Adjuvanted Fusion Protein Vaccine Induces Durable Immunity to Onchocerca volvulus in Mice and Non-Human Primates
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Parasites
2.2. Source of Mice and NHPs
2.3. Animal Ethics
2.4. Production of Ov-103 and Ov-RAL-2 Antigens
2.5. Cloning and Cell Banking of Ov-FUS-1 Antigen
2.6. Production of Ov-FUS-1 by Fermentation in P. pastoris
2.7. Purification of Ov-FUS-1 by Chromatography
2.8. Mouse Immunization and Challenge Protocol
2.9. NHP Immunization and Challenge Protocol
2.10. Recovery of Larvae from Mouse and NHP Diffusion Chambers
2.11. Passive Transfer of Mouse and NHP Serum into Naïve Mice
2.12. Analysis of Mouse and NHP Diffusion Chamber Cells by Microscopy
2.13. Analysis of Mouse Diffusion Chamber Cells by Flow Cytometry
2.14. Luminex Analysis of Cytokines in Ex Vivo Restimulated Mouse Spleen Cell Supernatants
2.15. Enzyme-Linked Immunosorbent Assay Analysis of Antigen-Specific Antibody in Mouse Serum
2.16. Enzyme-Linked Immunosorbent Assay Analysis of Antigen-Specific Antibody in NHP Serum
2.17. Statistics
3. Results
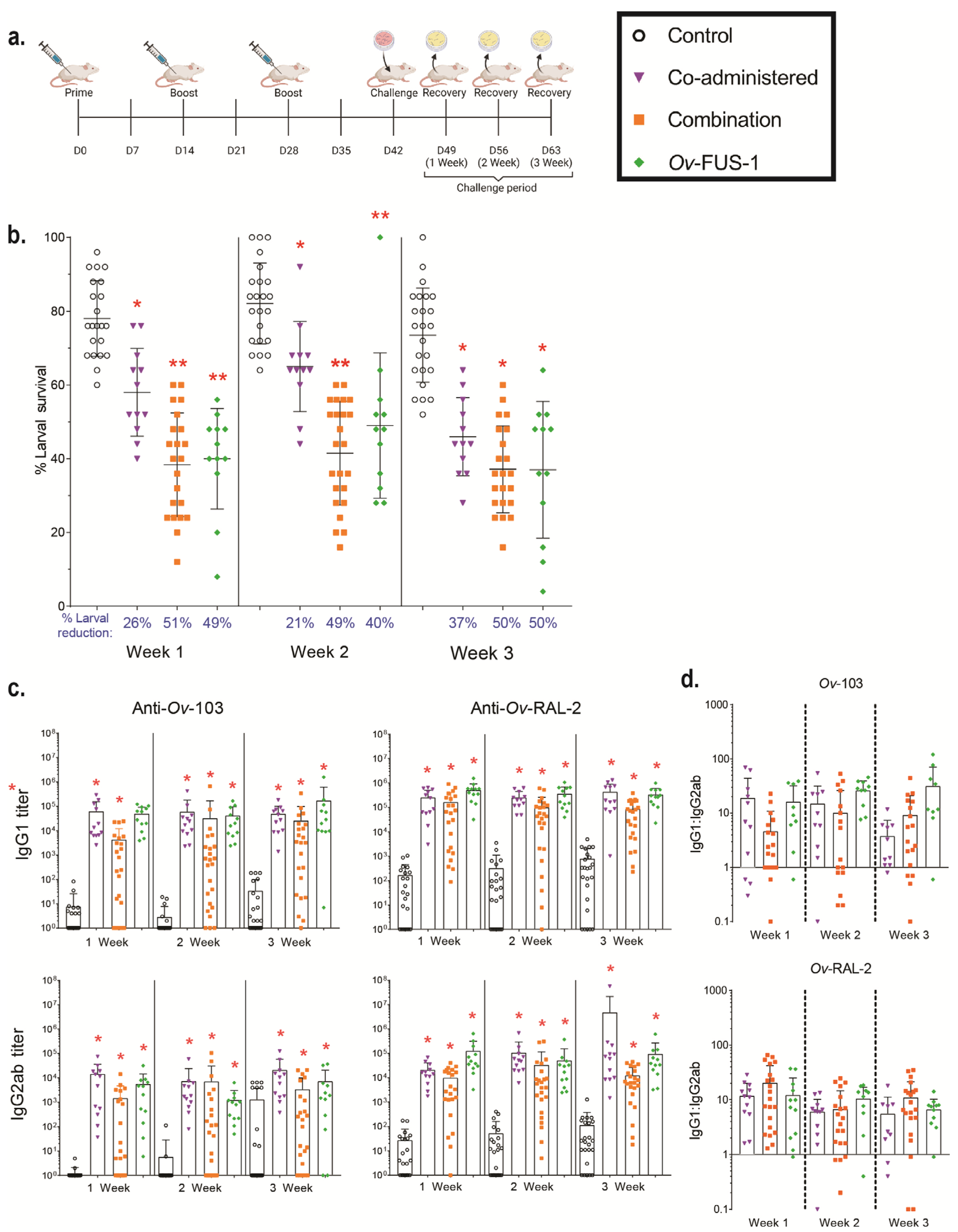
3.1. Immunization of Mice with Advax-CpG-Adjuvanted Ov-103 and Ov-RAL-2 Combined Antigens or with Bivalent Ov-FUS-1 Protein Induces Rapid Larval Killing
3.2. Ov-FUS-1 Formulated with Advax-CpG Induces Durable Protective Immunity in Mice
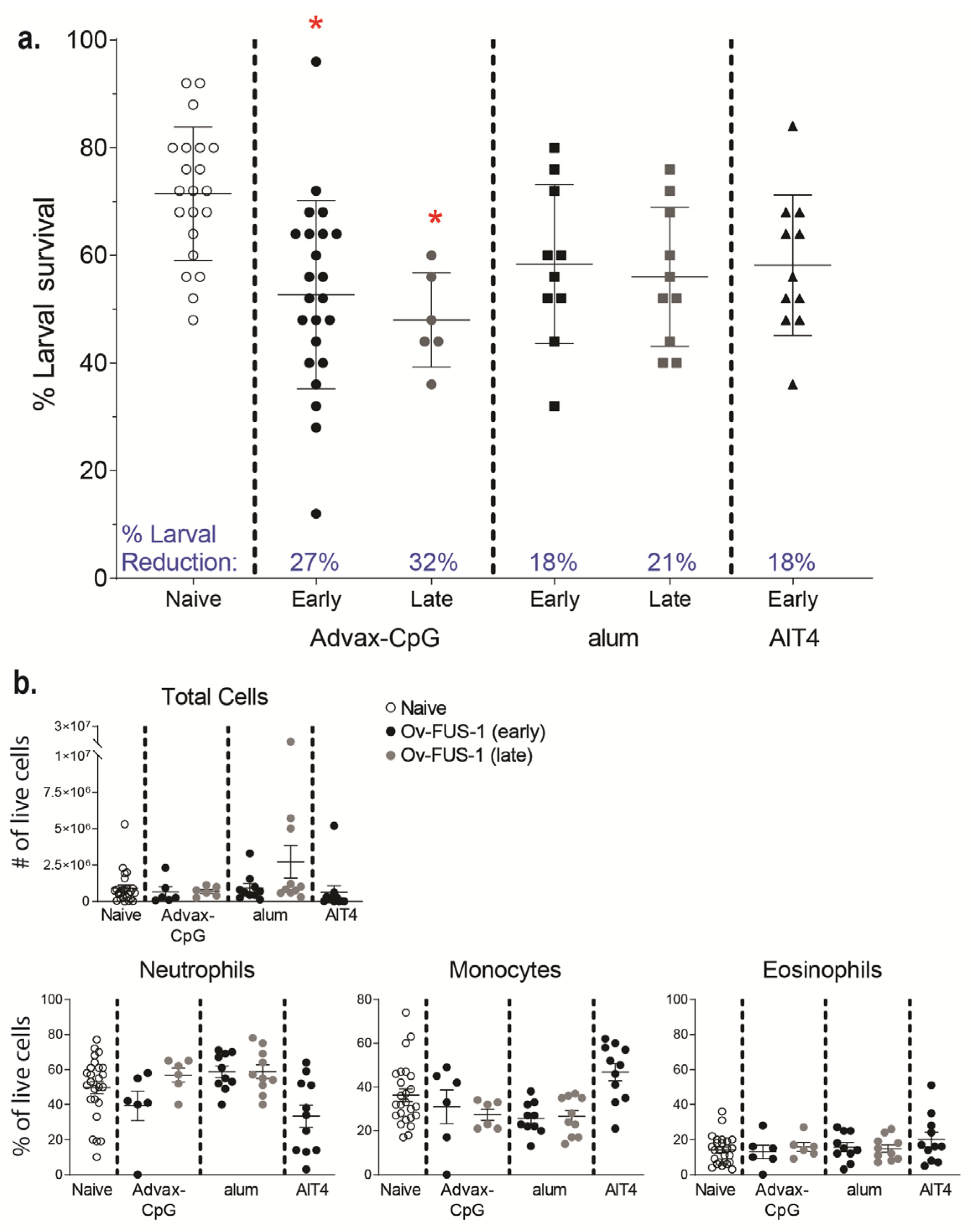
3.3. Passive Transfer of Sera from Mice Immunized with Ov-FUS-1 and Advax-CpG Protects Naïve Mice
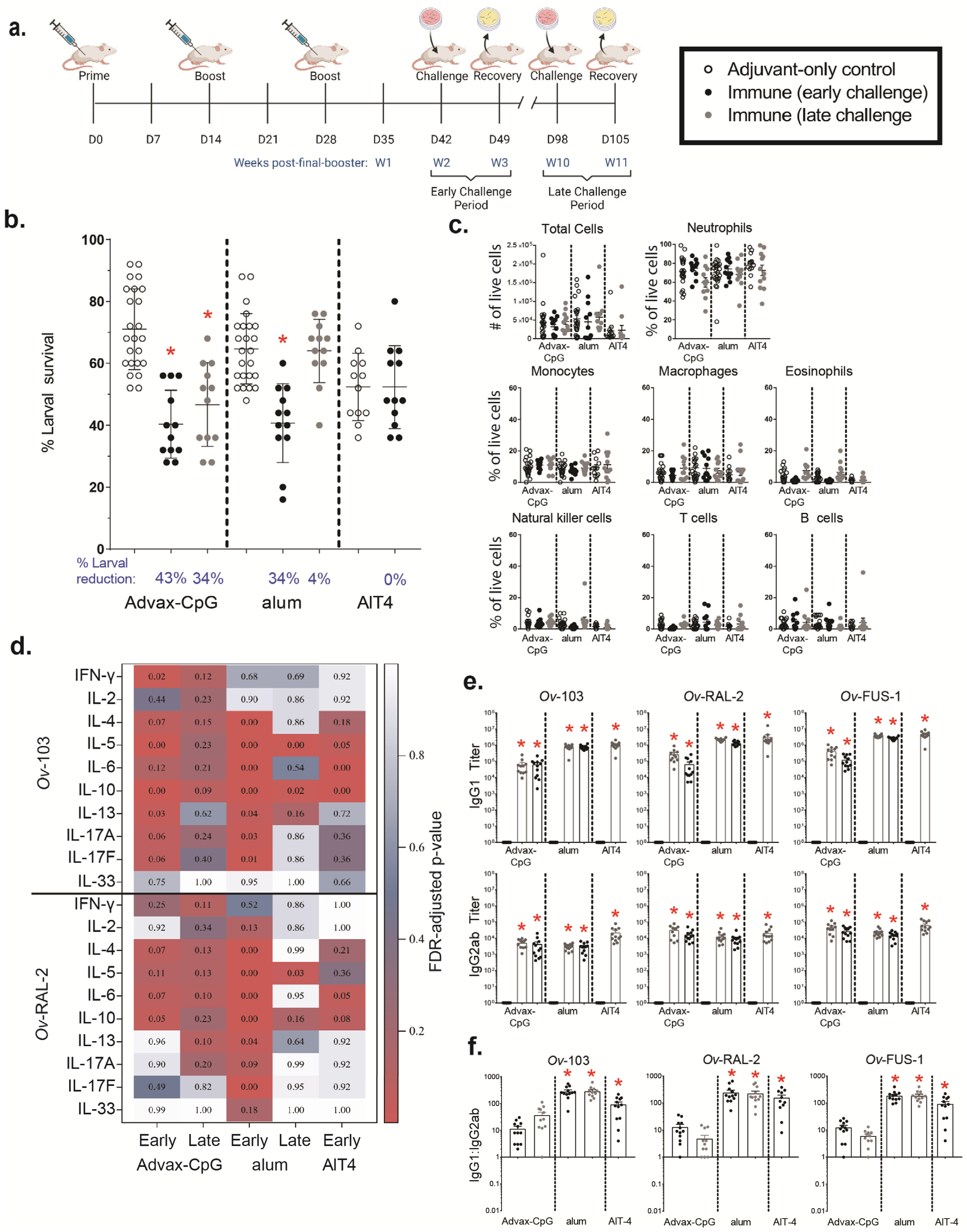
3.4. Ov-FUS-1 Formulated with Three Different Adjuvants Induces High-Titer Antibody Responses in NHPs
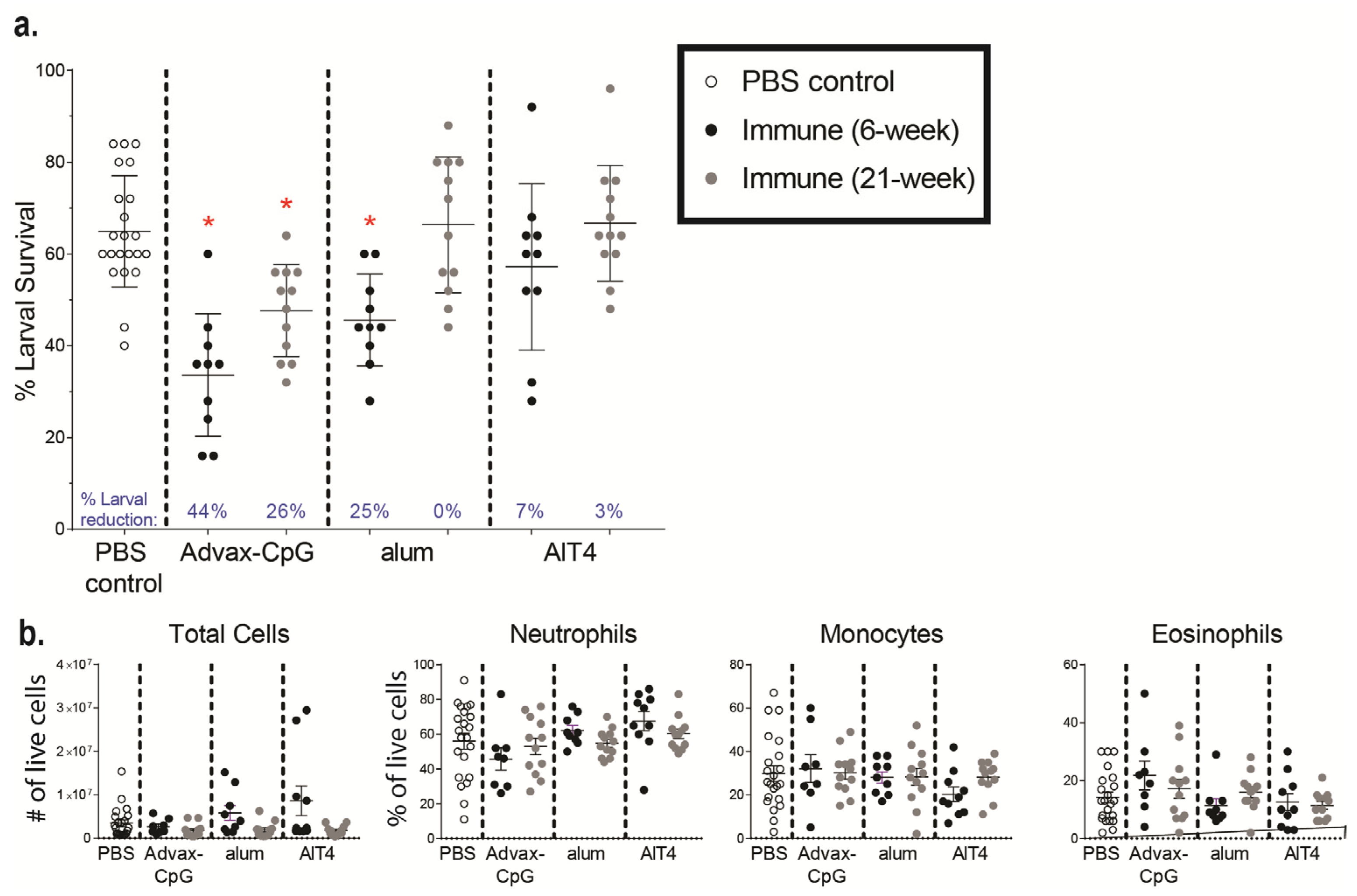
3.5. Passive Transfer of Serum from NHPs Immunized with Ov-FUS-1 and Advax-CpG into Naïve Mice Is Protective against O. volvulus L3 Challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melchers, N.V.S.V.; Stolk, W.A.; van Loon, W.; Pedrique, B.; Bakker, R.; Murdoch, M.E.; de Vlas, S.J.; Coffeng, L.E. The burden of skin disease and eye disease due to onchocerciasis in countries formerly under the African Programme for Onchocerciasis Control mandate for 1990, 2020, and 2030. PLoS Negl. Trop. Dis. 2021, 15, e0009604. [Google Scholar] [CrossRef]
- Winnen, M.; Plaisier, A.P.; Alley, E.S.; Nagelkerke, N.J.D.; Van Oortmarssen, G.; Boatin, B.A.; Habbema, J.D.F. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull. World Health Organ. 2002, 80, 384–391. [Google Scholar]
- Senyonjo, L.; Oye, J.; Bakajika, D.; Biholong, B.; Tekle, A.; Boakye, D.; Schmidt, E.; Elhassan, E. Factors Associated with Ivermectin Non-Compliance and Its Potential Role in Sustaining Onchocerca volvulus Transmission in the West Region of Cameroon. PLoS Negl. Trop. Dis. 2016, 10, e0004905. [Google Scholar] [CrossRef]
- Awadzi, K.; Attah, S.; Addy, E.; Opoku, N.; Quartey, B.; Lazdins-Helds, J.; Ahmed, K.; Boatin, B.; Boakye, D.; Edwards, G. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann. Trop. Med. Parasitol. 2004, 98, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Eyong, E.-E.J.; Tendongfor, N.; Ngwa, C.J.; Esuka, E.N.; Kengne-Ouafo, A.J.; Datchoua-Poutcheu, F.R.; Enyong, P.; Agnew, D.; Eversole, R.R.; et al. Ivermectin treatment of Loa loa hyper-microfilaraemic baboons (Papio anubis): Assessment of microfilarial load reduction, haematological and biochemical parameters and histopathological changes following treatment. PLoS Negl. Trop. Dis. 2017, 11, e0005576. [Google Scholar] [CrossRef]
- Taylor, H.R.; Pacqué, M.; Muñoz, B.; Greene, B.M. Impact of Mass Treatment of Onchocerciasis with Ivermectin on the Transmission of Infection. Science 1990, 250, 116–118. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E.; Zhan, B.; Makepeace, B.L.; Klei, T.R.; Abraham, D.; Taylor, D.W.; Lustigman, S. The Onchocerciasis Vaccine for Africa—TOVA—Initiative. PLoS Negl. Trop. Dis. 2015, 9, e0003422. [Google Scholar] [CrossRef] [PubMed]
- Makepeace, B.L.; Babayan, A.S.; Lustigman, S.; Taylor, D.W. The case for vaccine development in the strategy to eradicate river blindness (onchocerciasis) from Africa. Expert Rev. Vaccines 2015, 14, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Abraham, D.; Lange, A.M.; Yutanawiboonchai, W.; Trpiš, M.; Dickerson, J.W.; Swenson, B.; Eberhard, M.L. Survival and Development of Larval Onchocerca volvulus in Diffusion Chambers Implanted in Primate and Rodent Hosts. J. Parasitol. 1993, 79, 571. [Google Scholar] [CrossRef]
- Lustigman, S.; James, E.R.; Tawe, W.; Abraham, D. Towards a recombinant antigen vaccine against Onchocerca volvulus. Trends Parasitol. 2002, 18, 135–141. [Google Scholar] [CrossRef]
- Hess, J.A.; Zhan, B.; Bonne-Année, S.; Deckman, J.M.; Bottazzi, M.E.; Hotez, P.J.; Klei, T.R.; Lustigman, S.; Abraham, D. Vaccines to combat river blindness: Expression, selection and formulation of vaccines against infection with Onchocerca volvulus in a mouse model. Int. J. Parasitol. 2014, 44, 637–646. [Google Scholar] [CrossRef]
- Lustigman, S.; Brotman, B.; Johnson, E.H.; Smith, A.B.; Huima, T.; Prince, A.M. Identification and characterization of an Onchocerca volvulus cDNA clone encoding a microfilarial surface-associated antigen. Mol. Biochem. Parasitol. 1992, 50, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Gallin, M.Y.; Tan, M.; Kron, M.A.; Rechnitzer, D.; Greene, B.M.; Newland, H.S.; White, A.T.; Taylor, H.R.; Unnasch, T.R. Onchocerca volvulus Recombinant Antigen: Physical Characterization and Clinical Correlates with Serum Reactivity. J. Infect. Dis. 1989, 160, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.A.; Zhan, B.; Torigian, A.R.; Patton, J.B.; Petrovsky, N.; Zhan, T.; Bottazzi, M.E.; Hotez, P.J.; Klei, T.R.; Lustigman, S.; et al. The Immunomodulatory Role of Adjuvants in Vaccines Formulated with the Recombinant Antigens Ov-103 and Ov-RAL-2 against Onchocerca volvulus in Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004797. [Google Scholar] [CrossRef]
- Ryan, N.M.; Hess, J.A.; de Villena, F.P.-M.; Leiby, B.E.; Shimada, A.; Yu, L.; Yarmahmoodi, A.; Petrovsky, N.; Zhan, B.; Bottazzi, M.E.; et al. Onchocerca volvulus bivalent subunit vaccine induces protective immunity in genetically diverse collaborative cross recombinant inbred intercross mice. NPJ Vaccines 2021, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.; Bah, G.S.; Okah-Nnane, N.H.; Hartley, C.S.; Glover, A.F.; Walsh, T.R.; Lian, L.-Y.; Zhan, B.; Bottazzi, M.E.; Abraham, D.; et al. Co-Administration of Adjuvanted Recombinant Ov-103 and Ov-RAL-2 Vaccines Confer Protection against Natural Challenge in A Bovine Onchocerca ochengi Infection Model of Human Onchocerciasis. Vaccines 2022, 10, 861. [Google Scholar] [CrossRef]
- George, P.J.; Hess, J.A.; Jain, S.; Patton, J.B.; Zhan, T.; Tricoche, N.; Zhan, B.; Bottazzi, M.E.; Hotez, P.J.; Abraham, D.; et al. Antibody responses against the vaccine antigens Ov-103 and Ov-RAL-2 are associated with protective immunity to Onchocerca volvulus infection in both mice and humans. PLoS Negl. Trop. Dis. 2019, 13, e0007730. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.B.; Mellado-Sanchez, G.; Jorgensen, A.L.; Moore, S.; Nataro, J.P.; Pasetti, M.F.; Baillie, L.W. Development of a multiple-antigen protein fusion vaccine candidate that confers protection against Bacillus anthracis and Yersinia pestis. PLoS Negl. Trop. Dis. 2019, 13, e0007644. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Valley, U.; Rappuoli, R. Vaccine manufacturing: Challenges and solutions. Nat. Biotechnol. 2006, 24, 1377–1383. [Google Scholar] [CrossRef]
- Shrivastava, N.; Singh, P.K.; Nag, J.K.; Kushwaha, S.; Misra-Bhattacharya, S. Immunization with a multisubunit vaccine considerably reduces establishment of infective larvae in a rodent model of Brugia malayi. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Olsen, A.W.; Weischenfeldt, J.; Huygen, K.; D’souza, S.; Kondratieva, T.K.; Yeremeev, V.V.; Apt, A.S.; Raupach, B.; Grode, L.; et al. Comparative Analysis of Different Vaccine Constructs Expressing Defined Antigens from Mycobacterium tuberculosis. J. Infect. Dis. 2004, 190, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Wei, J.; Liu, Z.; Abraham, D.; Bell, A.; Bottazzi, M.E.; Hotez, P.J.; Zhan, B.; Lustigman, S.; Klei, T.R. Vaccination of Gerbils with Bm-103 and Bm-RAL-2 Concurrently or as a Fusion Protein Confers Consistent and Improved Protection against Brugia malayi Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004586. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Khatri, V.; Banerjee, P.; Kalyanasundaram, R. Evaluating the Vaccine Potential of a Tetravalent Fusion Protein (rBmHAXT) Vaccine Antigen Against Lymphatic Filariasis in a Mouse Model. Front. Immunol. 2018, 9, 1520. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Fecundity of adult female worms were affected when Brugia malayi infected Mongolian gerbils were immunized with a multivalent vaccine (rBmHAXT) against human lymphatic filarial parasite. Acta Trop. 2020, 208, 105487. [Google Scholar] [CrossRef]
- Khatri, V.; Chauhan, N.; Vishnoi, K.; von Gegerfelt, A.; Gittens, C.; Kalyanasundaram, R. Prospects of developing a prophylactic vaccine against human lymphatic filariasis—Evaluation of protection in non-human primates. Int. J. Parasitol. 2018, 48, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Melendez, V.; Turner, C.; Khatri, V.; Davis, J.; Chauhan, N.; Sudhakar, D.S.N.; Cabullos, R.; Carter, D.; Gray, S.A.; Kalyanasundaram, R. Pre-clinical development of a vaccine for human lymphatic filariasis. Front. Trop. Dis. 2022, 3, 998353. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Barnard, D.; Ong, C.H.; Peng, B.-H.; Tseng, C.-T.K.; Petrovsky, N. Severe Acute Respiratory Syndrome-Associated Coronavirus Vaccines Formulated with Delta Inulin Adjuvants Provide Enhanced Protection while Ameliorating Lung Eosinophilic Immunopathology. J. Virol. 2015, 89, 2995–3007. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, H.; Wu, C. The immune responses of HLA-A*0201 restricted SARS-CoV S peptide-specific CD8(+) T cells are augmented in varying degrees by CpG ODN, PolyI:C and R848. Vaccine 2011, 29, 6670–6678. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Baldwin, J.; Petrovsky, N. Advax-CpG Adjuvant Provides Antigen Dose-Sparing and Enhanced Immunogenicity for Inactivated Poliomyelitis Virus Vaccines. Pathogens 2021, 10, 500. [Google Scholar] [CrossRef]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Honda-Okubo, Y.; Wilks, S.H.; Aban, M.; Barr, I.G.; Petrovsky, N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine 2016, 34, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Woodman, R.J.; Honda-Okubo, Y.; Cox, M.M.; Heinzel, S.; Petrovsky, N. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine 2012, 30, 5407–5416. [Google Scholar] [CrossRef]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef]
- Heddle, R.; Smith, A.; Woodman, R.; Hissaria, P.; Petrovsky, N. Randomized controlled trial demonstrating the benefits of delta inulin adjuvanted immunotherapy in patients with bee venom allergy. J. Allergy Clin. Immunol. 2019, 144, 504–513.e16. [Google Scholar] [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. In DNA Vaccines: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 51–85. [Google Scholar] [CrossRef]
- Klinman, D.M.; Xie, H.; Little, S.F.; Currie, D.; Ivins, B.E. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 2004, 22, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springerplus 2015, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H. Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Front. Immunol. 2013, 3, 406. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine Adjuvants: Mode of Action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Kerepesi, L.A.; Leon, O.; Lustigman, S.; Abraham, D. Protective Immunity to the Larval Stages of Onchocerca volvulus Is Dependent on Toll-Like Receptor 4. Infect. Immun. 2005, 73, 8291–8297. [Google Scholar] [CrossRef] [PubMed]
- Trpis, M.; Scoles, G.A.; Struble, R.H. Cryopreservation of Infective Larvae of Onchocerca volvulus (Filarioidea: Onchocercidae). J. Parasitol. 1993, 79, 695. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Maurin, M.; Raoult, D. Wolbachia pipientis Growth Kinetics and Susceptibilities to 13 Antibiotics Determined by Immunofluorescence Staining and Real-Time PCR. Antimicrob. Agents Chemother. 2003, 47, 1665–1671. [Google Scholar] [CrossRef]
- Hermans, P.G.; Hart, C.A.; Trees, A.J. In vitro activity of antimicrobial agents against the endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 2001, 47, 659–663. [Google Scholar] [CrossRef]
- Hoerauf, A.; Volkmann, L.; Nissen-Paehle, K.; Schmetz, C.; Autenrieth, I.; Büttner, D.W.; Fleischer, B. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: Comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop. Med. Int. Health 2000, 5, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.B.; Bennuru, S.; Eberhard, M.L.; Hess, J.A.; Torigian, A.; Lustigman, S.; Nutman, T.B.; Abraham, D. Development of Onchocerca volvulus in humanized NSG mice and detection of parasite biomarkers in urine and serum. PLoS Negl. Trop. Dis. 2018, 12, e0006977. [Google Scholar] [CrossRef]
- Rao, R.U.; Moussa, H.; Weil, G.J. Brugia malayi: Effects of antibacterial agents on larval viability and development in vitro. Exp. Parasitol. 2002, 101, 77–81. [Google Scholar] [CrossRef]
- Smith, H.L.; Rajan, T. Tetracycline Inhibits Development of the Infective-Stage Larvae of Filarial Nematodes in Vitro. Exp. Parasitol. 2000, 95, 265–270. [Google Scholar] [CrossRef]
- Morgado, M.; Cam, P.; Gris-Liebe, C.; Cazenave, P.; Jouvin-Marche, E. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 1989, 8, 3245–3251. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, R.; Orwenyo, J.; Giddens, J.; Yang, Q.; Labranche, C.C.; Montefiori, D.C.; Wang, L.-X. Multivalent Antigen Presentation Enhances the Immunogenicity of a Synthetic Three-Component HIV-1 V3 Glycopeptide Vaccine. ACS Central Sci. 2018, 4, 582–589. [Google Scholar] [CrossRef]
- Cathcart, K.; Pinilla-Ibarz, J.; Korontsvit, T.; Schwartz, J.; Zakhaleva, V.; Papadopoulos, E.B.; Scheinberg, D.A. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood 2004, 103, 1037–1042. [Google Scholar] [CrossRef]
- Dakshinamoorthy, G.; Samykutty, A.K.; Munirathinam, G.; Reddy, M.V.; Kalyanasundaram, R. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine 2013, 31, 1616–1622. [Google Scholar] [CrossRef]
- Tabarsi, P.; Anjidani, N.; Shahpari, R.; Mardani, M.; Sabzvari, A.; Yazdani, B.; Kafi, H.; Fallah, N.; Ebrahimi, A.; Taheri, A. Evaluating the efficacy and safety of SpikoGen®, an Advax-CpG55.2-adjuvanted severe acute respiratory syndrome coronavirus 2 spike protein vaccine: A phase 3 randomized placebo-controlled trial. Clin. Microbiol. Infect. 2023, 29, 215–220. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Cartee, R.T.; Thanawastien, A.; Yang, J.S.; Killeen, K.P.; Petrovsky, N. A typhoid fever protein capsular matrix vaccine candidate formulated with Advax-CpG adjuvant induces a robust and durable anti-typhoid Vi polysaccharide antibody response in mice, rabbits and nonhuman primates. Vaccine 2022, 40, 4625–4634. [Google Scholar] [CrossRef] [PubMed]
- Jelínková, L.; Jhun, H.; Eaton, A.; Petrovsky, N.; Zavala, F.; Chackerian, B. An epitope-based malaria vaccine targeting the junctional region of circumsporozoite protein. NPJ Vaccines 2021, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.L.; Bossie, A.; Sanders, V.M.; Fernandez-Botran, R.; Coffman, R.L.; Mosmann, T.R.; Vitetta, E.S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 1988, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Diemert, D.J.; Pinto, A.G.; Freire, J.; Jariwala, A.; Santiago, H.; Hamilton, R.G.; Periago, M.V.; Loukas, A.; Tribolet, L.; Mulvenna, J.; et al. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: Implications for the development of vaccines against helminths. J. Allergy Clin. Immunol. 2012, 130, 169–176.e6. [Google Scholar] [CrossRef]
- Abraham, D.; Leon, O.; Schnyder-Candrian, S.; Wang, C.C.; Galioto, A.M.; Kerepesi, L.A.; Lee, J.J.; Lustigman, S. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect. Immun. 2004, 72, 810–817. [Google Scholar] [CrossRef]
- Abraham, D.; Graham-Brown, J.; Carter, D.; Gray, S.A.; Hess, J.A.; Makepeace, B.L.; Lustigman, S. Development of a recombinant vaccine against human onchocerciasis. Expert Rev. Vaccines 2021, 20, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Le, L.; Ahmad, G.; Molehin, A.J.; Siddiqui, A.J.; Torben, W.; Karmakar, S.; Rojo, J.U.; Sennoune, S.; Lazarus, S.; et al. Fifteen Years of Sm-p80-Based Vaccine Trials in Nonhuman Primates: Antibodies from Vaccinated Baboons Confer Protection In Vivo and In Vitro from Schistosoma mansoni and Identification of Putative Correlative Markers of Protection. Front. Immunol. 2020, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Ottenhoff, T.H.; Joosten, S.A. Antibody glycosylation in inflammation, disease and vaccination. Semin. Immunol. 2018, 39, 102–110. [Google Scholar] [CrossRef]
- Cribbs, D.H.; Ghochikyan, A.; Vasilevko, V.; Tran, M.; Petrushina, I.; Sadzikava, N.; Babikyan, D.; Kesslak, P.; Kieber-Emmons, T.; Cotman, C.W.; et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 2003, 15, 505–514. [Google Scholar] [CrossRef]
- Kenney, J.S.; Hughes, B.W.; Masada, M.P.; Allison, A.C. Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J. Immunol. Methods 1989, 121, 157–166. [Google Scholar] [CrossRef]
- Maeda, D.L.N.F.; Batista, M.T.; Pereira, L.R.; Cintra, M.D.J.; Amorim, J.H.; Mathias-Santos, C.; Pereira, S.A.; Boscardin, S.B.; Silva, S.D.R.; Faquim-Mauro, E.L.; et al. Adjuvant-Mediated Epitope Specificity and Enhanced Neutralizing Activity of Antibodies Targeting Dengue Virus Envelope Protein. Front. Immunol. 2017, 8, 1175. [Google Scholar] [CrossRef] [PubMed]
- Ajendra, J.; Allen, J.E. Neutrophils: Friend or foe in Filariasis? Parasite Immunol. 2022, 44, e12918. [Google Scholar] [CrossRef]
- Turner, H.C.; Walker, M.; Lustigman, S.; Taylor, D.W.; Basáñez, M.-G. Human Onchocerciasis: Modelling the Potential Long-term Consequences of a Vaccination Programme. PLoS Negl. Trop. Dis. 2015, 9, e0003938. [Google Scholar] [CrossRef]
- NTD Modelling Consortium Onchocerciasis Group. The World Health Organization 2030 goals for onchocerciasis: Insights and perspectives from mathematical modelling: NTD Modelling Consortium Onchocerciasis Group. Gates Open Res. 2019, 3, 1545. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryan, N.M.; Hess, J.A.; Robertson, E.J.; Tricoche, N.; Turner, C.; Davis, J.; Petrovsky, N.; Ferguson, M.; Rinaldi, W.J.; Wong, V.M.; et al. Adjuvanted Fusion Protein Vaccine Induces Durable Immunity to Onchocerca volvulus in Mice and Non-Human Primates. Vaccines 2023, 11, 1212. https://doi.org/10.3390/vaccines11071212
Ryan NM, Hess JA, Robertson EJ, Tricoche N, Turner C, Davis J, Petrovsky N, Ferguson M, Rinaldi WJ, Wong VM, et al. Adjuvanted Fusion Protein Vaccine Induces Durable Immunity to Onchocerca volvulus in Mice and Non-Human Primates. Vaccines. 2023; 11(7):1212. https://doi.org/10.3390/vaccines11071212
Chicago/Turabian StyleRyan, Nathan M., Jessica A. Hess, Erica J. Robertson, Nancy Tricoche, Cheri Turner, Jenn Davis, Nikolai Petrovsky, Melissa Ferguson, William J. Rinaldi, Valerie M. Wong, and et al. 2023. "Adjuvanted Fusion Protein Vaccine Induces Durable Immunity to Onchocerca volvulus in Mice and Non-Human Primates" Vaccines 11, no. 7: 1212. https://doi.org/10.3390/vaccines11071212
APA StyleRyan, N. M., Hess, J. A., Robertson, E. J., Tricoche, N., Turner, C., Davis, J., Petrovsky, N., Ferguson, M., Rinaldi, W. J., Wong, V. M., Shimada, A., Zhan, B., Bottazzi, M. E., Makepeace, B. L., Gray, S. A., Carter, D., Lustigman, S., & Abraham, D. (2023). Adjuvanted Fusion Protein Vaccine Induces Durable Immunity to Onchocerca volvulus in Mice and Non-Human Primates. Vaccines, 11(7), 1212. https://doi.org/10.3390/vaccines11071212






