Cellular and Humoral Immune Responses after Breakthrough Infection in Patients Undergoing Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Antibody Titers
2.3. Cytokine Production Stimulated by the SARS-CoV-2 Spike Protein
2.4. Flow Cytometry
2.5. Statistical Analysis
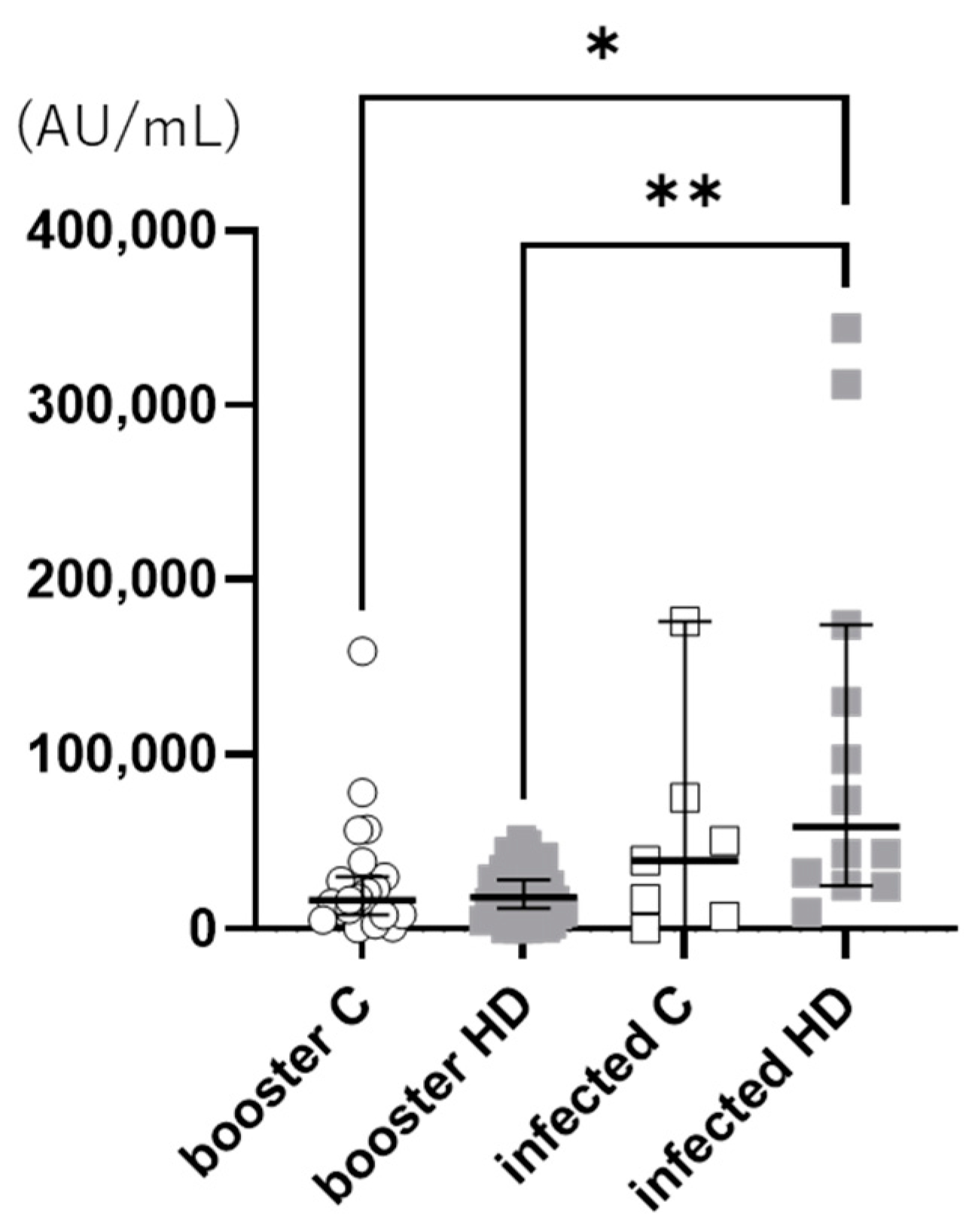
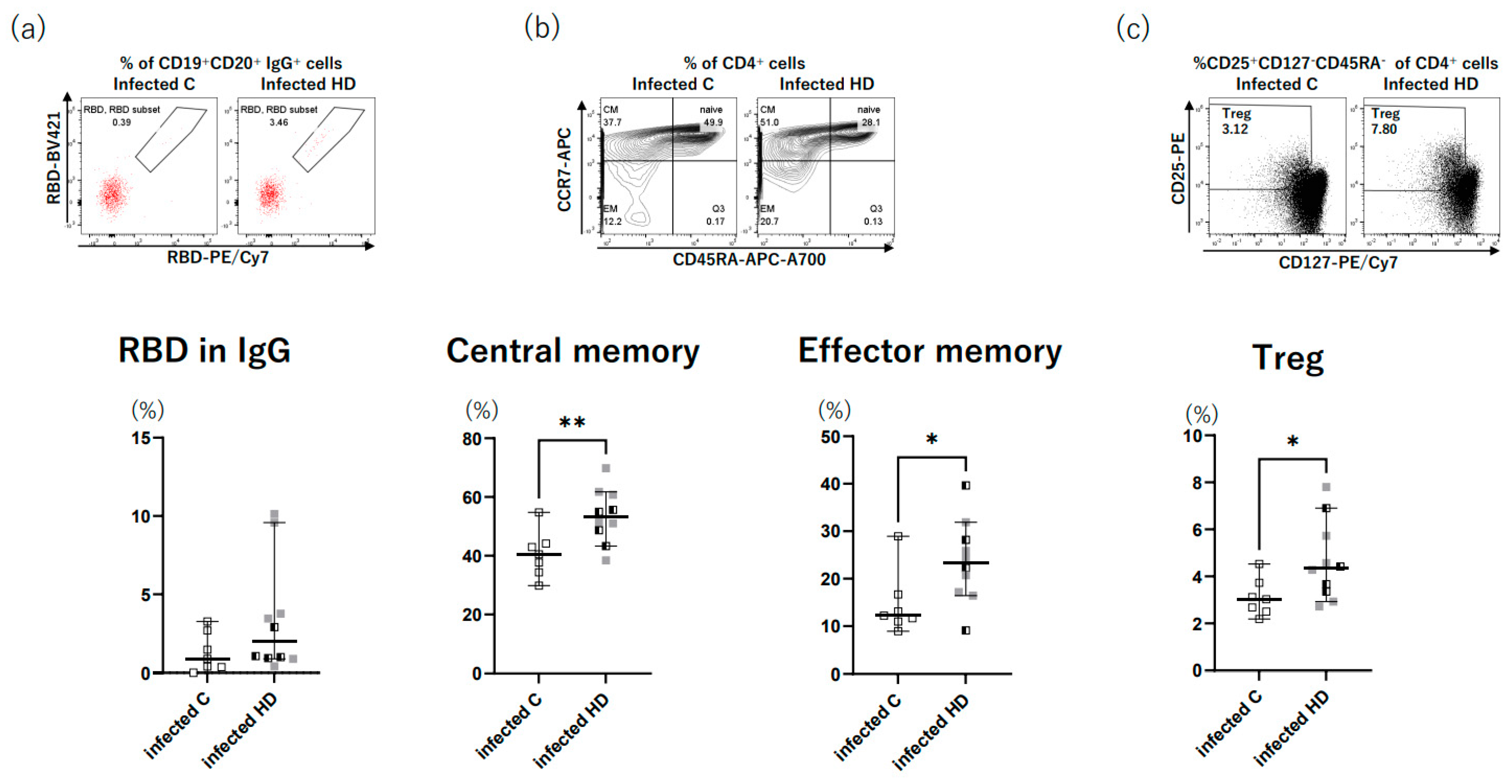
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Charmetant, X.; Barba, T.; Koppe, L.; Pelletier, C.; Kalbacher, E.; Chalencon, E.; Mathias, V.; Ovize, A.; Cart-Tanneur, E.; et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021, 100, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, A.; Toda, M.; Ryuzaki, M.; Kikuchi, K.; Kawai, T.; Sakai, K.; Oyama, E.; Koinuma, M.; Katayama, K.; Uehara, Y.; et al. Investigation for the efficacy of COVID-19 vaccine in Japanese CKD patients treated with hemodialysis. Ren. Replace. Ther. 2022, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Yoshifuji, A.; Kikuchi, K.; Koinuma, M.; Komatsu, M.; Fujii, K.; Kato, A.; Kikuchi, T.; Nakazawa, A.; Ryuzaki, M. Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin. Exp. Nephrol. 2022, 26, 571–580. [Google Scholar] [CrossRef]
- Curlin, M.E.; Bates, T.A.; Guzman, G.; Schoen, D.; McBride, S.K.; Carpenter, S.D.; Tafesse, F.G. Omicron neutralizing antibody response following booster vaccination compared with breakthrough infection. Med 2022, 3, 827–837.e3. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Simon, B.; Rubey, H.; Gromann, M.; Knopf-Völkerer, A.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. SARS-CoV-2 Antibody and T Cell Response after a Third Vaccine Dose in Hemodialysis Patients Compared with Healthy Controls. Vaccines 2022, 10, 694. [Google Scholar] [CrossRef]
- Yang, S.L.; Mat Ripen, A.; Leong, C.T.; Lee, J.V.; Yen, C.H.; Chand, A.K.; Koh, K.; Rahim, N.A.B.A.; Gokilavanan, V.; Mohamed, N.N.E.B.; et al. COVID-19 breakthrough infections and humoral immune response among BNT162b2 vaccinated healthcare workers in Malaysia. Emerg. Microbes Infect. 2022, 11, 1262–1271. [Google Scholar] [CrossRef]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.-Y.; et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell 2022, 185, 1539–1548.e5. [Google Scholar] [CrossRef]
- Kim, J.U.; Kim, M.; Kim, S.; Nguyen, T.T.; Kim, E.; Lee, S.; Kim, S.; Kim, H. Dendritic Cell Dysfunction in Patients with End-stage Renal Disease. Immune Netw. 2017, 17, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Haskin, O.; Ashkenazi-Hoffnung, L.; Ziv, N.; Borovitz, Y.; Dagan, A.; Levi, S.; Koren, G.; Hamdani, G.; Levi-Erez, D.; Landau, D.; et al. Serological Response to the BNT162b2 COVID-19 mRNA Vaccine in Adolescent and Young Adult Kidney Transplant Recipients. Transplantation 2021, 105, e226–e233. [Google Scholar] [CrossRef]
- Ziv, N.; Gimelraikh, Y.; Ashkenazi-Hoffnung, L.; Alfandary, H.; Borovitz, Y.; Dagan, A.; Levi, S.; Hamdani, G.; Levy-Erez, D.; Landau, D.; et al. Serologic response to COVID-19 infection or vaccination in pediatric kidney transplant recipients compared to healthy children. Transpl. Immunol. 2023, 78, 101839. [Google Scholar] [CrossRef]
- Kang, E.K.; Lim, J.S.; Lee, J.A.; Kim, D.H. Comparison of immune response by virus infection and vaccination to 2009 pandemic influenza A/H1N1 in children. J. Korean Med. Sci. 2013, 28, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Brueggeman, J.M.; Zhao, J.; Schank, M.; Yao, Z.Q.; Moorman, J.P. Trained Immunity: An Overview and the Impact on COVID-19. Front. Immunol. 2022, 13, 837524. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kato, Y.; Edahiro, R.; Søndergaard, J.N.; Murakami, T.; Amiya, S.; Nameki, S.; Yoshimine, Y.; Morita, T.; Takeshima, Y.; et al. Consecutive BNT162b2 mRNA vaccination induces short-term epigenetic memory in innate immune cells. JCI Insight 2022, 7, e163347. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. 2021, 36, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Fujii, K.; Yoshifuji, A.; Kondo, Y.; Itoh, K.; Sekine, K.; Kikuchi, T.; Ryuzaki, M. Clinical efficacy and safety of combination therapy of tocilizumab and steroid pulse therapy for critical COVID-19 in HD patients. Clin. Exp. Nephrol. 2022, 26, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Kahan, S.M.; Zajac, A.J. Anti-viral CD8 T cells and the cytokines that they love. Virology 2013, 435, 157–169. [Google Scholar] [CrossRef]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Chen, R.; Cao, X.; Zou, J.; Ji, J.; Ding, X.; Yu, X. The difference of T cell phenotypes in end stage renal disease patients under different dialysis modality. BMC Nephrol. 2019, 20, 301. [Google Scholar]
- Sampani, E.; Vagiotas, L.; Daikidou, D.V.; Nikolaidou, V.; Xochelli, A.; Kasimatis, E.; Lioulios, G.; Dimitriadis, C.; Fylaktou, A.; Papagianni, A.; et al. End stage renal disease has an early and continuous detrimental effect on regulatory T cells. Nephrology 2022, 27, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Samaan, E.; Elmaria, M.O.; Khedr, D.; Gaber, T.; Elsayed, A.G.; Shenouda, R.N.; Gamal, H.; Shahin, D.; Abousamra, N.K.; Shemies, R. Characterization of regulatory T cells in SARS-CoV-2 infected hemodialysis patients: Relation to clinical and radiological severity. BMC Nephrol. 2022, 23, 391. [Google Scholar] [CrossRef] [PubMed]

| Breakthrough Infection | Booster Immunization | ||||
|---|---|---|---|---|---|
| Control (n = 7) | HD (n = 10) | Control (n = 22) | HD (n = 37) | ||
| Age (years) | 58.9 ± 14.2 | 69.5 ± 11.4 | 70.5 ± 8.8 | 78.7 ± 9.0 | |
| Males (n, (%)) | 7 (77.7) | 10 (90.9) | 17 (77.3) | 22 (59.5) | |
| BMI | 25.3 ± 3.9 | 23.6 ± 5.4 | 23.5 ± 3.4 | 22.2 ± 3.5 | |
| Diabetes mellitus (n, (%)) | 6 (85.7) | 8 (80.0) | 4 (18.2) | 15 (40.5) | |
| Dyslipidemia (n, (%)) | 2 (27.6) | 4 (40.0) | 5 (22.7) | 16 (43.2) | |
| Malignant tumor (n, (%)) | 0 | 2 (20.0) | 0 | 1 (2.7) | |
| Cardiovascular disease (n, (%)) | 0 | 5 (50.0) | 1 (4.5) | 11 (29.7) | |
| Cerebrovascular disease (n, (%)) | 1 (14.3) | 2 (20.0) | 1 (4.5) | 6 (16.2) | |
| COVID-19 | Mild | 7 | 4 | ||
| Moderate | 0 | 6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toda, M.; Yoshifuji, A.; Nakayama, T.; Mise-Omata, S.; Oyama, E.; Uwamino, Y.; Namkoong, H.; Komatsu, M.; Yoshimura, A.; Hasegawa, N.; et al. Cellular and Humoral Immune Responses after Breakthrough Infection in Patients Undergoing Hemodialysis. Vaccines 2023, 11, 1214. https://doi.org/10.3390/vaccines11071214
Toda M, Yoshifuji A, Nakayama T, Mise-Omata S, Oyama E, Uwamino Y, Namkoong H, Komatsu M, Yoshimura A, Hasegawa N, et al. Cellular and Humoral Immune Responses after Breakthrough Infection in Patients Undergoing Hemodialysis. Vaccines. 2023; 11(7):1214. https://doi.org/10.3390/vaccines11071214
Chicago/Turabian StyleToda, Masataro, Ayumi Yoshifuji, Tetsuo Nakayama, Setsuko Mise-Omata, Emi Oyama, Yoshifumi Uwamino, Ho Namkoong, Motoaki Komatsu, Akihiko Yoshimura, Naoki Hasegawa, and et al. 2023. "Cellular and Humoral Immune Responses after Breakthrough Infection in Patients Undergoing Hemodialysis" Vaccines 11, no. 7: 1214. https://doi.org/10.3390/vaccines11071214
APA StyleToda, M., Yoshifuji, A., Nakayama, T., Mise-Omata, S., Oyama, E., Uwamino, Y., Namkoong, H., Komatsu, M., Yoshimura, A., Hasegawa, N., Kikuchi, K., & Ryuzaki, M. (2023). Cellular and Humoral Immune Responses after Breakthrough Infection in Patients Undergoing Hemodialysis. Vaccines, 11(7), 1214. https://doi.org/10.3390/vaccines11071214






