COVID-19 Vaccination Hesitancy in Autoimmune Disease Patients: Policy Action and Ethical Considerations
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
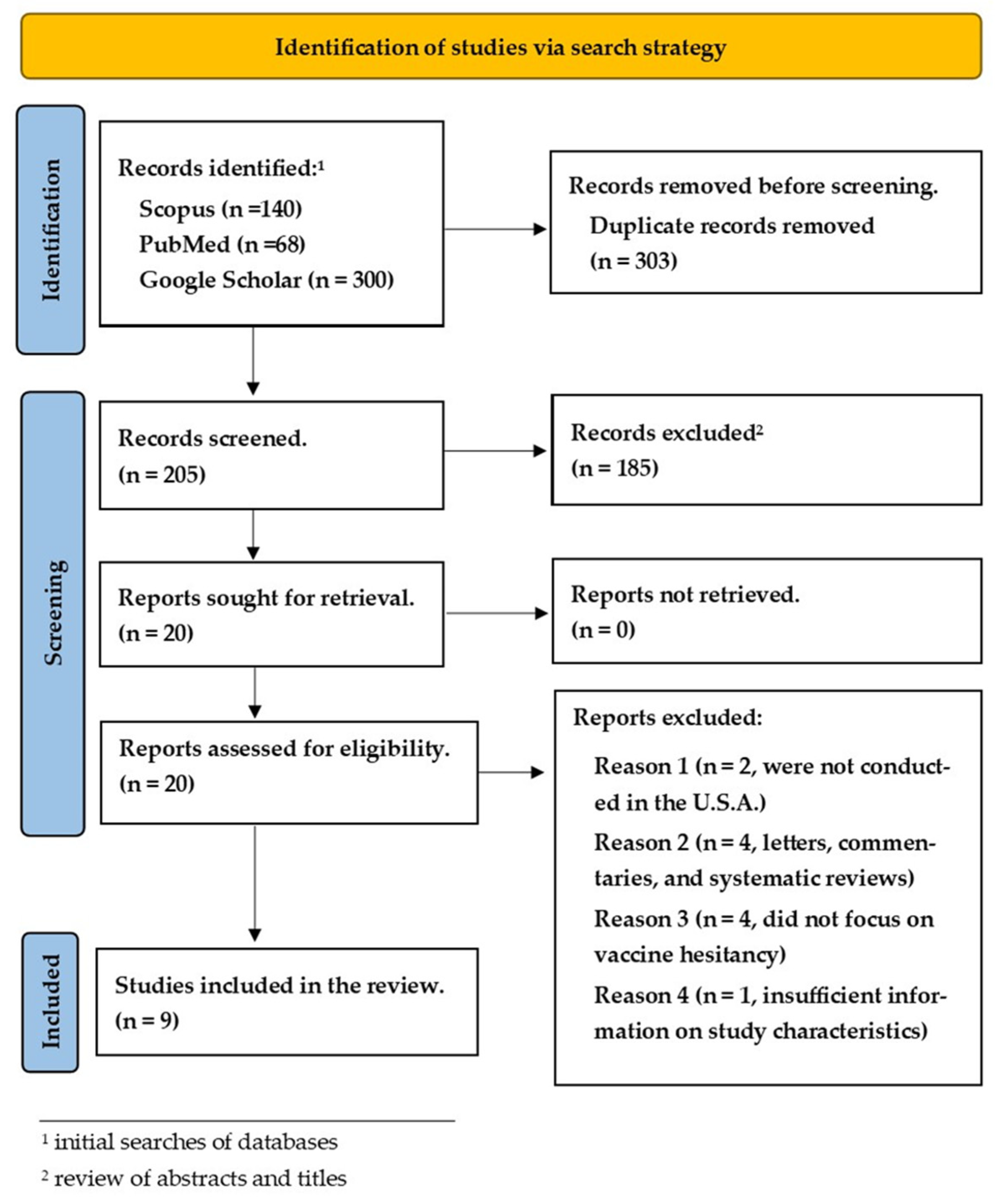
2.2. Study Selection
3. Results
3.1. Study Characteristics
3.2. Quality Appraisal
3.3. Factors Associated with COVID-19 Vaccine Hesitancy
3.4. Vaccine Safety, Efficacy, Concern Vaccine May Interfere with Medication and Fear of Adverse Reaction
| Reference | Study Design/Data Collection | Sample Size | Respondents by Sex (%) | Age Range (Mean) | Race | Main Findings | Reasons for Hesitancy |
|---|---|---|---|---|---|---|---|
| Bogart et al., 2021 [20] | Telephone interview | 101 | Cisgender female—16%, Cisgender male—80, transgender female—3%, gender nonconforming—1%, Gay or bisexual—77%. | Mean age 50.3 | X | 50% showed hesitancy regarding the COVID-19 vaccine or treatment, and a third of participants said they would not get vaccinated or treated. 97% percent endorsed at least one mistrust belief. About 50% or more major prevalent mistrust belief was a concern of withholding information on the vaccine or lack of honesty by the government. | Mistrust beliefs |
| Duculan et al., 2022 [18] | Telephone interview | 112 | Female—83, Male—13, Missing—14 | 22–87 (50) | Asian—8, Black—10, White—82, Latino—13 | 77% stated they would receive it, 28% had received the first dose, 6% would not get the vaccine, and 17% were hesitant. | Fear of adverse effects, no reason to be vaccinated, distrust of vaccine information, and fear of worsening rheumatic disease symptoms |
| Ehde et al., 2021 [19] | Cross-sectional online survey | 491 | Female—81.3, Male—17.3, non-binary—0.4, Transgender—0.2, other/prefer not to say/no answer—0.8 | X | White—90.5, Black—2.5, more than one race—4.1, prefer not to say—1.4, other—0.8, American Indian/Alaska Native—0.4, and Asian—0.2 | 73.8% planned to get the vaccine, 6% had received one vaccine dose, and 20.3% were hesitant. | Efficacy, long-term effects of the vaccine, the vaccine approval process, wanting more information about the vaccine, and the potential impact of the vaccine on their own health conditions. |
| Herman et al., 2022 [17] | Electronic survey and telephone interview | 210 | X | Mean age 46.6 | White—76.2%, Black—21%, Asian pacific islander or Native Hawaiian—2.4%. Other—1% | 88.10% were already vaccinated or wanted to be as soon as possible. The vaccine hesitancy rate was 11.9% and higher in younger black and Hispanic patients. | Adverse reaction, concern that the vaccine may interfere with medication efficiency, concern that medication may make the vaccine ineffective, and safety of the vaccine. |
| Shaw et al., 2022 [21] | Online survey/thematic analysis | 537 | Female—84%, Male-unknown, others-unknown | 64% were 65 years or older | White—94%, other races-unknown | 93% received or intend to receive at least a dose of the vaccine, 83% had concerns about the vaccine among vaccinated and unvaccinated, 71% had concerns about side effects, and 20% had concerns about the effect of the vaccine on DMARD management and flares. | Concern about side effects, doubts about vaccine effectiveness, mistrust, perception of low risk, and concerns about DMARD management /flares |
| Smith et al., 2022 [16] | Survey through a prolific survey platform | 2535 from the initial survey: 478—autoimmune disorder; 618—respiratory diseases; 136—autoimmune disease and chronic respiratory condition; 1303—no condition (healthy control); 589—other chronic conditions. 55% from initial respondents participated; 54% with respiratory diseases, 61% with autoimmune disorders, and 57% with autoimmune and respiratory diseases | X | X | Non-Hispanic White: Respiratory—70.2, Autoimmune—80.4, both—79.4, None—67.7; Black: Respiratory—7.38, Autoimmune—4.6, both—6.4, None—4.4; Hispanic or Latin0: Respiratory—4.1, Autoimmune—4.6, both—2.9, None—4.9; Asian: Respiratory—9.5, Autoimmune—4.4, both—5.2, None—14; Native American: Respiratory—0.98, Autoimmune—0.2, both—1.5, None—0.5; Two or more: Respiratory—7.9, Autoimmune—5.7, both—6.6, None—6.7. | Participants with autoimmune diseases were the only group to have a significant association with a specific cause for vaccine hesitancy or fear or adverse vaccine reaction. | Adverse reaction for those with an autoimmune disorder |
| Tsai et al., 2022 [4] | Survey | 21,943; 74.2% reside in the US, 8.5% in Canada, 8.1% in the UK, 3.1% in Australia, and 6.1% in Europe, Central, South America, and the Caribbean, the Middle East, the Russian Federation, Africa or the Far East. Cancer—27.3%, autoimmune disease—23.2%, chronic lung disease—35.4% | Female—75.9%, Male-unknown, others-unknown | Mean age 56–65 | X | 18.6% indicated COVID-19 vaccine hesitancy, 10.3% stated they would not receive the vaccine, 3.5% stated they would probably not receive the vaccine, and 4.8% stated they were not sure they would agree to be vaccinated. 25.8% reported they had received one dose of COVID-19 vaccine. 29.6% of US participants had already undergone vaccination. 19.4% with autoimmune diseases reported vaccine hesitancy compared with 18% of those not treated with autoimmune diseases. | Apprehension regarding the newness of the vaccine concerns about the safety of the vaccine and distrust of the development process |
| Uhr et al., 2022 [14] | Survey via the online iConquerMS platform | 1662 active users, and 789 responded. 15 were excluded due to lack of MS diagnosis, and 73 failed to respond to key questions making 701 analyzed respondents. 87.2% of respondents live in the US, and the remaining live in other countries in North America, Africa, Asia, Europe, Oceania, and South America. | X | 20 years and older | Race was categorized as white and others. Whites were 656, and other races were 41. | Younger age, racial minorities, and higher functional disability were independently associated with vaccine hesitancy. | Adverse reactions, safety and efficacy, and effect of vaccine on MS symptoms. |
| Wu et al., 2022 [22] | Survey | 306 | Female—77.45%, Male—22.45% | ≤24–≥75. The median age was 50 years, and the prevalent age group was 45–54 years. | X | 66.24% had received the vaccines or planned to be vaccinated, and 33.99% were unlikely to be vaccinated | Vaccine safety concerns, fast vaccine approval, vaccine efficacy, concern about vaccine causing MS relapse, and concern about the vaccine causing other diseases. |
| Reference | Score | Representativeness of Sample | Sample Size | Non-Respondents | Design and Analysis | Statistical Test | Ascertainment of Exposure | Assessment of Outcome |
|---|---|---|---|---|---|---|---|---|
| Bogart et al., 2021 [20] | 8 | * | * | * | * | * | * | * |
| Duculan et al., 2022 [18] | 7 | * | * | * | * | * | * | |
| Ehde et al., 2021 [19] | 7 | * | * | * | * | * | * | |
| Herman et al., 2022 [17] | 6 | * | * | * | * | * | ||
| Shaw et al., 2022 [21] | 6 | * | * | * | * | * | ||
| Smith et al., 2022 [16] | 6 | * | * | * | * | * | ||
| Tsai et al., 2022 [4] | 7 | * | * | * | * | * | * | |
| Uhr et al., 2022 [14] | 8 | * | * | * | * | * | * | * |
| Wu et al., 2022 [22] | 8 | * | * | * | * | * |
3.5. Fear of Worsening Symptoms, Mistrust, and Vaccine Causing Other Diseases
3.6. Apprehension about the Newness of the Vaccine, Needing More Information, the Fast Approval Process of the Vaccine
4. Discussion
4.1. Factors Related to Vaccine Hesitancy
4.2. Policy Action & Future Implications
4.3. The Common-Good Approach to Ethical Decision-Making
5. Conclusions
6. Limitations and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The COVID-19 Pandemic in 2023: Far from over. Lancet 2023, 401, 79. [CrossRef] [PubMed]
- Lazarus, J.V.; Wyka, K.; White, T.M. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat. Med. 2023, 29, 366–375. [Google Scholar] [CrossRef]
- Tharwat, S.; Abdelsalam, H.A.; Abdelsalam, A.; Nassar, M.K. COVID-19 vaccination intention and vaccine hesitancy among patients with autoimmune and autoinflammatory rheumatological diseases: A survey. Int. J. Clin. Pr. 2022, 2022, 5931506. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.; Hervey, J.; Hoffman, K.; Wood, J.; Johnson, J.; Deighton, D.; Clermont, D.; Loew, B.; Goldberg, S.L. COVID-19 vaccine hesitancy and acceptance among individuals with cancer, autoimmune diseases, or other serious comorbid conditions: Cross-sectional, internet-based survey. JMIR Public Health Surveill. 2022, 8, e29872. [Google Scholar] [CrossRef] [PubMed]
- Mohanasundaram, K.; Santhanam, S.; Natarajan, R.; Murugesan, H.; Nambi, T.; Chilikuri, B.; Nallasivan, S. COVID-19 vaccination in autoimmune rheumatic diseases: A multi-center survey from southern India. Int. J. Rheum. Dis. 2022, 25, 1046–1052. [Google Scholar] [CrossRef]
- Peshevska-Sekulovska, M.; Bakalova, P.; Snegarova, V.; Lazova, S.; Velikova, T. COVID-19 vaccines for adults and children with autoimmune gut or liver disease. Vaccines 2022, 10, 2075. [Google Scholar] [CrossRef]
- Boekel, L.; Hooijberg, F.; Van Kempen, Z.L.E.; Vogelzang, E.H.; Tas, S.W.; Killestein, J.; Nurmohamed, M.T.; Boers, M.; Kuijpers, T.W.; Van Ham, S.M.; et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021, 3, e241–e243. [Google Scholar] [CrossRef]
- Gaur, P.; Agrawat, H.; Shukla, A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: An interview-based survey. Rheum. Int. 2021, 41, 1601–1605. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Rueda-Medina, B.; Callejas-Rubio, J.L. COVID-19 vaccine literacy in patients with systemic autoimmune diseases. Curr. Psychol. 2022, 42, 13769–13784. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Schwarz, L.; Lorgelly, P. A rapid review of COVID-19 vaccine prioritization in the U.S.: Alignment between federal guidance and state practice. Int. J. Environ. Res. Public Health 2021, 18, 3483. [Google Scholar] [CrossRef]
- John, P.; Heith, K.; Johnson, E.; Gaeta, M. Ethical considerations for a COVID-19 vaccine mandate. Soc. Crit. Care Med. 2021. Available online: https://www.sccm.org/Blog/June-2021/Ethical-Considerations-for-a-COVID-19-Vaccine-Mand (accessed on 1 April 2023).
- Sekalala, S.; Perehudoff, K.; Parker, M.; Forman, L.; Rawson, B.; Smith, M. An intersectional human rights approach to prioritising access to COVID-19 vaccines. BMJ Glob. Health 2021, 6, e004462. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Uhr, L.; Mateen, F.J. COVID-19 vaccine hesitancy in multiple sclerosis: A cross-sectional survey. Mult. Scler. J. 2021, 28, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In Proceedings of the 3rd Symposium on Systematic Reviews: Beyond the Basics, Oxford, UK, 3–5 July 2000. [Google Scholar]
- Smith, B.A.; Ricotta, E.E.; Kwan, J.; Evans, N.G. COVID-19 risk perception and vaccine acceptance in individuals with chronic disease. MedRxiv 2022, 19, 37. [Google Scholar]
- Herman, H.S.; Rosenthaler, M.P.; Elhassan, N.; Weinberg, J.M.; Satyam, V.R.; Wasan, S.K. COVID-19 vaccine hesitancy among patients with inflammatory bowel diseases at a diverse safety net hospital. Dig. Dis. Sci. 2022, 67, 5029–5033. [Google Scholar] [CrossRef]
- Duculan, R.; Mancuso, C.A. Perceived risk of SARS-CoV-2 at the start of the COVID-19 pandemic and subsequent vaccination attitudes in patients with rheumatic diseases: A Longitudinal Analysis. J. Clin. Rheumatol. 2022, 28, 190. [Google Scholar] [CrossRef]
- Ehde, D.M.; Roberts, M.K.; Humbert, A.T.; Herring, T.E.; Alschuler, K.N. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: A follow up survey during the initial vaccine rollout in 2021. Mult. Scler. Relat. Disord. 2021, 54, 103163. [Google Scholar] [CrossRef]
- Bogart, L.M.; Ojikutu, B.O.; Tyagi, K.; Klein, D.J.; Mutchler, M.G.; Dong, L.; Lawrence, S.J.; Thomas, D.R.; Kellman, S. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. J. Acquir. Immune Defic. Syndr. 2021, 86, 200. [Google Scholar] [CrossRef]
- Shaw, Y.P.; Hustek, S.; Nguyen, N.; Starlin, M.; Wipfler, K.; Wallace, B.I.; Michaud, K. Rheumatic disease patient decision-making about COVID-19 vaccination: A qualitative analysis. BMC Rheumatol. 2022, 6, 76. [Google Scholar] [CrossRef]
- Wu, H.; Ward, M.; Brown, A.; Blackwell, E.; Umer, A. COVID-19 vaccine intent in Appalachian patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103450. [Google Scholar] [CrossRef]
- Felten, R.; Dubois, M.; Ugarte-Gil, M.F.; Chaudier, A.; Kawka, L.; Bergier, H.; Costecalde, C.; Pijnenburg, L.; Fort, J.; Chatelus, E.; et al. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021, 3, e243–e245. [Google Scholar] [CrossRef] [PubMed]
- Van Oost, P.; Yzerbyt, V.; Schmitz, M.; Vansteenkiste, M.; Luminet, O.; Morbée, S.; Van den Bergh, O.; Waterschoot, J.; Klein, O. The relation between conspiracism, government trust, and COVID-19 vaccination intentions: The key role of motivation. Soc. Sci. Med. 2022, 301, 114926. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Sarwar, M.; Ansar, S.; Awan, U.A.; Ahmed, H.; Aftab, N.; Afzal, M.S. COVID-19 vaccination hesitancy in patients with autoimmune diseases: A mystery that needs an immediate solution. Soc. Sci. Med. 2021, 93, 5216–5218. [Google Scholar] [CrossRef] [PubMed]
- Momplaisir, F.M.; Kuter, B.J.; Ghadimi, F.; Browne, S.; Nkwihoreze, H.; Feemster, K.A.; Frank, I.; Faig, W.; Shen, A.K.; Offit, P.A.; et al. Racial/ethnic differences in COVID-19 vaccine hesitancy among health care workers in 2 large academic hospitals. JAMA 2021, 4, e2121931. [Google Scholar] [CrossRef]
- Agazzi, E. The Coronavirus pandemic and the principle of common. Bioeth. Updat. 2020, 6, 63–66. [Google Scholar] [CrossRef]
- Tolbert, J.; Kates, J.; Michaud, J. The COVID-19 vaccine priority line continues to change as States make further updates. KFF 2021. Available online: https://www.kff.org/policy-watch/the-covid-19-vaccine-priority-line-continues-to-change-as-states-make-further-updates/ (accessed on 1 April 2023).
- Islam, N.; Lacey, B.; Shabnam, S.; Erzurumluoglu, A.M.; Dambha-Miller, H.; Chowell, G.; Kawachi, I.; Marmot, M. Social inequality and the syndemic of chronic disease and COVID-19: County-level analysis in the USA. JECH 2021, 75, 496–500. [Google Scholar]
- Velasquez, M.; Andre, C.; Shanks, T.; Meyer, M.J. Thinking Ethically; Markkula Center for Applied Ethics: Santa Clara, CA, USA, 2015. [Google Scholar]
- Kadambari, S.; Vanderslott, S. Lessons about COVID-19 vaccine hesitancy among minority ethnic people in the UK. Lancet Infect. Dis. 2021, 21, 1204–1206. [Google Scholar] [CrossRef]
- Velikova, T.; Georgiev, T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021, 41, 509–518. [Google Scholar] [CrossRef]
- Truong, M.; Paradies, Y.; Priest, N. Interventions to improve cultural competency in healthcare: A systematic review of reviews. BMC Health Serv. Res. 2014, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- El Amin, A.N.; Parra, M.T.; Kim-Farley, R.; Fielding, J.E. Ethical Issues Concerning Vaccination Requirements. Public Health Rev. 2012, 34, 14. [Google Scholar] [CrossRef][Green Version]
- Sween, L.; Ekeoduru, R.; Mann, D. Ethics and pitfalls of Vaccine Mandates. ASA Monit. 2022, 86, 24–25. [Google Scholar] [CrossRef]
| No. | Search String | Results |
|---|---|---|
| Database: PUBMED | ||
| 1 | covid 19 OR covid-19 OR sars-cov2 OR “novel coronavirus” OR ncov OR 2019ncov OR hcov-19 OR covid19 OR “sarscov 2 infection” OR “severe acute respiratory syndrome coronavirus” OR “wuhan coronavirus” | 334,326 |
| 2 | vaccination OR vaccine OR immunization OR vaccin * OR immune * | 4,829,086 |
| 3 | hesitan* OR anti-vaccin* OR unwillingness | 11,182 |
| 4 | (Autoimmune Diseases[MeSH] OR autoimmun* OR Autoimmunity[MeSH] OR Autoantibodies[MeSH] OR Autoimmune Diseases of the Nervous System[MeSH] OR Neurologic Autoimmun * [tiab] OR Nervous System Immune * [tiab]) | 684,101 |
| 5 | # 1 AND # 2 AND #3 AND #4 | 68 |
| Database: SCOPUS | ||
| 6 | (“COVID-19” OR “COVID” OR “COVID 19” OR “COVID19” OR “Coronavirus” OR “2019-nCoV” OR “2019 nCoV” OR “2019 Novel Coronavirus” OR “SARS-CoV-2” OR “SARS CoV 2” OR “SARS Coronavirus 2”)) AND (vaccination * OR vaccine * OR immunization * OR vaccin * OR Immun *)) AND (hesitan * OR doubt* OR distrust OR anti-vaccin *)) AND (“Au-toimmune disease” OR “inflammatory bowel disease” OR psoriasis * OR “rheumatic diseases”, OR “systemic lupus erythematosus”)) | 140 |
| Database: Google Scholar | ||
| 7 | “Covid 19” OR COVID-19 OR SARS-CoV2 * OR “novel coronavirus” OR ncov* OR “covid19” OR “sarscov 2 infection” OR “severe acute respiratory syndrome” AND vaccination OR immunization AND hesitancy OR “anti-vaccine” AND “Autoim-mune Diseases” | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafik, N.; Akpo, J.E.; Waterfield, K.C.; Mase, W.A. COVID-19 Vaccination Hesitancy in Autoimmune Disease Patients: Policy Action and Ethical Considerations. Vaccines 2023, 11, 1283. https://doi.org/10.3390/vaccines11081283
Shafik N, Akpo JE, Waterfield KC, Mase WA. COVID-19 Vaccination Hesitancy in Autoimmune Disease Patients: Policy Action and Ethical Considerations. Vaccines. 2023; 11(8):1283. https://doi.org/10.3390/vaccines11081283
Chicago/Turabian StyleShafik, Nardeen, Jennifer E. Akpo, Kristie C. Waterfield, and William A. Mase. 2023. "COVID-19 Vaccination Hesitancy in Autoimmune Disease Patients: Policy Action and Ethical Considerations" Vaccines 11, no. 8: 1283. https://doi.org/10.3390/vaccines11081283
APA StyleShafik, N., Akpo, J. E., Waterfield, K. C., & Mase, W. A. (2023). COVID-19 Vaccination Hesitancy in Autoimmune Disease Patients: Policy Action and Ethical Considerations. Vaccines, 11(8), 1283. https://doi.org/10.3390/vaccines11081283







