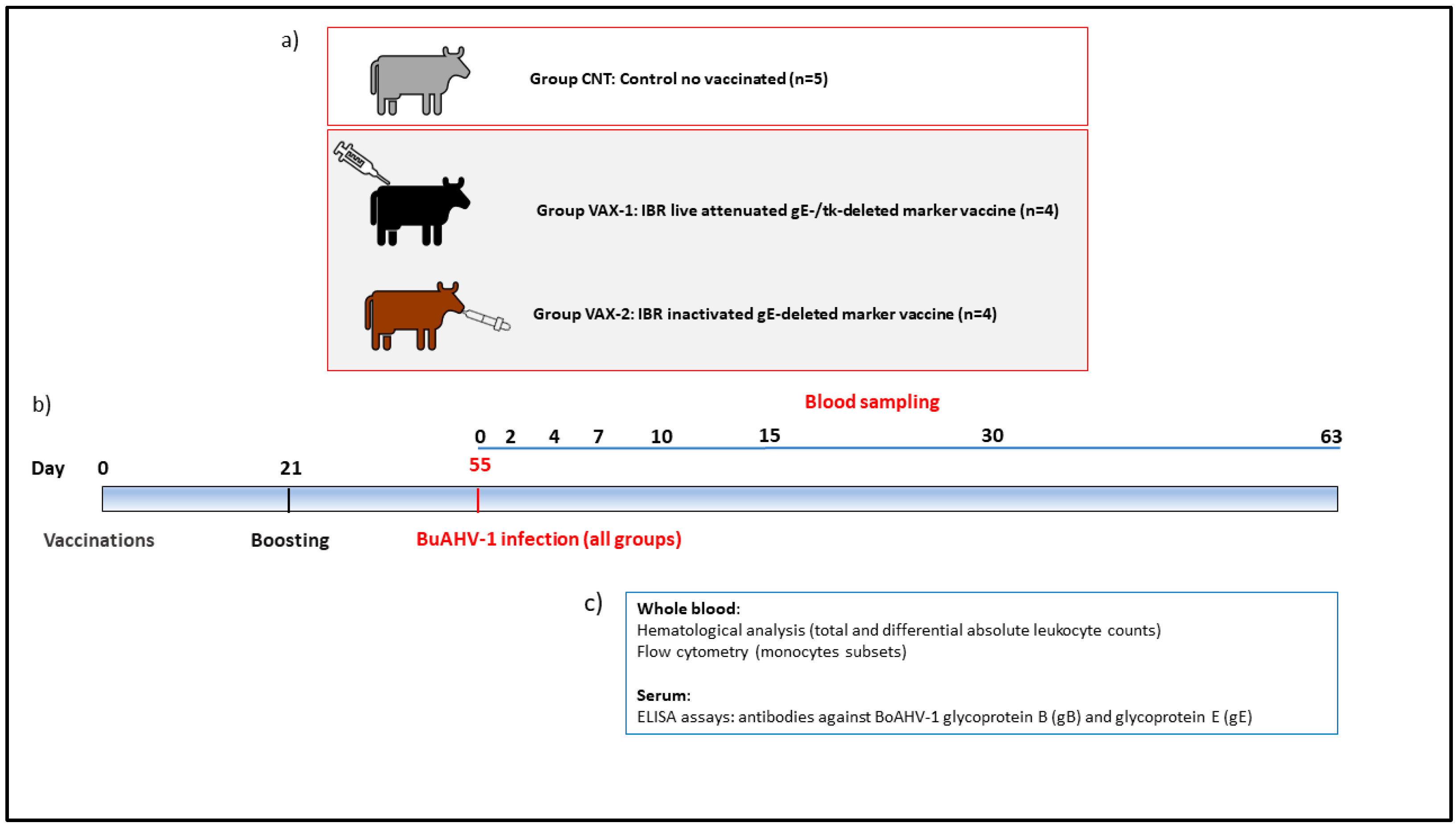
2.3. Experimental Design
2.4. Hematological and Flow Cytometry Analysis
2.5. ELISA Test
2.6. Statistical Analysis
3. Results
3.1. Clinical Observations
3.2. Serological Investigations
3.3. Relative and Absolute Count of the Main Populations of Blood Leukocytes
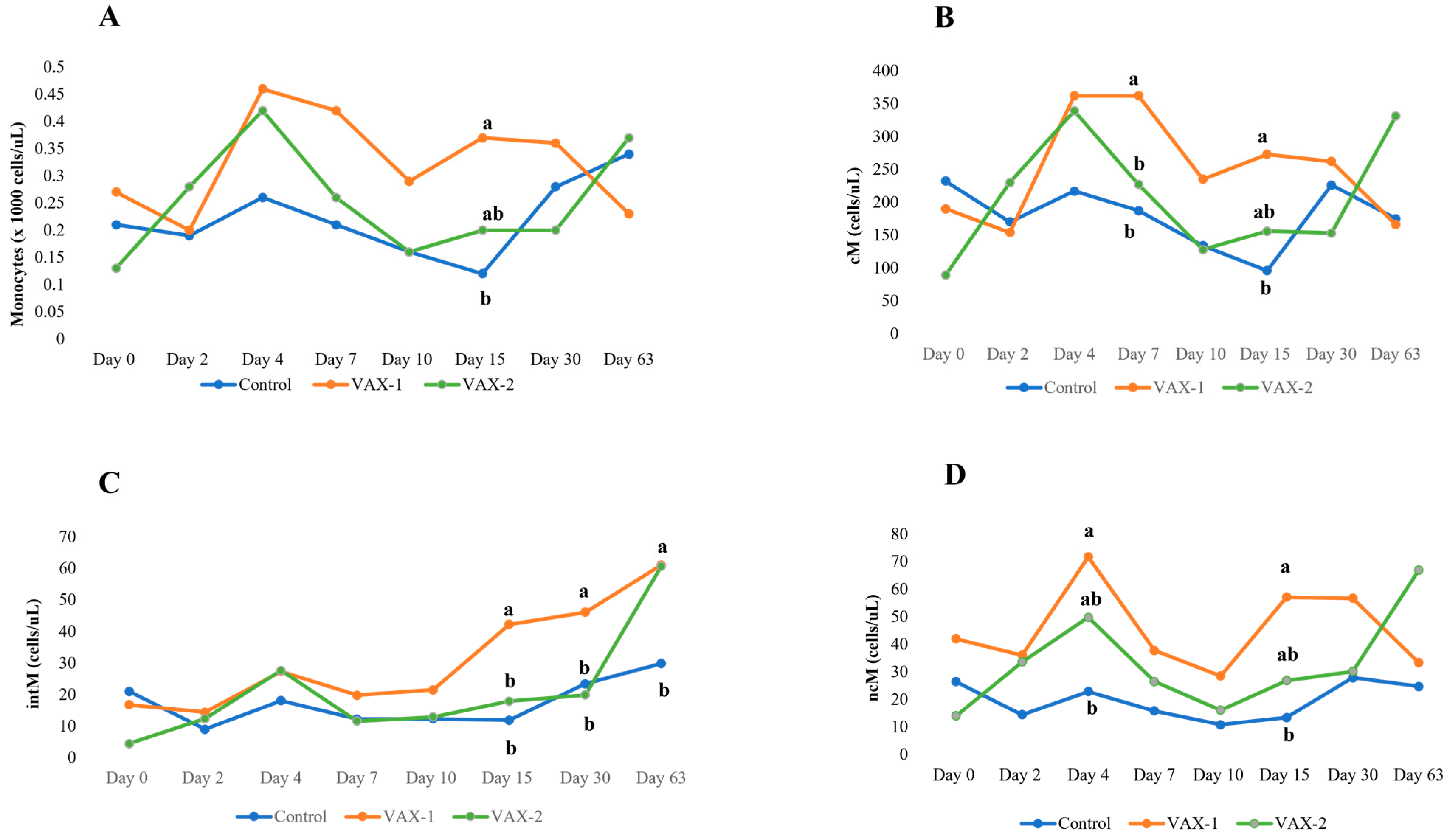
3.4. Flow Cytometric Profiling of Bubaline Monocyte Subsets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2016. [Google Scholar]
- Minervino, A.H.H.; Zava, M.; Vecchio, D.; Borghese, A. Bubalus bubalis: A Short Story. Front. Vet. Sci. 2020, 7, 570413. [Google Scholar] [CrossRef] [PubMed]
- Montagnaro, S.; De Martinis, C.; Iovane, V.; Ciarcia, R.; Damiano, S.; Nizza, S.; De Martino, L.; Iovane, G.; Pagnini, U. Bovine herpesvirus type 1 marker vaccine induces cross-protection against bubaline herpesvirus type 1 in water buffalo. Prev. Vet. Med. 2014, 116, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Maidana, S.S.; Delgado, F.; Vagnoni, L.; Mauroy, A.; Thiry, E.; Romera, S. Cattle are a potential reservoir of bubaline herpesvirus 1 (BuHV1). Vet. Rec. Open 2016, 3, e000162. [Google Scholar] [CrossRef]
- Nandi, S.; Kumar, M.; Manohar, M.; Chauhan, R.S. Bovine herpes virus infections in cattle. Anim. Health Res. Rev. 2009, 10, 85–98. [Google Scholar] [CrossRef]
- Caruso, C.; Prato, R.; Ingravalle, F.; Vecchio, D.; Sciarra, A.; Ternavasio, M.; Ceccarelli, L.; Martucciello, A.; Galiero, G.; De Carlo, E.; et al. Prevalence of antibodies against Bubaline herpesvirus (BuHV-1) among Mediterranean water buffalo (Bubalus bubalis) with implications in buffalo trade. Vet. Q. 2016, 36, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet J. 2014, 201, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Iscaro, C.; Righi, C. Antibody Responses to Bovine Alphaherpesvirus 1 (BoHV-1) in Passively Immunized Calves. Viruses 2019, 11, 23. [Google Scholar] [CrossRef]
- Brock, J.; Lange, M.; Guelbenzu-Gonzalo, M.; Meunier, N.; Vaz, A.M.; Tratalos, J.A.; Dittrich, P.; Gunn, M.; More, S.J.; Graham, D.; et al. Epidemiology of age-dependent prevalence of Bovine Herpes Virus Type 1 (BoHV-1) in dairy herds with and without vaccination. Veter Res. 2020, 51, 124. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, C.A.; Miller, J.M. Two different strains of an alphaherpesvirus can establish latency in the same tissue of the host animal: Evidence from bovine herpesvirus 1. Arch. Virol. 1989, 107, 27–34. [Google Scholar] [CrossRef]
- Babiuk, L.A.; van Drunen Littel-van den Hurk, S.; Tikoo, S.K. Immunology of bovine herpesvirus 1 infection. Vet. Microbiol. 1996, 53, 31–42. [Google Scholar] [CrossRef]
- Collins, J.K.; Butcher, A.C.; Riegel, C.A. Immune response to bovine herpes herpesvirus type 1 infections: Virus-specific antibodies in sera from infected animals. J. Clin. Microbiol. 1985, 21, 546–552. [Google Scholar] [CrossRef]
- Jones, C. Bovine Herpesvirus 1 Counteracts Immune Responses and Immune-Surveillance to Enhance Pathogenesis and Virus Transmission. Front. Immunol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Renjifo, X.; Letellier, C.; Keil, G.M.; Ismaili, J.; Vanderplasschen, A.; Michel, P.; Godfroid, J.; Walravens, K.; Charlier, G.; Pastoret, P.P.; et al. Susceptibility of bovine antigen-presenting cells to infection by bovine herpesvirus 1 and in vitro presentation to T cells: Two independent events. J. Virol. 1999, 73, 4840–4846. [Google Scholar] [CrossRef] [PubMed]
- Righi, C.; Franzoni, G.; Feliziani, F.; Jones, C.; Petrini, S. The Cell-Mediated Immune Response against Bovine alphaherpesvirus 1 (BoHV-1) Infection and Vaccination. Vaccines 2023, 11, 785. [Google Scholar] [CrossRef]
- Nyaga, P.N.; McKercher, D.G. Pathogenesis of bovine herpesvirus-1 (BHV-1) infections: Interactions of the virus with peripheral bovine blood cellular components. Comp. Immunol. Microbiol. Infect. Dis. 1979, 2, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.J.; Qualtiere, L.; Davis, W.C.; Lawman, M.J.; Babiuk, L.A. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1987, 1, 267–286. [Google Scholar] [CrossRef]
- Grandoni, F.; Fraboni, D.; Canonico, B.; Papa, S.; Buccisano, F.; Schuberth, H.J.; Hussen, J. Flow Cytometric Identification and Enumeration of Monocyte Subsets in Bovine and Water Buffalo Peripheral Blood. Curr. Protoc. 2023, 3, e676. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Martucciello, A.; Grandoni, F.; De Matteis, G.; Cappelli, G.; Giammarioli, M.; Scoccia, E.; Grassi, C.; Righi, C.; Fusco, G.; et al. Evaluation of Safety and Efficacy of an Inactivated Marker Vaccine against Bovine alphaherpesvirus 1 (BoHV-1) in Water Buffalo (Bubalus bubalis). Vaccines 2021, 9, 355. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Corrado, F.; De Carlo, E.; Lucibelli, M.G.; Martucciello, A.; Guarino, A.; Galiero, G. Bubaline herpesvirus 1 associated with abortion in a Mediterranean water buffalo. Res. Vet. Sci. 2013, 94, 813–816. [Google Scholar] [CrossRef]
- Internet, N.L.o.M. Bubaline Herpesvirus 1 Strain BuHV-1 Izsm.13 Glycoprotein C (UL44) Gene, Partial Cds. National Library of Medicine. 2013. Available online: https://www.ncbi.nlm.nih.gov/nuccore/kf679678.1 (accessed on 11 July 2023).
- Reed, L.J.; Muench, H.A. A simple method for estimating 50% end points. Am. J. Hyg. 1933, 27, 493–497. [Google Scholar]
- Martucciello, A.; Balestrieri, A.; Righi, C.; Cappelli, G.; Scoccia, E.; Grassi, C.; Brandi, S.; Rossi, E.; Galiero, G.; Gioia, D.; et al. Evaluation of an Immunization Protocol Using Bovine Alphaherpesvirus 1 gE-Deleted Marker Vaccines against Bubaline Alphaherpesvirus 1 in Water Buffaloes. Vaccines 2023, 11, 981. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, M.T.; Caprioli, A.; Saralli, G.; Manna, G.; Barone, A.; Cersini, A.; Cardeti, G.; Condoleo, R.U.; Autorino, G.L. Should the domestic buffalo (Bubalus bubalis) be considered in the epidemiology of Bovine Herpesvirus 1 infection? Vet. Microbiol. 2010, 143, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Maidana, S.S.; Konrad, J.L.; Craig, M.I.; Zabal, O.; Mauroy, A.; Thiry, E.; Crudeli, G.; Romera, S.A. First report of isolation and molecular characterization of bubaline herpesvirus 1 (BuHV1) from Argentinean water buffaloes. Arch. Virol. 2014, 159, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.; Foell, D. Anti-inflammatory monocytes-interplay of innate and adaptive immunity. Mol. Cell. Pediatr. 2018, 5, 5. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; van Vloten, J.P.; Mould, R.C.; Klafuric, E.M.; Minott, J.A.; Wootton, S.K.; Bridle, B.W.; Karimi, K. Myeloid Cells during Viral Infections and Inflammation. Viruses 2019, 11, 168. [Google Scholar] [CrossRef]
- Molina, V.; Risalde, M.A.; Sanchez-Cordon, P.J.; Pedrera, M.; Romero-Palomo, F.; Luzzago, C.; Gomez-Villamandos, J.C. Effect of infection with BHV-1 on peripheral blood leukocytes and lymphocyte subpopulations in calves with subclinical BVD. Res. Vet. Sci. 2013, 95, 115–122. [Google Scholar] [CrossRef]
- Griebel, P.J.; Qualtiere, L.; Davis, W.C.; Gee, A.; Bielefeldt Ohmann, H.; Lawman, M.J.; Babiuk, L.A. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1987, 1, 287–304. [Google Scholar] [CrossRef]
- Filion, L.G.; McGuire, R.L.; Babiuk, L.A. Nonspecific suppressive effect of bovine herpesvirus type 1 on bovine leukocyte functions. Infect. Immun. 1983, 42, 106–112. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef]
- Hussen, J.; Schuberth, H.J. Heterogeneity of Bovine Peripheral Blood Monocytes. Front. Immunol. 2017, 8, 1875. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell. Immunol. 2014, 289, 135–139. [Google Scholar] [CrossRef]
- Rutkowska, E.; Kwiecien, I.; Klos, K.; Rzepecki, P.; Chcialowski, A. Intermediate Monocytes with PD-L1 and CD62L Expression as a Possible Player in Active SARS-CoV-2 Infection. Viruses 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Radrizzani, D.; Vigano, P.; Mazzone, A.; Brando, B. Decrease of Non-Classical and Intermediate Monocyte Subsets in Severe Acute SARS-CoV-2 Infection. Cytometry A 2020, 97, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Talker, S.C.; Barut, G.T.; Lischer, H.E.L.; Rufener, R.; von Munchow, L.; Bruggmann, R.; Summerfield, A. Monocyte biology conserved across species: Functional insights from cattle. Front. Immunol. 2022, 13, 889175. [Google Scholar] [CrossRef]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.R.; Fliser, D.; Heine, G.H. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 2011, 118, e50–e61. [Google Scholar] [CrossRef]
- Hussen, J.; Duvel, A.; Sandra, O.; Smith, D.; Sheldon, I.M.; Zieger, P.; Schuberth, H.J. Phenotypic and functional heterogeneity of bovine blood monocytes. PLoS ONE 2013, 8, e71502. [Google Scholar] [CrossRef]
- Tak, T.; Drylewicz, J.; Conemans, L.; de Boer, R.J.; Koenderman, L.; Borghans, J.A.M.; Tesselaar, K. Circulatory and maturation kinetics of human monocyte subsets in vivo. Blood 2017, 130, 1474–1477. [Google Scholar] [CrossRef]

| Group | Test | Post-Challenge Day (PCD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 7 | 10 | 15 | 30 | 63 | ||
| VAX-1 | gE-ELISA 1 | − | − | − | − | − | − | − | − |
| gB-ELISA 2 | + | + | + | + | + | + | + | + | |
| VAX-2 | gE-ELISA 1 | − | − | − | − | − | − | + | + |
| gB-ELISA 2 | + | + | + | + | + | + | + | + | |
| CNT | gE-ELISA 1 | − | − | − | − | − | − | + | + |
| gB-ELISA 2 | − | − | − | − | + | + | + | + | |
| Post-Challenge Day (PCD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 0 | 2 | 4 | 7 | 10 | 15 | 30 | 63 | |
| Leukocytes (×1000 cells/μL) | CNT | 4.90 ± 0.61 B | 4.28 ± 0.61 | 4.68 ± 0.61 | 4.59 ± 0.61 | 4.74 ± 0.61 | 3.70 ± 0.61 C | 5.96 ± 0.61 A | 6.15 ± 0.61 A |
| VAX-1 | 5.51 ± 0.68 B | 4.88 ± 0.68 | 5.44 ± 0.68 | 5.69 ± 0.68 | 5.21 ± 0.68 | 4.48 ± 0.68 C | 7.16 ± 0.68 A | 6.22 ± 0.68 | |
| VAX-2 | 5.04 ± 0.68 B | 4.45 ± 0.68 | 4.66 ± 0.68 | 5.15 ± 0.68 | 5.15 ± 0.68 | 3.73 ± 0.68 C | 6.90 ± 0.68 A | 6.69 ± 0.68 A | |
| Neutrophils (%) | CNT | 41.0 ± 4.1 B | 45.2 ± 4.1 | 43.5 ± 4.1 | 46.3 ± 4.1 | 49.3 ± 4.1 A | 43.9 ± 4.1 | 52.7 ± 4.1 A | 50.3 ± 4.1 A |
| VAX-1 | 50.7 ± 4.5 B | 53.5 ± 4.5 | 52.9 ± 4.5 | 56.6 ± 4.5 | 54.1 ± 4.5 | 51.0 ± 4.5 | 57.7 ± 4.5 A | 52.0 ± 4.5 | |
| VAX-2 | 52.1 ± 4.5 B | 54.4 ± 4.5 | 49.6 ± 4.5 | 57.7 ± 4.5 | 60.5 ± 4.5 A | 49.8 ± 4.5 | 61.2 ± 4.5 A | 56.3 ± 4.5 | |
| Neutrophils (cells/μL) | CNT | 1.91 ± 0.27 b-B | 1.86 ± 0.27 | 1.95 ± 0.27 b | 2.02 ± 0.27 b | 2.24 ± 0.27 b | 1.53 ± 0.27 | 3.12 ± 0.27 b-A | 3.03 ± 0.27 A |
| VAX-1 | 2.78 ± 0.30 a-B | 2.60 ± 0.30 | 2.87 ± 0.30 a | 3.22 ± 0.30 a | 2.80 ± 0.30 ab | 2.28 ± 0.30 | 4.13 ± 0.30 a-A | 3.23 ± 0.30 | |
| VAX-2 | 2.61 ± 0.30 ab-B | 2.41 ± 0.30 | 2.32 ± 0.30 ab | 2.93 ± 0.30 a | 3.10 ± 0.30 a | 1.85 ± 0.30 C | 4.19 ± 0.30 a-A | 3.75 ± 0.30 A | |
| Lymphocytes (%) | CNT | 50.9 ± 4.2 A | 49.5 ± 4.2 | 50.3 ± 4.2 a | 46.0 ± 4.2 a | 46.1 ± 4.2 | 51.3 ± 4.2 | 41.3 ± 4.2 B | 42.5 ± 4.2 B |
| VAX-1 | 44.0 ± 4.7 A | 41.5 ± 4.7 | 36.9 ± 4.7 b-B | 34.6 ± 4.7 b-B | 39.2 ± 4.7 | 39.6 ± 4.7 | 35.9 ± 4.7 B | 43.6 ± 4.7 | |
| VAX-2 | 45.2 ± 4.7 A | 38.6 ± 4.7 B | 40.6 ± 4.7 ab | 36.0 ± 4.7 ab-B | 35.9 ± 4.7 B | 44.4 ± 4.7 | 34.3 ± 4.7 B | 37.5 ± 4.7 | |
| Lymphocytes (cells/μL) | CNT | 2.65 ± 0.41 A | 2.19 ± 0.41 B | 2.44 ± 0.41 | 2.31 ± 0.41 | 2.29 ± 0.41 | 2.01 ± 0.41 B | 2.51 ± 0.41 | 2.70 ± 0.41 |
| VAX-1 | 2.43 ± 0.46 A | 2.02 ± 0.46 | 2.01 ± 0.46 | 1.98 ± 0.46 | 2.07 ± 0.46 | 1.79 ± 0.46 B | 2.58 ± 0.46 | 2.71 ± 0.46 | |
| VAX-2 | 2.30 ± 0.46 A | 1.72 ± 0.46 B | 1.90 ± 0.46 | 1.92 ± 0.46 | 1.86 ± 0.46 | 1.67 ± 0.46 B | 2.40 ± 0.46 | 2.50 ± 0.46 | |
| Monocytes (%) | CNT | 5.1 ± 1.4 | 4.2 ± 1.4 | 5.6 ± 1.4 | 5.6 ± 1.4 | 3.6 ± 1.4 | 3.6 ± 1.4 b | 5.2 ± 1.4 | 6.0 ± 1.4 |
| VAX-1 | 4.6 ± 1.6 | 4.0 ± 1.6 | 8.6 ± 1.6 | 7.3 ± 1.6 | 5.7 ± 1.6 | 8.2 ± 1.6 a | 5.2 ± 1.6 | 3.8 ± 1.6 | |
| VAX-2 | 2.7 ± 1.6 B | 6.2 ± 1.6 | 9.2 ± 1.6 A | 5.6 ± 1.6 | 3.1 ± 1.6 | 5.3 ± 1.6 ab | 3.0 ± 1.6 | 5.3 ± 1.6 | |
| Monocytes (×1000 cells/μL) | CNT | 0.21 ± 0.07 | 0.19 ± 0.07 | 0.26 ± 0.07 | 0.21 ± 0.07 | 0.16 ± 0.07 | 0.12 ± 0.07 b | 0.28 ± 0.07 | 0.34 ± 0.07 |
| VAX-1 | 0.27 ± 0.08 | 0.20 ± 0.06 | 0.46 ± 0.08 | 0.42 ± 0.08 | 0.29 ± 0.08 | 0.37 ± 0.08 a | 0.36 ± 0.08 | 0.23 ± 0.08 | |
| VAX-2 | 0.13 ± 0.08 B | 0.28 ± 0.08 | 0.42 ± 0.08 A | 0.26 ± 0.08 | 0.16 ± 0.08 | 0.20 ± 0.08 ab | 0.20 ± 0.08 | 0.37 ± 0.08 A | |
| Post-Challenge Day (PCD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 0 | 2 | 4 | 7 | 10 | 15 | 30 | 63 | |
| cM cells (%) | CNT | 79.9 ± 3.3 B | 86.8 ± 3.3 a-A | 84.1 ± 3.3 | 87.5 ± 3.3 A | 84.4 ± 3.3 | 81.1 ± 3.3 | 81.1 ± 3.3 | 81.8 ± 4.1 a |
| VAX-1 | 75.6 ± 3.6 B | 74.0 ± 3.6 b | 78.9 ± 3.6 | 86.2 ± 3.6 A | 83.7 ± 3.6 A | 75.9 ± 3.6 | 74.4 ± 3.6 | 65.8 ± 4.3 b-C | |
| VAX-2 | 81.2 ± 3.6 A | 83.6 ± 3.6 ab | 82.4 ± 3.6 | 84.8 ± 3.6 | 81.5 ± 3.6 | 75.7 ± 3.6 | 80.1 ± 3.6 | 73.2 ± 3.9 ab-B | |
| cM cells (cells/μL) | CNT | 232 ± 53 A | 170 ± 53 | 217 ± 53 | 187 ± 53 b | 134 ± 53 | 96 ± 53 b-B | 226 ± 53 | 175 ± 83 |
| VAX-1 | 190 ± 60 B | 154 ± 60 | 362 ± 60 A | 362 ± 60 a-A | 235 ± 60 | 273 ± 60 a | 262 ± 60 | 166 ± 83 | |
| VAX-2 | 89 ± 60 B | 230 ± 60 | 339 ± 60 A | 227 ± 60 ab | 128 ± 60 | 156 ± 60 ab | 153 ± 60 | 331 ± 68 A | |
| intM cells (%) | CNT | 7.3 ± 1.8 | 5.1 ± 1.8 | 6.9 ± 1.8 | 5.8 ± 1.8 | 8.6 ± 1.8 | 9.1 ± 1.8 | 8.5 ± 1.8 | 10.3 ± 2.4 b |
| VAX-1 | 6.8 ± 2.0 B | 8.2 ± 2.0 | 6.0 ± 2.0 | 4.7 ± 2.0 | 6.4 ± 2.0 | 11.3 ± 2.0 A | 12.1 ± 2.0 A | 20.6 ± 2.5 a-A | |
| VAX-2 | 5.0 ± 2.0 B | 4.7 ± 2.0 | 6.6 ± 2.0 | 5.0 ± 2.0 | 8.4 ± 2.0 | 9.0 ± 2.0 A | 8.3 ± 2.0 | 12.8 ± 2.2 b-A | |
| intM cells (cells/μL) | CNT | 20.96 ± 7.97 | 8.96 ± 7.97 | 18.05 ± 7.97 | 12.19 ± 7.97 | 12.28 ± 7.97 | 11.83 ± 7.97 b | 23.38 ± 7.97 b | 29.85 ± 11.43 b |
| VAX-1 | 16.71 ± 8.91 B | 14.37 ± 8.91 | 27.28 ± 8.91 | 19.78 ± 8.91 | 21.47 ± 8.91 | 42.23 ± 8.91 a-A | 46.04 ± 8.91 a-A | 61.07 ± 11.63 a-A | |
| VAX-2 | 4.36 ± 8.91 B | 12.29 ± 8.91 | 27.58 ± 8.91 A | 11.56 ± 8.91 | 12.86 ± 8.91 | 17.86 ± 8.91 b | 19.83 ± 8.91 b | 60.69 ± 9.90 a-A | |
| ncM cells (%) | CNT | 13.0 ± 2.4 A | 8.2 ± 2.4 b | 9.1 ± 2.4 | 6.8 ± 2.4 B | 7.0 ± 2.4 B | 10.2 ± 2.4 | 10.5 ± 2.4 | 8.3 ± 3.2 |
| VAX-1 | 17.8 ± 2.6 A | 18.0 ± 2.6 a | 15.2 ± 2.6 | 9.1 ± 2.6 B | 9.9 ± 2.6 B | 13.3 ± 2.6 | 13.6 ± 2.6 | 12.2 ± 3.3 B | |
| VAX-2 | 14.0 ± 2.6 | 11.9 ± 2.6 ab | 11.1 ± 2.6 | 10.2 ± 2.6 | 10.1 ± 2.6 | 15.4 ± 2.6 | 11.8 ± 2.6 | 13.9 ± 2.9 | |
| ncM cells (cells/μL) | CNT | 26.39 ± 11.75 | 14.45 ± 11.75 | 22.79 ± 11.75 b | 15.80 ± 11.75 | 10.81 ± 11.75 | 13.41 ± 11.75 b | 27.85 ± 11.75 | 24.65 ± 17.20 |
| VAX-1 | 41.96 ± 13.13 | 36.06 ± 13.13 | 71.65 ± 13.13 a | 37.74 ± 13.13 | 28.50 ± 13.13 | 57.08 ± 13.13 a | 56.66 ± 13.13 | 33.27 ± 17.44 | |
| VAX-2 | 14.04 ± 13.13 B | 33.58 ± 13.13 | 49.71 ± 13.13 ab-A | 26.48 ± 13.13 | 16.06 ± 13.13 | 26.79 ± 13.13 ab | 30.12 ± 13.13 | 66.94 ± 14.71 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandoni, F.; Hussen, J.; Signorelli, F.; Napolitano, F.; Scatà, M.C.; De Donato, I.; Cappelli, G.; Galiero, G.; Grassi, C.; De Carlo, E.; et al. Evaluation of Hematological Profiles and Monocyte Subpopulations in Water Buffalo Calves after Immunization with Two Different IBR Marker Vaccines and Subsequent Infection with Bubaline alphaherpesvirus-1. Vaccines 2023, 11, 1405. https://doi.org/10.3390/vaccines11091405
Grandoni F, Hussen J, Signorelli F, Napolitano F, Scatà MC, De Donato I, Cappelli G, Galiero G, Grassi C, De Carlo E, et al. Evaluation of Hematological Profiles and Monocyte Subpopulations in Water Buffalo Calves after Immunization with Two Different IBR Marker Vaccines and Subsequent Infection with Bubaline alphaherpesvirus-1. Vaccines. 2023; 11(9):1405. https://doi.org/10.3390/vaccines11091405
Chicago/Turabian StyleGrandoni, Francesco, Jamal Hussen, Federica Signorelli, Francesco Napolitano, Maria Carmela Scatà, Immacolata De Donato, Giovanna Cappelli, Giorgio Galiero, Carlo Grassi, Esterina De Carlo, and et al. 2023. "Evaluation of Hematological Profiles and Monocyte Subpopulations in Water Buffalo Calves after Immunization with Two Different IBR Marker Vaccines and Subsequent Infection with Bubaline alphaherpesvirus-1" Vaccines 11, no. 9: 1405. https://doi.org/10.3390/vaccines11091405
APA StyleGrandoni, F., Hussen, J., Signorelli, F., Napolitano, F., Scatà, M. C., De Donato, I., Cappelli, G., Galiero, G., Grassi, C., De Carlo, E., Petrini, S., De Matteis, G., & Martucciello, A. (2023). Evaluation of Hematological Profiles and Monocyte Subpopulations in Water Buffalo Calves after Immunization with Two Different IBR Marker Vaccines and Subsequent Infection with Bubaline alphaherpesvirus-1. Vaccines, 11(9), 1405. https://doi.org/10.3390/vaccines11091405






