Influence of the Single Nucleotide Polymorphisms rs12252 and rs34481144 in IFITM3 on the Antibody Response after Vaccination against COVID-19
Abstract
1. Introduction
2. Materials and Methods
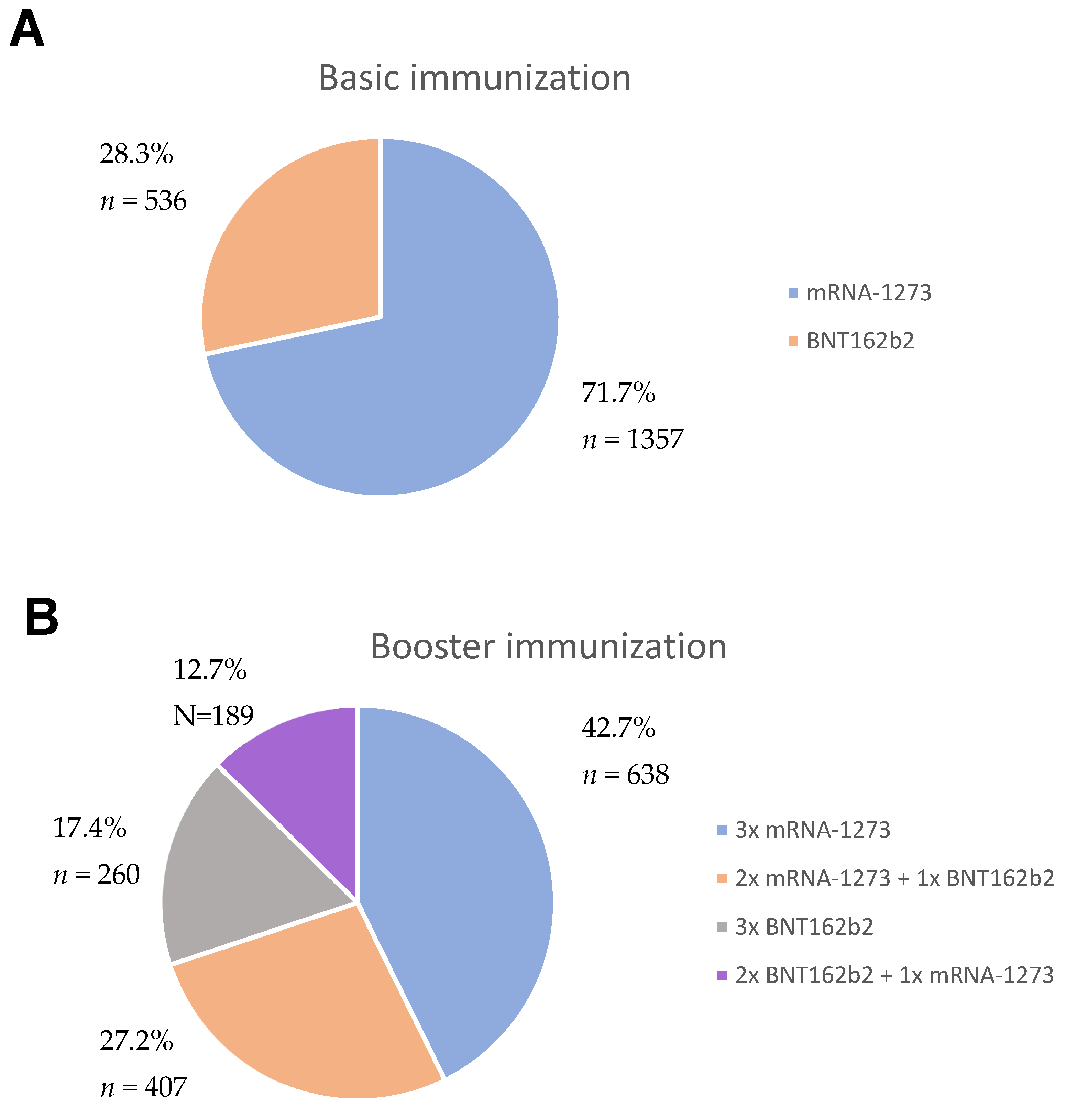
2.1. Study Group
2.2. Study Design
2.3. Genotyping of IFITM3
2.4. Detection of Antibodies against SARS-CoV-2 Spike Protein
2.5. Detection of Antibodies against SARS-CoV-2 Nucleocapsid Protein
2.6. Statistical Analysis
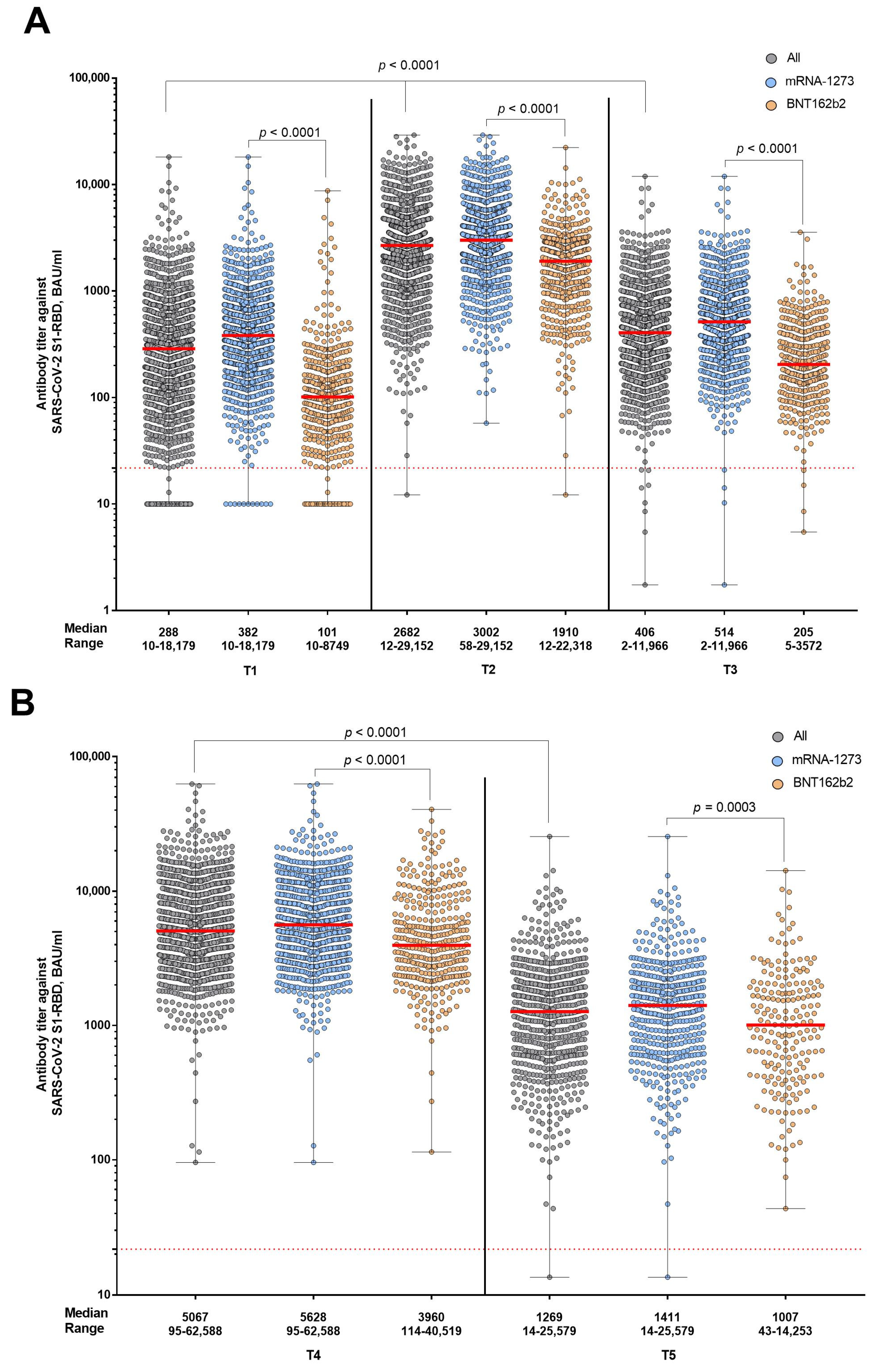
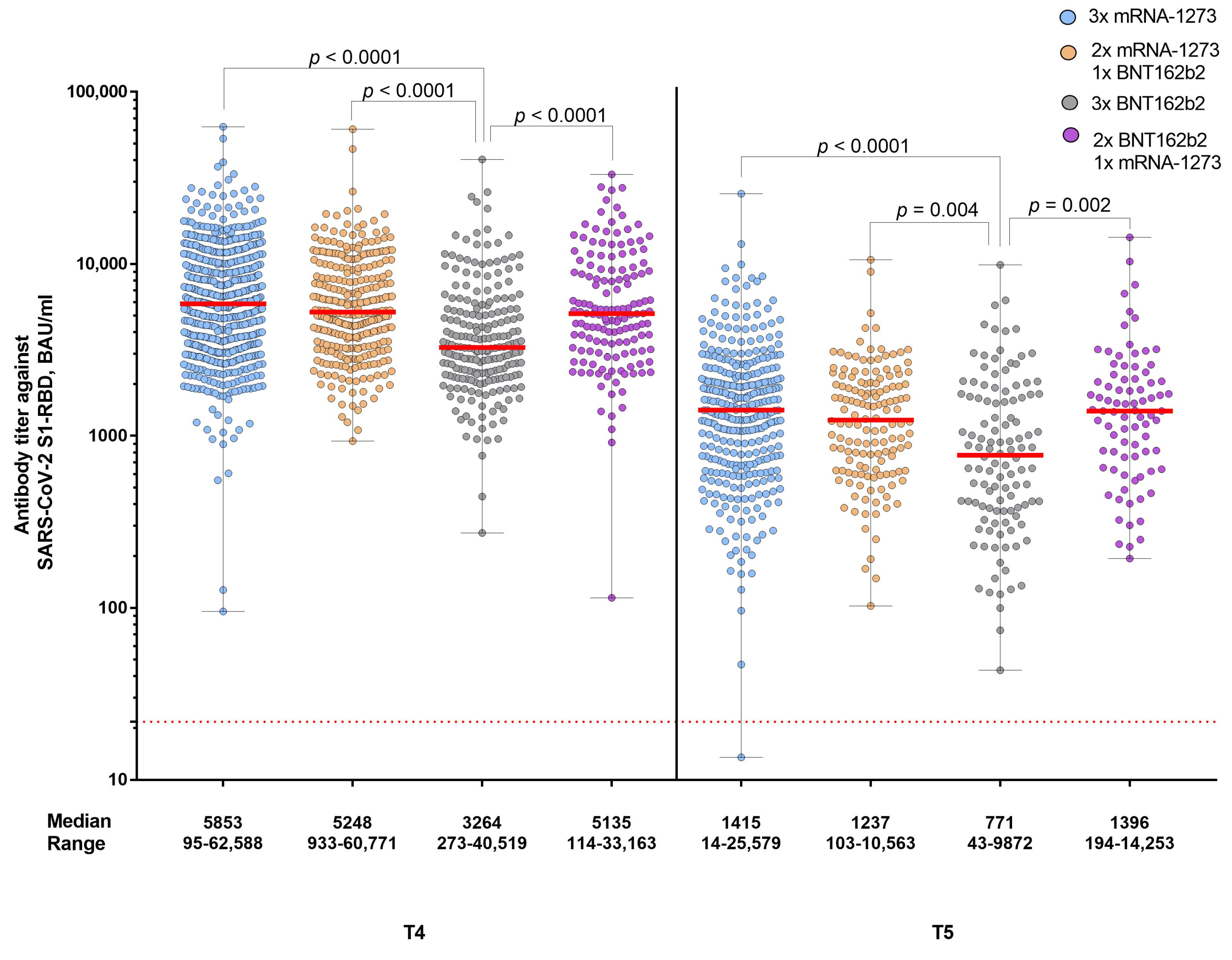
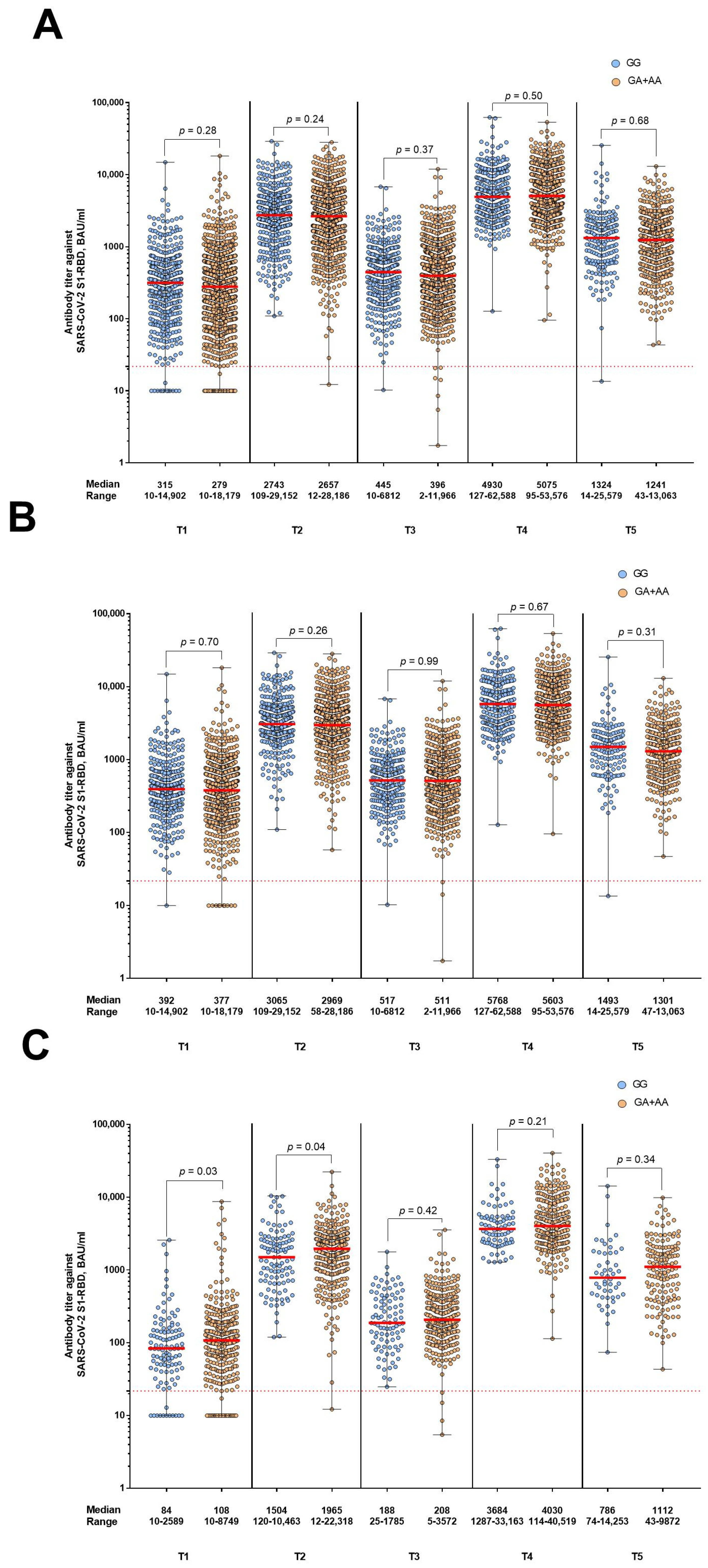
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Coronavirus Resource Center. COVID-19 Dashboard. Available online: https://coronavirus.jhu.edu/map.html (accessed on 27 April 2022).
- Lupia, T.; Scabini, S.; Mornese Pinna, S.; Di Perri, G.; De Rosa, F.G.; Corcione, S. 2019 novel coronavirus (2019-nCoV) outbreak: A new challenge. J. Glob. Antimicrob. Resist. 2020, 21, 22–27. [Google Scholar] [CrossRef]
- Wei, X.; Li, L.; Zhang, F. The impact of the COVID-19 pandemic on socio-economic and sustainability. Environ. Sci. Pollut. Res. 2021, 28, 68251–68260. [Google Scholar] [CrossRef]
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- Paul-Ehrlich-Institut. COVID-19 Vaccines. Available online: https://www.pei.de/EN/medicinal-products/vaccines-human/covid-19/covid-19-node.html (accessed on 27 April 2022).
- Bai, J.; Chiba, A.; Murayama, G.; Kuga, T.; Tamura, N.; Miyake, S. Sex, Age, and Ethnic Background Shape Adaptive Immune Responses Induced by the SARS-CoV-2 mRNA Vaccine. Front. Immunol. 2022, 13, 786586. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Klisanin, V.; Thummler, L.; Fisenkci, N.; Tsachakis-Muck, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Simon, D.; Tascilar, K.; Fagni, F.; Kronke, G.; Kleyer, A.; Meder, C.; Atreya, R.; Leppkes, M.; Kremer, A.E.; Ramming, A.; et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann. Rheum. Dis. 2021, 80, 1312–1316. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Collier, A.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by COVID-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef]
- Gallagher, K.M.E.; Leick, M.B.; Larson, R.C.; Berger, T.R.; Katsis, K.; Yam, J.Y.; Maus, M.V. Differential T-Cell Immunity to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in mRNA-1273- and BNT162b2-Vaccinated Individuals. Clin. Infect. Dis. 2022, 75, e869–e873. [Google Scholar] [CrossRef]
- Hussein, K.; Dabaja-Younis, H.; Szwarcwort-Cohen, M.; Almog, R.; Leiba, R.; Weissman, A.; Mekel, M.; Hyams, G.; Horowitz, N.A.; Gepstein, V.; et al. Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers. Vaccines 2022, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Di Franco, M.; Angeloni, A.; Zicari, A.; Cardinale, V.; Visentini, M.; Antonelli, G.; Napoli, A.; Anastasi, E.; Romiti, G.F.; et al. Serological Response and Relationship with Gender-Sensitive Variables among Healthcare Workers after SARS-CoV-2 Vaccination. J. Pers. Med. 2022, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Crocchiolo, R.; Gallina, A.M.; Pani, A.; Campisi, D.; Cento, V.; Sacchi, N.; Miotti, V.; Gagliardi, O.M.; D’Amico, F.; Vismara, C.; et al. Polymorphism of the HLA system and weak antibody response to BNT162b2 mRNA vaccine. HLA 2022, 99, 183–191. [Google Scholar] [CrossRef]
- Gutierrez-Bautista, J.F.; Sampedro, A.; Gomez-Vicente, E.; Rodriguez-Granger, J.; Reguera, J.A.; Cobo, F.; Ruiz-Cabello, F.; Lopez-Nevot, M.A. HLA Class II Polymorphism and Humoral Immunity Induced by the SARS-CoV-2 mRNA-1273 Vaccine. Vaccines 2022, 10, 402. [Google Scholar] [CrossRef]
- Ciuciulkaite, I.; Mohlendick, B.; Thummler, L.; Fisenkci, N.; Elsner, C.; Dittmer, U.; Siffert, W.; Lindemann, M. GNB3 c.825c>T polymorphism influences T-cell but not antibody response following vaccination with the mRNA-1273 vaccine. Front. Genet. 2022, 13, 932043. [Google Scholar] [CrossRef]
- Colucci, M.; De Santis, E.; Totti, B.; Miroballo, M.; Tamiro, F.; Rossi, G.; Piepoli, A.; De Vincentis, G.; Greco, A.; Mangia, A.; et al. Associations between Allelic Variants of the Human IgH 3’ Regulatory Region 1 and the Immune Response to BNT162b2 mRNA Vaccine. Vaccines 2021, 9, 1207. [Google Scholar] [CrossRef]
- Lei, N.; Li, Y.; Sun, Q.; Lu, J.; Zhou, J.; Li, Z.; Liu, L.; Guo, J.; Qin, K.; Wang, H.; et al. IFITM3 affects the level of antibody response after influenza vaccination. Emerg. Microbes Infect. 2020, 9, 976–987. [Google Scholar] [CrossRef]
- Diamond, M.S.; Farzan, M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013, 13, 46–57. [Google Scholar] [CrossRef]
- Perreira, J.M.; Chin, C.R.; Feeley, E.M.; Brass, A.L. IFITMs restrict the replication of multiple pathogenic viruses. J. Mol. Biol. 2013, 425, 4937–4955. [Google Scholar] [CrossRef]
- Xu, F.; Wang, G.; Zhao, F.; Huang, Y.; Fan, Z.; Mei, S.; Xie, Y.; Wei, L.; Hu, Y.; Wang, C.; et al. IFITM3 Inhibits SARS-CoV-2 Infection and Is Associated with COVID-19 Susceptibility. Viruses 2022, 14, 2553. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xiao, M.; Yang, J.; Chen, Y.K.; Bai, T.; Tang, X.J.; Shu, Y.L. Association between rs12252 and influenza susceptibility and severity: An updated meta-analysis. Epidemiol. Infect. 2018, 147, e39. [Google Scholar] [CrossRef]
- Prabhu, S.S.; Chakraborty, T.T.; Kumar, N.; Banerjee, I. Association between IFITM3 rs12252 polymorphism and influenza susceptibility and severity: A meta-analysis. Gene 2018, 674, 70–79. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhao, Y.; Li, N.; Peng, Y.C.; Giannoulatou, E.; Jin, R.H.; Yan, H.P.; Wu, H.; Liu, J.H.; Liu, N.; et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat. Commun. 2013, 4, 1418. [Google Scholar] [CrossRef] [PubMed]
- Compton, A.A.; Roy, N.; Porrot, F.; Billet, A.; Casartelli, N.; Yount, J.S.; Liang, C.; Schwartz, O. Natural mutations in IFITM3 modulate post-translational regulation and toggle antiviral specificity. EMBO Rep. 2016, 17, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Everitt, A.R.; Clare, S.; Pertel, T.; John, S.P.; Wash, R.S.; Smith, S.E.; Chin, C.R.; Feeley, E.M.; Sims, J.S.; Adams, D.J.; et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 2012, 484, 519–523. [Google Scholar] [CrossRef]
- Jia, R.; Pan, Q.; Ding, S.; Rong, L.; Liu, S.L.; Geng, Y.; Qiao, W.; Liang, C. The N-terminal region of IFITM3 modulates its antiviral activity by regulating IFITM3 cellular localization. J. Virol. 2012, 86, 13697–13707. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- Allen, E.K.; Randolph, A.G.; Bhangale, T.; Dogra, P.; Ohlson, M.; Oshansky, C.M.; Zamora, A.E.; Shannon, J.P.; Finkelstein, D.; Dressen, A.; et al. SNP-mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat. Med. 2017, 23, 975–983. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Al Heialy, S.; Hachim, I.Y.; Halwani, R.; Senok, A.C.; Maghazachi, A.A.; Hamid, Q. Interferon-Induced Transmembrane Protein (IFITM3) Is Upregulated Explicitly in SARS-CoV-2 Infected Lung Epithelial Cells. Front. Immunol. 2020, 11, 1372. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Llavona, E.; Albaiceta, G.M.; Garcia-Clemente, M.; Duarte-Herrera, I.D.; Amado-Rodriguez, L.; Hermida-Valverde, T.; Enriquez-Rodriguez, A.I.; Hernandez-Gonzalez, C.; Melon, S.; Alvarez-Arguelles, M.E.; et al. Association between the interferon-induced transmembrane protein 3 gene (IFITM3) rs34481144/rs12252 haplotypes and COVID-19. Curr. Res. Virol. Sci. 2021, 2, 100016. [Google Scholar] [CrossRef]
- Alghamdi, J.; Alaamery, M.; Barhoumi, T.; Rashid, M.; Alajmi, H.; Aljasser, N.; Alhendi, Y.; Alkhalaf, H.; Alqahtani, H.; Algablan, O.; et al. Interferon-induced transmembrane protein-3 genetic variant rs12252 is associated with COVID-19 mortality. Genomics 2021, 113, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, L.; Zhao, Y.; Zhang, P.; Xu, B.; Li, K.; Liang, L.; Zhang, C.; Dai, Y.; Feng, Y.; et al. Interferon-Induced Transmembrane Protein 3 Genetic Variant rs12252-C Associated With Disease Severity in Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 34–37. [Google Scholar] [CrossRef]
- Gomez, J.; Albaiceta, G.M.; Cuesta-Llavona, E.; Garcia-Clemente, M.; Lopez-Larrea, C.; Amado-Rodriguez, L.; Lopez-Alonso, I.; Melon, S.; Alvarez-Arguelles, M.E.; Gil-Pena, H.; et al. The Interferon-induced transmembrane protein 3 gene (IFITM3) rs12252 C variant is associated with COVID-19. Cytokine 2021, 137, 155354. [Google Scholar] [CrossRef]
- Cagigi, A.; Lore, K. Immune Responses Induced by mRNA Vaccination in Mice, Monkeys and Humans. Vaccines 2021, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Tre-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Antoine-Moussiaux, T.; Della Vecchia, A.; Horeanga, A.; Papleux, E.; Vekemans, M.; Beukinga, I.; Blairon, L. Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. J. Infect. 2021, 83, 559–564. [Google Scholar] [CrossRef]
- Desmecht, S.; Tashkeev, A.; El Moussaoui, M.; Marechal, N.; Peree, H.; Tokunaga, Y.; Fombellida-Lopez, C.; Polese, B.; Legrand, C.; Wery, M.; et al. Kinetics and Persistence of the Cellular and Humoral Immune Responses to BNT162b2 mRNA Vaccine in SARS-CoV-2-Naive and -Experienced Subjects: Impact of Booster Dose and Breakthrough Infections. Front. Immunol. 2022, 13, 863554. [Google Scholar] [CrossRef]
- Almendro-Vazquez, P.; Chivite-Lacaba, M.; Utrero-Rico, A.; Gonzalez-Cuadrado, C.; Laguna-Goya, R.; Moreno-Batanero, M.; Sanchez-Paz, L.; Luczkowiak, J.; Labiod, N.; Folgueira, M.D.; et al. Cellular and humoral immune responses and breakthrough infections after three SARS-CoV-2 mRNA vaccine doses. Front. Immunol. 2022, 13, 981350. [Google Scholar] [CrossRef]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.L. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef] [PubMed]
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex Disparities in Efficacy in COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Vassilaki, N.; Gargalionis, A.N.; Bletsa, A.; Papamichalopoulos, N.; Kontou, E.; Gkika, M.; Patas, K.; Theodoridis, D.; Manolis, I.; Ioannidis, A.; et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms 2021, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. eClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Holtkamp, C.; Scholer, L.; Anastasiou, O.E.; Brune, B.; Fessmann, K.; Elsner, C.; Mohlendick, B.; Ciuciulkaite, I.; Dudda, M.; Trilling, M.; et al. Antibody responses elicited by mRNA vaccination in firefighters persist six months and correlate inversely with age and directly with BMI. Heliyon 2023, 9, e12746. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Masi, D.; Tozzi, R.; Risi, R.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Biagi, F.; Anastasi, E.; et al. Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine. Vaccines 2022, 10, 79. [Google Scholar] [CrossRef]
- Schonfelder, K.; Breuckmann, K.; Elsner, C.; Dittmer, U.; Fistera, D.; Herbstreit, F.; Risse, J.; Schmidt, K.; Sutharsan, S.; Taube, C.; et al. The influence of IFITM3 polymorphisms on susceptibility to SARS-CoV-2 infection and severity of COVID-19. Cytokine 2021, 142, 155492. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Mohlendick, B.; Ciuciulkaite, I.; Elsner, C.; Anastasiou, O.E.; Trilling, M.; Wagner, B.; Zwanziger, D.; Jockel, K.H.; Dittmer, U.; Siffert, W. Individuals With Weaker Antibody Responses After Booster Immunization Are Prone to Omicron Breakthrough Infections. Front. Immunol. 2022, 13, 907343. [Google Scholar] [CrossRef] [PubMed]

| T1 | p-Value | T2 | p-Value | T3 | p-Value | T4 | p-Value | T5 | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||||
| All | |||||||||||
| female | 323 (10-18179) | <0.0001 | 2642 (29-29152) | 0.03 | 397 (9-11966) | 0.02 | 4957 (127-62588) | 0.15 | 1243 (14-25579) | 0.32 | |
| male | 223 (10-5465) | 2797 (12-17888) | 465 (2-9189) | 5265 (95-60771) | 1387 (96-13063) | ||||||
| mRNA-1273 | |||||||||||
| female | 413 (10-18179) | <0.0001 | 2895 (109-29152) | 0.0002 | 482 (10-11966) | <0.0001 | 5419 (127-62588) | 0.05 | 1407 (14-25579) | 0.45 | |
| male | 322 (10-5465) | 3329 (58-17888) | 652 (2-9189) | 6259 (95-60771) | 1482 (96-13063) | ||||||
| BNT162b2 | |||||||||||
| female | 109 (10-8749) | 0.15 | 1891 (29-22318) | 0.89 | 198 (9-3078) | 0.46 | 3820 (273-40519) | 0.49 | 926 (43-14253) | 0.24 | |
| male | 98 (10-2754) | 1942 (12-11229) | 221 (5-3572) | 4239 (114-27694) | 1287 (128-6725) | ||||||
| Age | |||||||||||
| All | |||||||||||
| 18–30 | 435 (10-14902) | <0.0001 | 3138 (135-28186) | <0.0001 | 597 (51-9149) | <0.0001 | 5131 (443-46487) | 0.32 | 1267 (103-10563) | 0.17 | |
| 31–49 | 248 (10-18179) | 2537 (12-29152) | 350 (5-11966) | 4911 (114-60771) | 1206 (47-8473) | ||||||
| 50–72 | 133 (10-8749) | 2369 (29-26264) | 298 (2-6812) | 5134 (95-62588) | 1370 (14-25579) | ||||||
| mRNA-1273 | |||||||||||
| 18–30 | 474 (10-14902) | <0.0001 | 3384 (338-28186) | <0.0001 | 662 (51-9249) | <0.0001 | 5463 (933-46487) | 0.08 | 1366 (103-10563) | 0.01 | |
| 31–49 | 345 (10-18179) | 2890 (117-29152) | 433 (21-11966) | 5276 (606-60771) | 1259 (47-8473) | ||||||
| 50–72 | 233 (10-2452) | 2648 (58-26264) | 424 (2-6812) | 6058 (95-62588) | 1552 (14-25579) | ||||||
| BNT162b2 | |||||||||||
| 18–30 | 177 (28-7137) | <0.0001 | 2481 (135-10463) | 0.0004 | 357 (52-1785) | <0.0001 | 3944 (443-40519) | 0.61 | 1052 (120-9872) | 0.87 | |
| 31–49 | 108 (10-4900) | 1884 (12-22318) | 196 (5-3572) | 4208 (114-27694) | 964 (74-7569) | ||||||
| 50–72 | 67 (10-8749) | 1636 (29-8939) | 167 (9-3078) | 3792 (273-33163) | 1000 (43-14253) | ||||||
| BMI | |||||||||||
| All | |||||||||||
| <30 | 306 (10-18179) | <0.0001 | 2697 (12-29152) | 0.29 | 425 (2-11966) | 0.001 | 4918 (95-62588) | 0.005 | 1240 (14-25579) | 0.26 | |
| ≥30.0 | 203 (10-8749) | 2658 (68-28186) | 324 (25-9249) | 6157 (606-53576) | 1522 (74-13063) | ||||||
| mRNA-1273 | |||||||||||
| <30 | 392 (10-18179) | 0.005 | 3061 (58-29152) | 0.76 | 535 (2-11966) | 0.005 | 5463 (95-62588) | 0.01 | 1337 (14-25579) | 0.11 | |
| ≥30.0 | 332 (10-3093) | 2865 (147-28186) | 425 (57-9249) | 6644 (606-53576) | 1602 (215-13063) | ||||||
| BNT162b2 | |||||||||||
| <30 | 109 (10-7137) | 0.02 | 1945 (12-22318) | 0.80 | 209 (5-3572) | 0.28 | 3820 (114-40519) | 0.12 | 978 (43-14253) | 0.87 | |
| ≥30.0 | 87 (10-8749) | 1747 (68-8939) | 183 (25-3078) | 5252 (1243-23523) | 1138 (74-4842) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čiučiulkaitė, I.; Siffert, W.; Elsner, C.; Dittmer, U.; Wichert, M.; Wagner, B.; Volbracht, L.; Mosel, F.; Möhlendick, B. Influence of the Single Nucleotide Polymorphisms rs12252 and rs34481144 in IFITM3 on the Antibody Response after Vaccination against COVID-19. Vaccines 2023, 11, 1257. https://doi.org/10.3390/vaccines11071257
Čiučiulkaitė I, Siffert W, Elsner C, Dittmer U, Wichert M, Wagner B, Volbracht L, Mosel F, Möhlendick B. Influence of the Single Nucleotide Polymorphisms rs12252 and rs34481144 in IFITM3 on the Antibody Response after Vaccination against COVID-19. Vaccines. 2023; 11(7):1257. https://doi.org/10.3390/vaccines11071257
Chicago/Turabian StyleČiučiulkaitė, Ieva, Winfried Siffert, Carina Elsner, Ulf Dittmer, Marc Wichert, Bernd Wagner, Lothar Volbracht, Frank Mosel, and Birte Möhlendick. 2023. "Influence of the Single Nucleotide Polymorphisms rs12252 and rs34481144 in IFITM3 on the Antibody Response after Vaccination against COVID-19" Vaccines 11, no. 7: 1257. https://doi.org/10.3390/vaccines11071257
APA StyleČiučiulkaitė, I., Siffert, W., Elsner, C., Dittmer, U., Wichert, M., Wagner, B., Volbracht, L., Mosel, F., & Möhlendick, B. (2023). Influence of the Single Nucleotide Polymorphisms rs12252 and rs34481144 in IFITM3 on the Antibody Response after Vaccination against COVID-19. Vaccines, 11(7), 1257. https://doi.org/10.3390/vaccines11071257






