No Significant Association between 25-OH Vitamin D Status and SARS-CoV-2 Antibody Response after COVID-19 Vaccination in Nursing Home Residents and Staff
Abstract
1. Background
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethics
2.3. Sample Collection
2.4. Antibody Detection
2.5. Pseudovirus Neutralization Assay
2.6. 25-Hydroxyvitamin D Detection and Supplementation
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
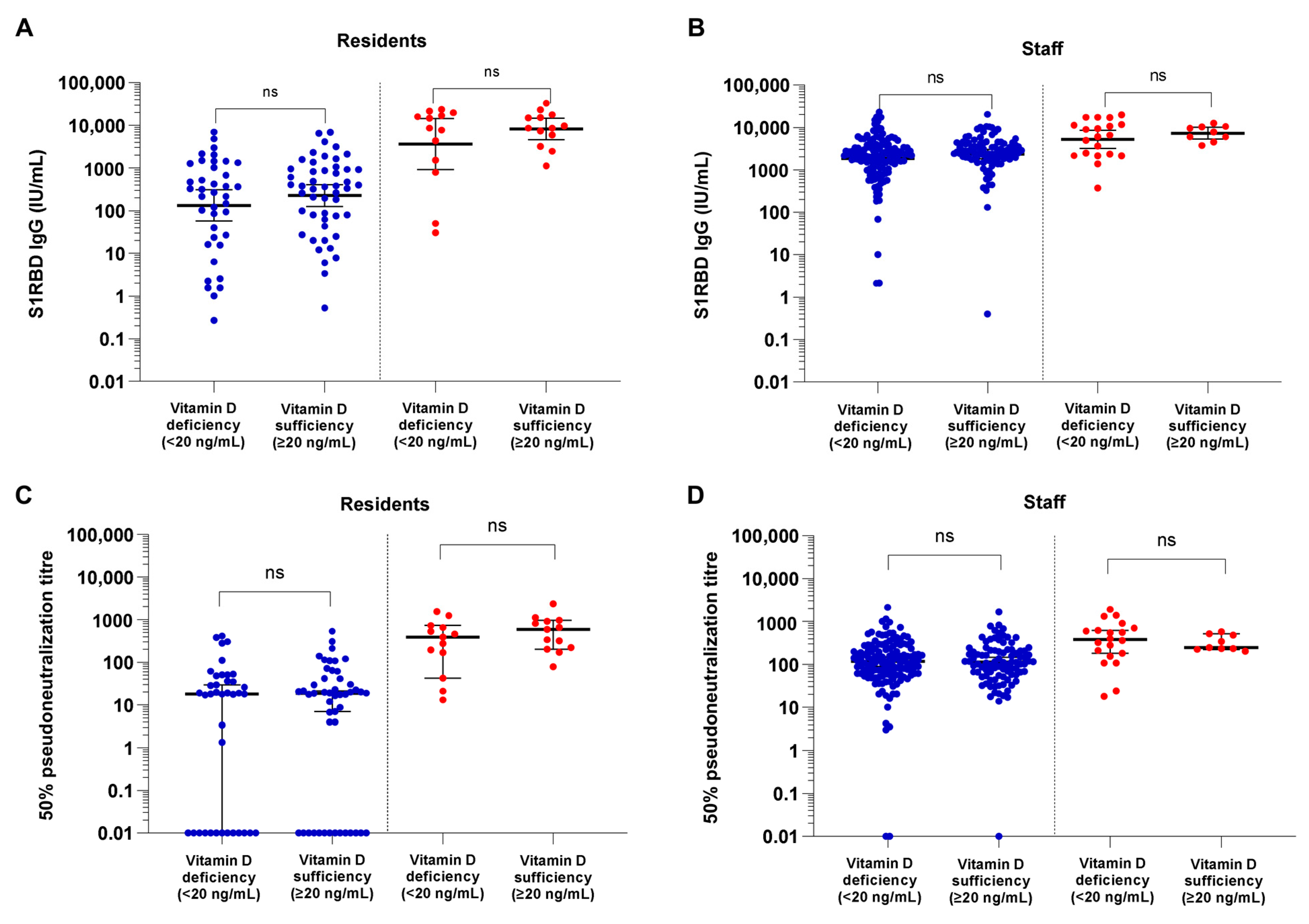
3.2. COVID-19 Vaccine Binding and Neutralizing Antibody Responses in Vitamin-D-Deficient versus Sufficient NHRs and NHS
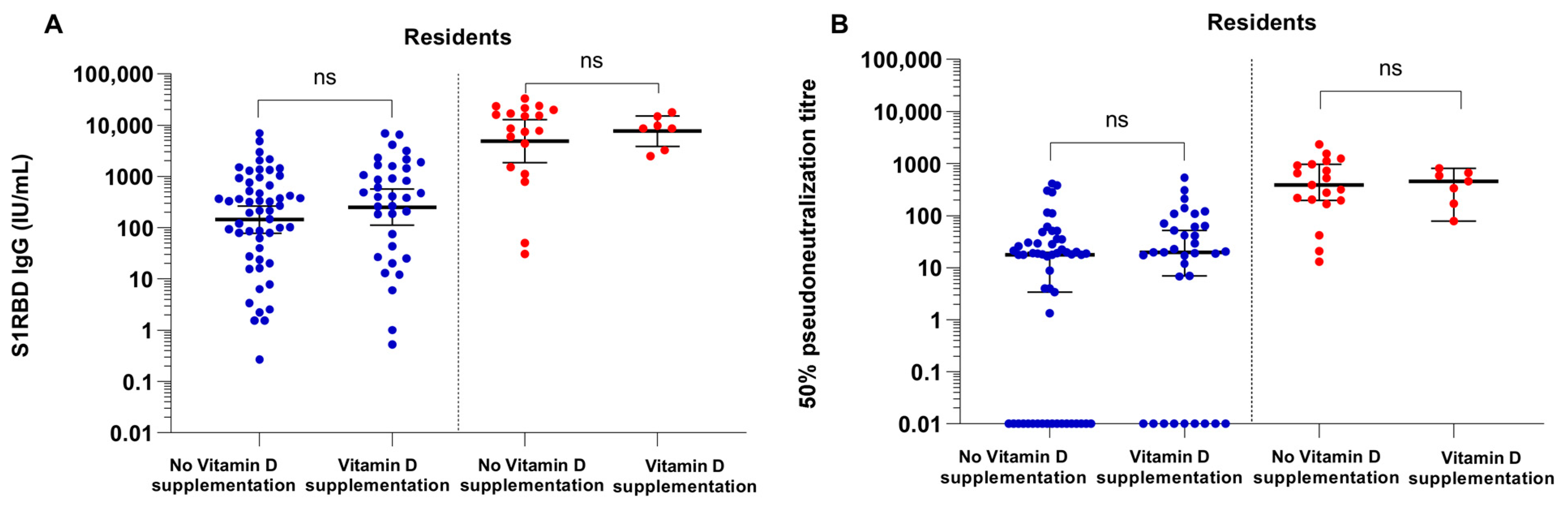
3.3. COVID-19 Vaccine Binding and Neutralizing Antibody Responses in Vitamin D Supplemented NHRs versus Non-Supplemented NHR
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikle, D.D. Vitamin D and Bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Łukaszkiewicz, J.; Wrzosek, M.; Jakubczyk, A.; Matsumoto, H.; Piątkiewicz, P.; Radziwoń-Zaleska, M.; Wojnar, M.; Nowicka, G. Vitamin D and the central nervous system. Pharmacol. Rep. 2013, 65, 271–278. [Google Scholar] [CrossRef]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; AP Steegers, E.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on Bone Regeneration: A Review. Medicina 2022, 58, 1337. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Gunville, C.F.; Mourani, P.M.; Ginde, A.A. The Role of Vitamin D in Prevention and Treatment of Infection. Inflamm. Allergy-Drug Targets 2013, 12, 239–245. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.E.; Dahlback, A.; Ma, L.; Juzeniene, A. Influenza, solar radiation and vitamin D. Dermato-Endocrinology 2009, 1, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.R.; Wang, M.; Aboud, S.; Giovannucci, E.L.; Mugusi, F.M.; Fawzi, W.W. Vitamin D and HIV Progression among Tanzanian Adults Initiating Antiretroviral Therapy. PLoS ONE 2012, 7, e40036. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Sampson, M.; Matheson, L.A.; Hutton, B.; Little, J. Vitamin D receptor (VDR) polymorphisms and severe RSV bronchiolitis: A systematic review and meta-analysis. Pediatr. Pulmonol. 2013, 49, 790–799. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Campi, I.; Gennari, L.; Merlotti, D.; Mingiano, C.; Frosali, A.; Giovanelli, L.; Torlasco, C.; Pengo, M.F.; Heilbron, F.; Soranna, D.; et al. Vitamin D and COVID-19 severity and related mortality: A prospective study in Italy. BMC Infect. Dis. 2021, 21, 566. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Zahari, N.; Shah, S.; Kandiah, P.; Van den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2020, 97, 1149. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.D.; Galvão Azevedo, L.M.G.; de Almeida Oliveira, T.D.A.; da Mota Santana, J.D.M. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2021, 96, 281–287. [Google Scholar] [CrossRef]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Cohen, A.G.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Vivaldi, G.; Chambers, E.S.; Cai, W.; Li, W.; Faustini, S.E.; Gibbons, J.M.; Pade, G.; Coussens, A.K.; Richter, A.K.; et al. Vitamin D Supplementation Does Not Influence SARS-CoV-2 Vaccine Efficacy or Immunogenicity: Sub-Studies Nested within the CORONAVIT Randomised Controlled Trial. Nutrients 2022, 14, 3821. [Google Scholar] [CrossRef]
- Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines 2021, 9, 1714. [Google Scholar] [CrossRef]
- Öztürk, R.; Yılmaz, N.S.; Ulukanlıgil, M. The relationship between serum vitamin D and antibody response following two doses of inactivated COVID-19 vaccine. Turk. J. Biochem. 2022, 47, 665–671. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef] [PubMed]
- Piec, I.; Cook, L.; Dervisevic, S.; Fraser, W.D.; Ruetten, S.; Berman, M.; English, E.; John, W.G. Age and vitamin D affect the magnitude of the antibody response to the first dose of the SARS-CoV-2 BNT162b2 vaccine. Curr. Res. Transl. Med. 2022, 70, 103344. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Faustini, S.E.; Holt, H.; Perdek, N.; Maltby, S.; Talaei, M.; Greenig, M.; Vivaldi, G.; Tydeman, F.; Symons, J.; et al. Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK). Vaccines 2022, 10, 1601. [Google Scholar] [CrossRef]
- Feehan, O.; Magee, P.J.; Pourshahidi, L.K.; Armstrong, D.J.; McSorley, E.M. Vitamin D deficiency in nursing home residents: A systematic review. Nutr. Rev. 2022, 81, 804–822. [Google Scholar] [CrossRef]
- Arnljots, R.; Thorn, J.; Elm, M.; Moore, M.; Sundvall, P.-D. Vitamin D deficiency was common among nursing home residents and associated with dementia: A cross sectional study of 545 Swedish nursing home residents. BMC Geriatr. 2017, 17, 229. [Google Scholar] [CrossRef]
- Goldberg, T.H.; Hassan, T.; Grant, R. High Prevalence of Vitamin D Deficiency in Elderly Nursing Home Patients Despite Vitamin Supplements. J. Am. Med. Dir. Assoc. 2008, 9, B15. [Google Scholar] [CrossRef]
- Bruyere, O.; Decock, C.; Delhez, M.; Collette, J.; Reginster, J.-Y. Highest Prevalence of Vitamin D Inadequacy in Institutionalized Women Compared with Noninstitutionalized Women: A Case–Control Study. Women Health 2009, 5, 49–54. [Google Scholar] [CrossRef]
- Meyers, E.; Deschepper, E.; Duysburgh, E.; De Rop, L.; De Burghgraeve, T.; Van Ngoc, P.; Digregorio, M.; Delogne, S.; Coen, A.; De Clercq, N.; et al. Declining Prevalence of SARS-CoV-2 Antibodies among Vaccinated Nursing Home Residents and Staff Six Months after the Primary BNT162b2 Vaccination Campaign in Belgium: A Prospective Cohort Study. Viruses 2022, 14, 2361. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, W.; Gerlo, S.; De Smet, E.; Wejda, M.; Acar, D.; Callens, S.; Heytens, S.; Padalko, E.; Vercruysse, H.; Cools, P.; et al. Humoral and Cellular Responses to COVID-19 Vaccination Indicate the Need for Post-Vaccination Testing in Frail Population. Vaccines 2022, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.; Coen, A.; De Sutter, A.; Padalko, E.; Callens, S.; Vandekerckhove, L.; Witkowski, W.; Heytens, S.; Cools, P. Diagnostic performance of the SARS-CoV-2 S1RBD IgG ELISA (Immuno Diagnostics) for the quantitative detection of SARS-CoV-2 antibodies on dried blood spots. J. Clin. Virol. 2022, 155, 105270. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. Impact of Vitamin D Supplementation on Influenza Vaccine Response and Immune Functions in Deficient Elderly Persons: A Randomized Placebo-Controlled Trial. Front. Immunol. 2019, 10, 65. [Google Scholar] [CrossRef]
- Lee, R.U.; Won, S.H.; Hansen, C.; Crum-Cianflone, N.F. 25-hydroxyvitamin D, influenza vaccine response and healthcare encounters among a young adult population. PLoS ONE 2018, 13, e0192479. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Talbot, H.K.; Zhu, Y.; Griffin, M.R.; Spencer, S.; Shay, D.K.; Coleman, L.A. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 2013, 31, 2057–2061. [Google Scholar] [CrossRef]
- Sadarangani, S.P.; Young, B.E.; Lian, W.; Phua, H.P.; Chen, M.I.-C.; Barr, I.; Yeo, T.W.; Dalan, R.; Chow, A. DYNAMIC cohort study evaluating metabolic predictors of influenza vaccine immune response in older adults. NPJ Vaccines 2022, 7, 135. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, V.; Vanpuyenbroeck, K.; Lopez-Hartmann, M.; Wens, J.; Remmen, R. Walk on the sunny side of life—Epidemiology of hypovitaminosis D and mental health in elderly nursing home residents. J. Nutr. Health Aging 2011, 16, 417–420. [Google Scholar] [CrossRef]
- BCFI. Formularium Ouderenzorg: Vitamine D. Available online: https://farmaka.bcfi.be/nl/formularium/282 (accessed on 14 June 2023).
- Arshad, S.; Zaidi, S.J.A. Vitamin D levels among children, adolescents, adults, and elders in Pakistani population: A cross-sectional study. BMC Public Health 2022, 22, 2040. [Google Scholar] [CrossRef]
- Kweder, H.; Eidi, H. Vitamin D deficiency in elderly: Risk factors and drugs impact on vitamin D status. Avicenna J. Med. 2018, 8, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, N.; Turan, H.; Bayraktar, M.; Ozturk, A.; Erdoğdu, H. Analysis of serum cytokine and protective vitamin D levels in severe cases of COVID-19. J. Med. Virol. 2021, 94, 154–160. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022, 13, 836738. [Google Scholar] [CrossRef]
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Xiong, A.; Sun, R.; Qiu, L.; Xie, Z. Vitamin D supplementation reduces the risk of fall in the vitamin D deficient elderly: An updated meta-analysis. Clin. Nutr. 2021, 40, 5531–5537. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Muniz-Terrera, G.; Phillips, C.L.; Cherubini, A.; Ferrucci, L.; Melzer, D. Vitamin D and Risk of Cognitive Decline in Elderly Persons. Arch. Intern. Med. 2010, 170, 1135–1141. [Google Scholar] [CrossRef]
- Miller, J.W.; Harvey, D.J.; Beckett, L.A.; Green, R.; Farias, S.T.; Reed, B.R.; Olichney, J.M.; Mungas, D.M.; DeCarli, C. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 2015, 72, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Lapid, M.; Takahashi, P.Y.; Cha, S.S. Vitamin D and depression in geriatric primary care patients. Clin. Interv. Aging 2013, 8, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.W.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed]
| Nursing Home Residents (n = 115) | Nursing Home Staff (n = 254) | |
|---|---|---|
| Sociodemographic characteristics | ||
| Female, n (%) | 93 (81%) | 208 (82%) |
| Age, median (interquartile range) | 89 (86–93) | 51 (36–63) |
| 25(OH)D | ||
| 25(OH)D serum concentration (ng/mL), median (interquartile range) | 20.80 (16.85–24.75) | 18.19 (14.94–23.02) |
| 25(OH)D serum concentration non-supplemented (ng/mL), median (interquartile range) | 13.39 (10.82–22.42) | NA |
| 25(OH)D serum concentration supplemented (ng/mL), median (interquartile range) | 27.97 (24.82–33.49) | NA |
| 25(OH)D serum concentration previous SARS-CoV-2 infection (ng/mL), median (interquartile range) | 19.71 (11.52–28.29) | 18.52 (16.90–20.82) |
| 25(OH)D serum concentration no history of SARS-CoV-2 infection (ng/mL), median (interquartile range) | 22.56 (13.13–26.69) | 18.22 (14.94–23.26) |
| 25-OH Vitamin D deficiency (<20 ng/mL), n (%; 95% CI) | 52 (45%; 36–54%) | 153 (60%; 54–66%) |
| 25-OH Vitamin D severe deficiency (<12 ng/mL), n (%; 95% CI) | 28 (24%; 17–33%) | 27 (11%; 7–15%) |
| Vitamin D supplementation, n (%) | 42 (37%) | NA |
| SARS-CoV-2 | ||
| Previous SARS-CoV-2 infection, n (%) a | 26 (23%) | 29 (11%) |
| SARS-CoV-2 IgG concentration post-vaccination, geometric mean (interquartile range) | 388.24 (82.15–2237.43) | 2286.64 (1630.52–4521.25) |
| SARS-CoV-2 50% pseudoneutralization titer, median (interquartile range) | 20.96 (2.35–153.3) | 124.00 (61.71–271.48) |
| Prevalence seroconversion after vaccination, n (%) | 102 (89%) | 250 (98%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyers, E.; De Smet, E.; Vercruysse, H.; Callens, S.; Padalko, E.; Heytens, S.; Vandekerckhove, L.; Cools, P.; Witkowski, W. No Significant Association between 25-OH Vitamin D Status and SARS-CoV-2 Antibody Response after COVID-19 Vaccination in Nursing Home Residents and Staff. Vaccines 2023, 11, 1343. https://doi.org/10.3390/vaccines11081343
Meyers E, De Smet E, Vercruysse H, Callens S, Padalko E, Heytens S, Vandekerckhove L, Cools P, Witkowski W. No Significant Association between 25-OH Vitamin D Status and SARS-CoV-2 Antibody Response after COVID-19 Vaccination in Nursing Home Residents and Staff. Vaccines. 2023; 11(8):1343. https://doi.org/10.3390/vaccines11081343
Chicago/Turabian StyleMeyers, Eline, Evelien De Smet, Hanne Vercruysse, Steven Callens, Elizaveta Padalko, Stefan Heytens, Linos Vandekerckhove, Piet Cools, and Wojciech Witkowski. 2023. "No Significant Association between 25-OH Vitamin D Status and SARS-CoV-2 Antibody Response after COVID-19 Vaccination in Nursing Home Residents and Staff" Vaccines 11, no. 8: 1343. https://doi.org/10.3390/vaccines11081343
APA StyleMeyers, E., De Smet, E., Vercruysse, H., Callens, S., Padalko, E., Heytens, S., Vandekerckhove, L., Cools, P., & Witkowski, W. (2023). No Significant Association between 25-OH Vitamin D Status and SARS-CoV-2 Antibody Response after COVID-19 Vaccination in Nursing Home Residents and Staff. Vaccines, 11(8), 1343. https://doi.org/10.3390/vaccines11081343





