Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Selection
2.2. Processing of Serum and PBMC Samples
2.3. SARS-CoV-2 Antibody Multiplex Immunoassay
2.4. SARS-CoV-2 Antibody Avidity Assay
2.5. SARS-CoV-2 Neutralization Assay
2.6. SARS-CoV-2 Memory B Cell ELISpot
2.7. Flow Cytometry
2.8. Statistics
3. Results
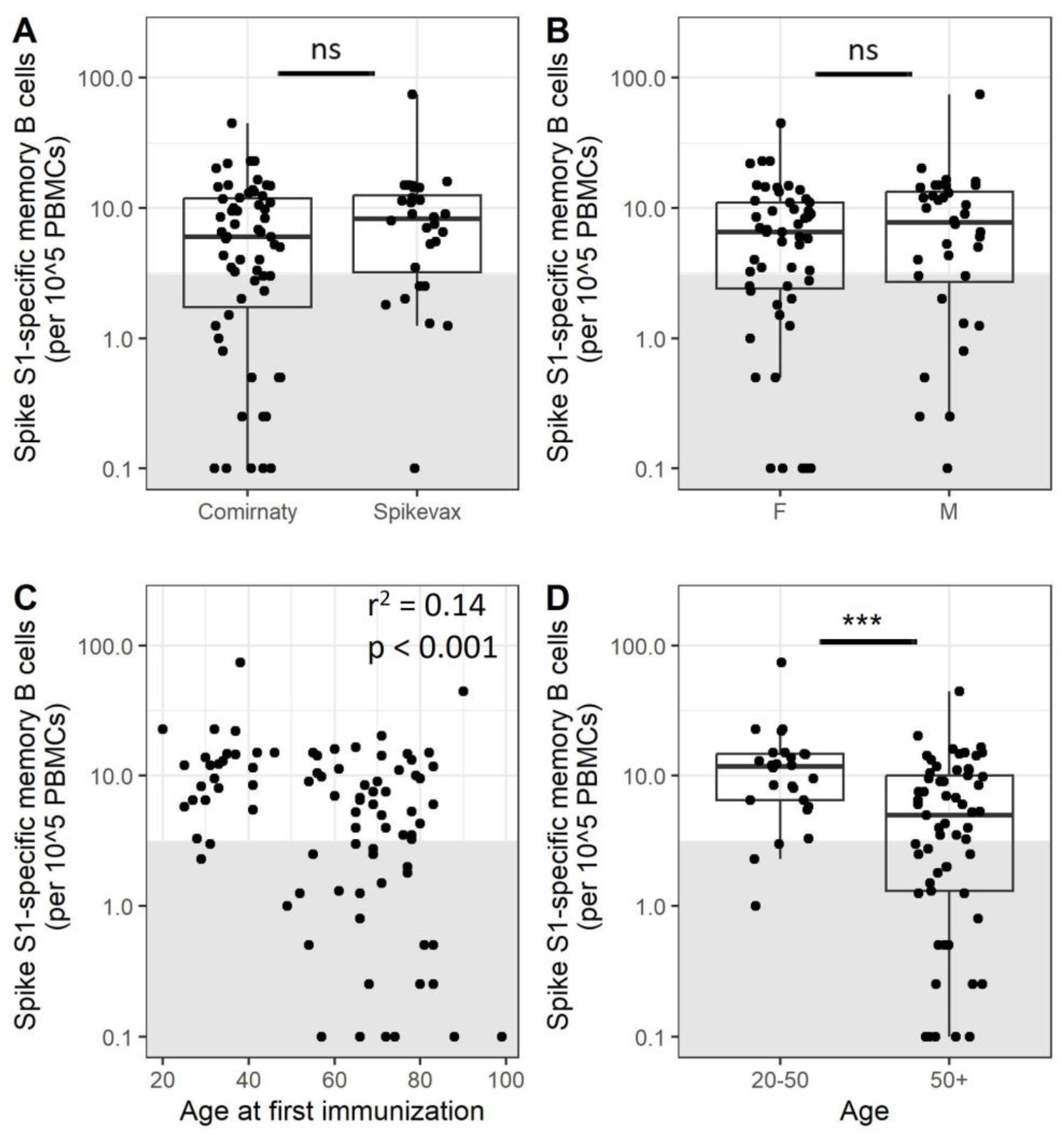
3.1. A Subset of Adults Does Not Have Detectable Frequencies of Specific Memory B Cells after Completion of the Primary Vaccination Series
3.2. Older Adults Exhibit Less SARS-CoV-2-Specific Memory B Cells after Immunization
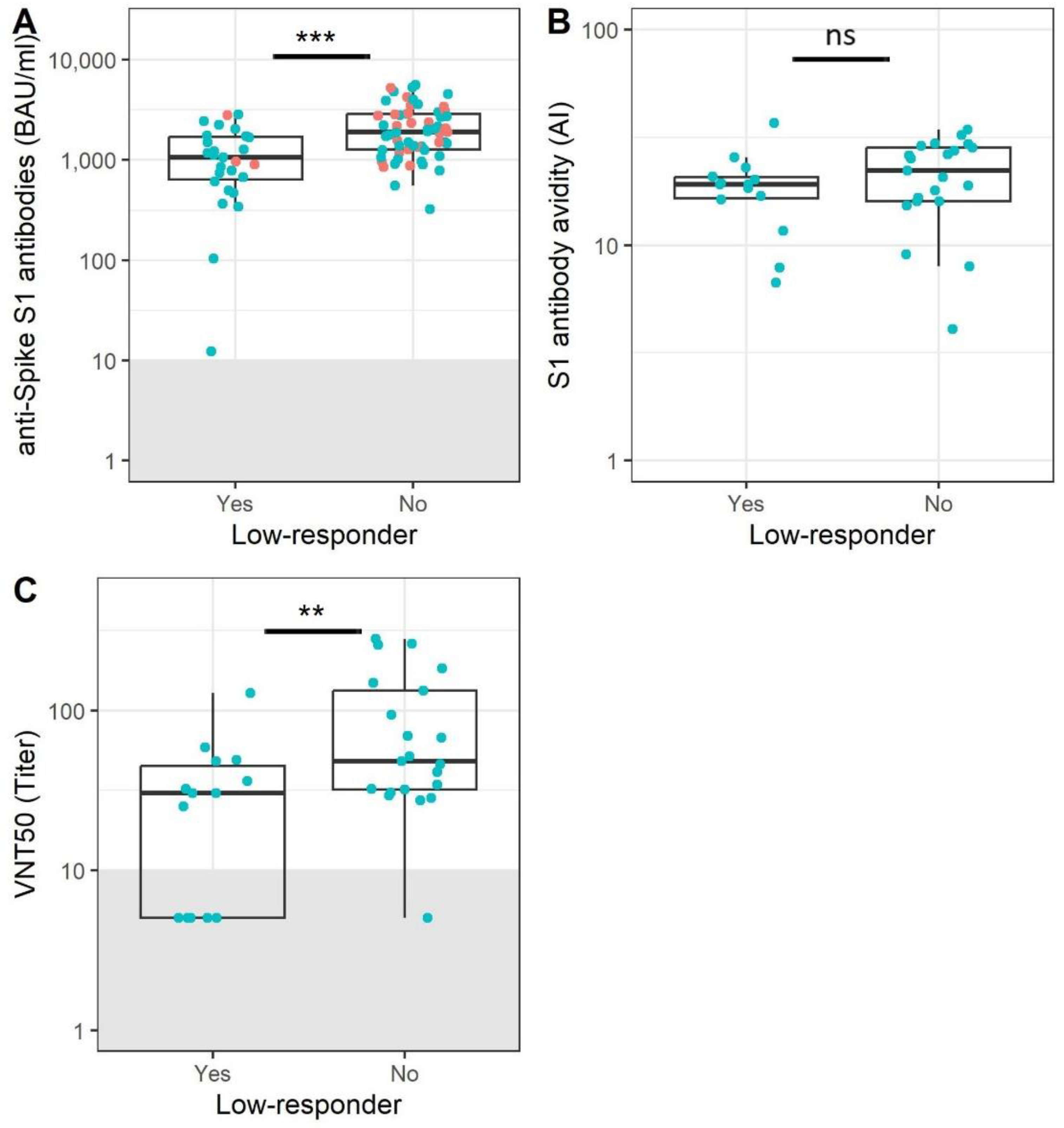
3.3. Participants without Memory B Cells Show Reduced Antibody Levels and Functionality
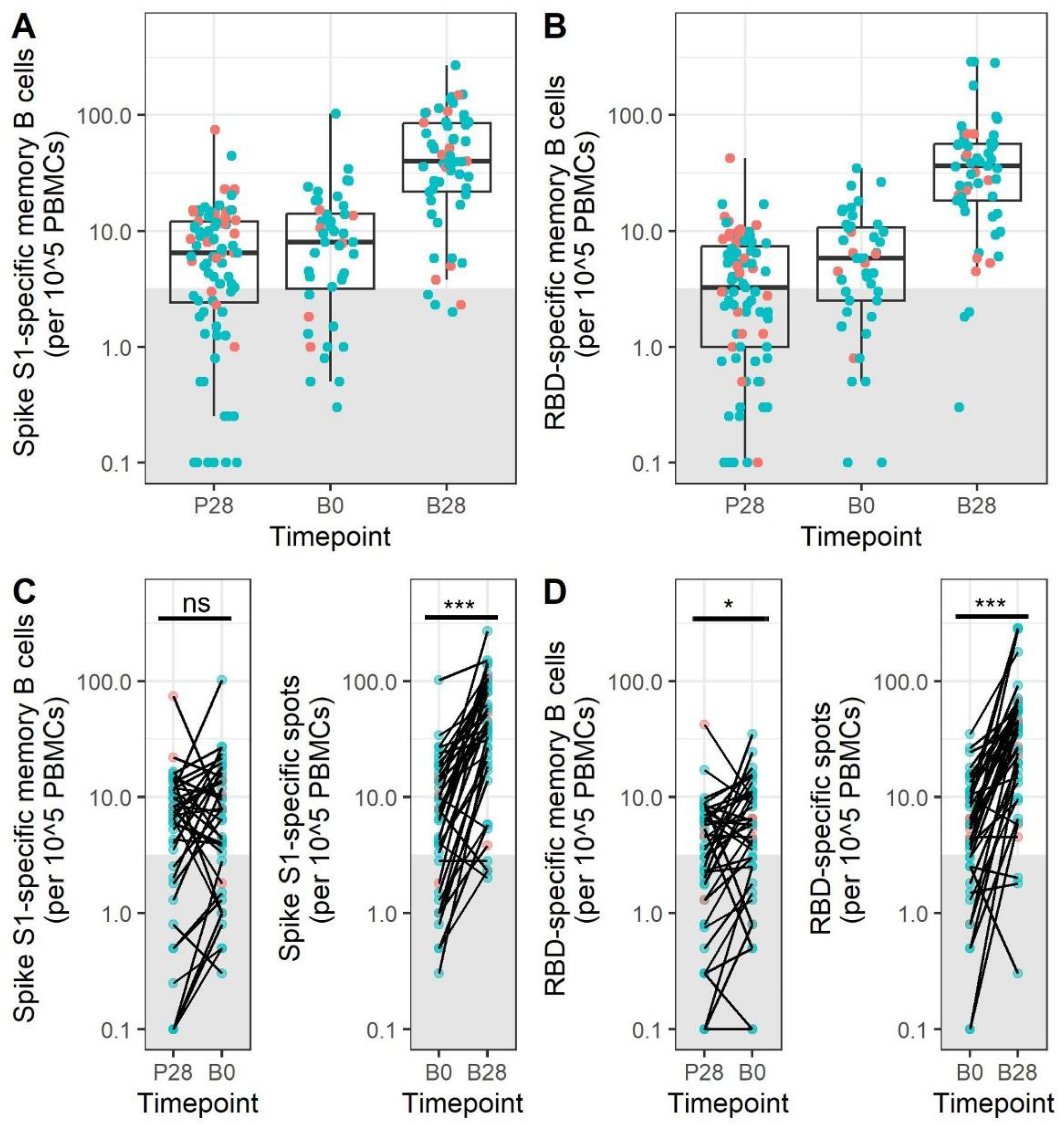
3.4. Memory B Cell Responses after Primary mRNA Vaccination Do Not Decrease during the First 5–9 Months after Vaccination
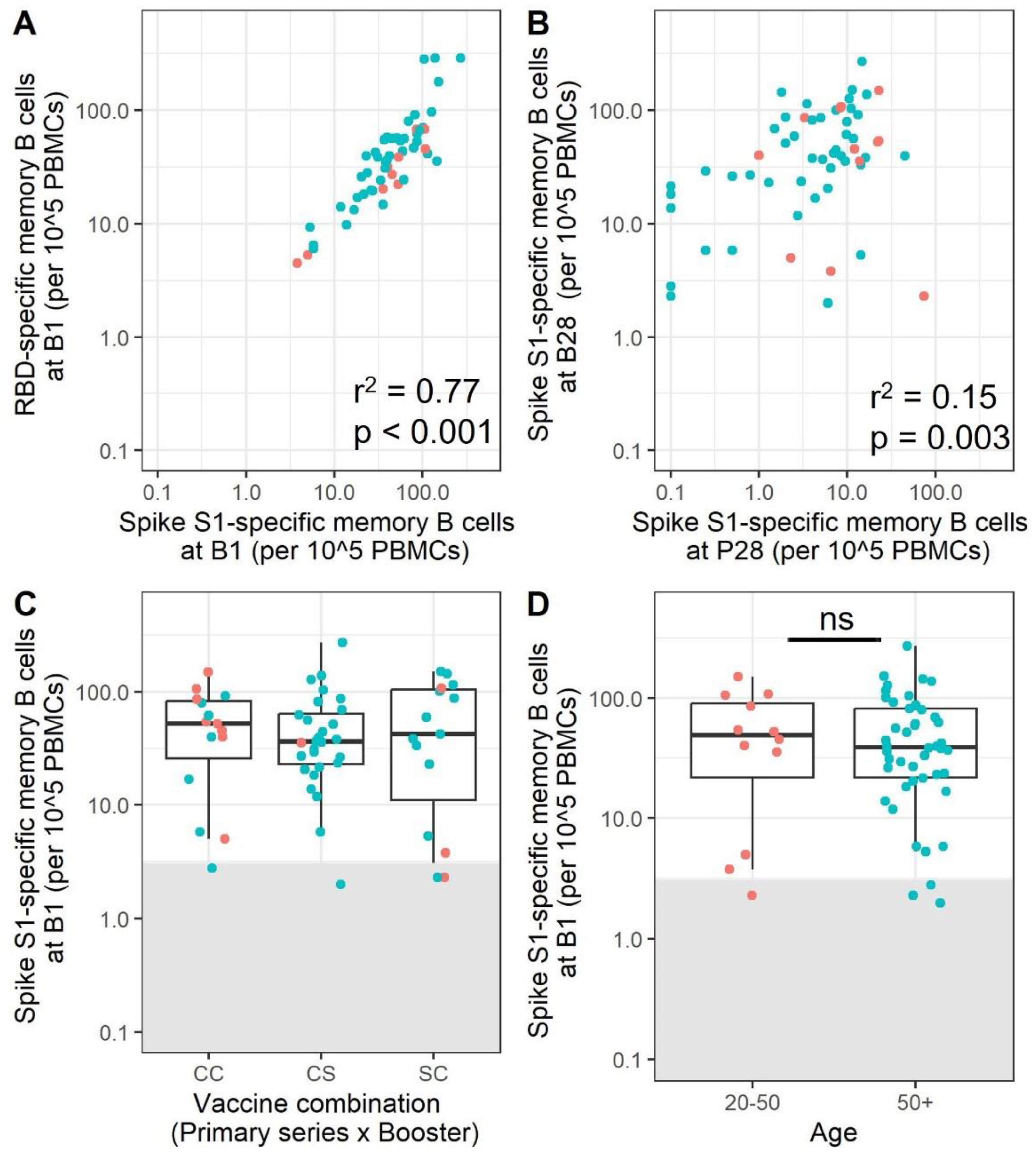
3.5. mRNA Booster Immunization Induces a Sharp Increase in Frequencies of SARS-CoV-2-Specific Memory B Cells and Reduces the Overall Number of Low-Responders
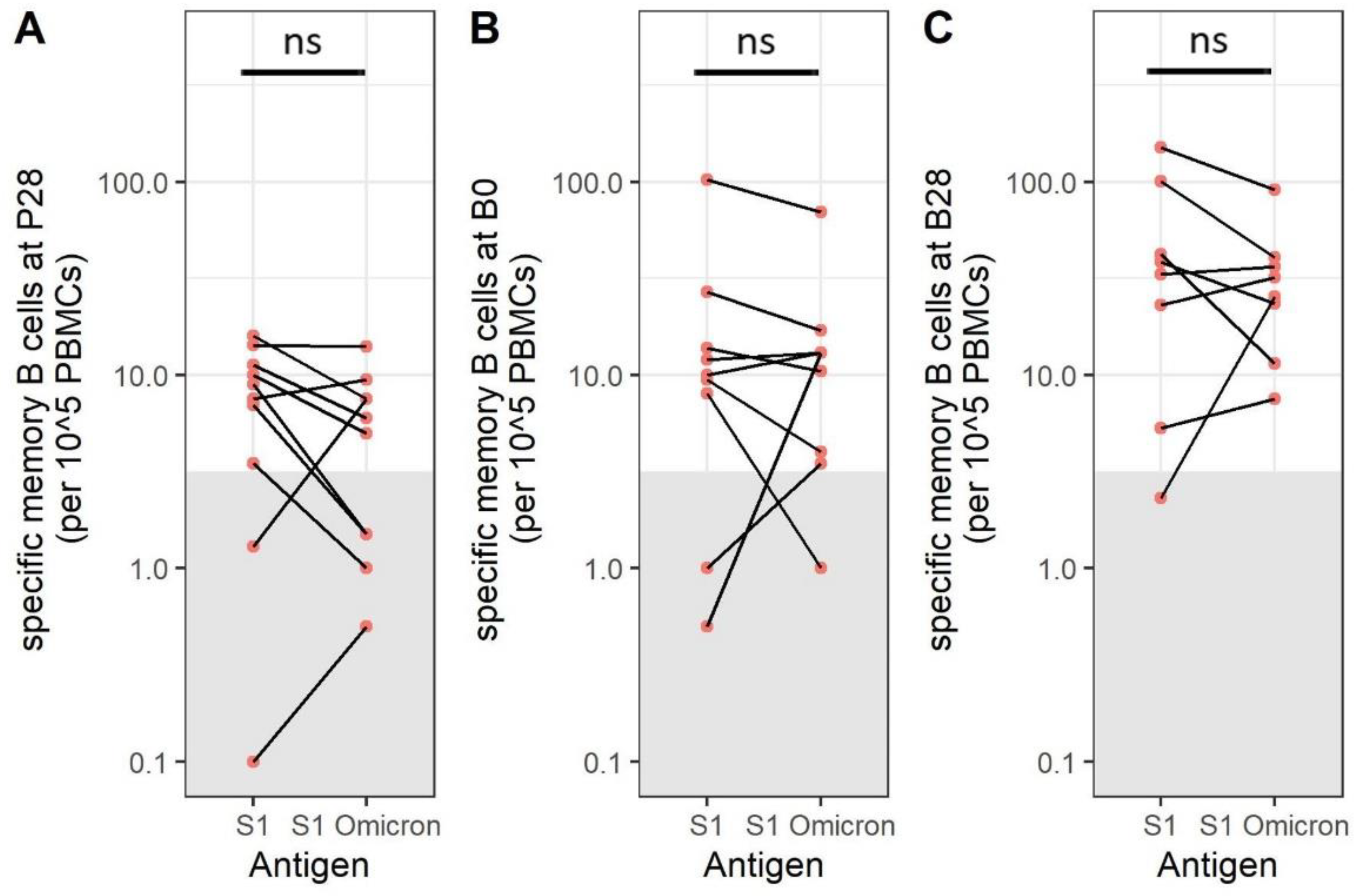
3.6. SARS-CoV-2 Omicron BA.1-Specific Memory B Cells Are Present after Immunization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, R.M.; Sorensen, R.J.D.; Pigott, D.M.; Bisignano, C.; Carter, A.; Amlag, J.O.; Collins, J.K.; Abbafati, C.; Adolph, C.; Allorant, A.; et al. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: A statistical analysis. Lancet 2022, 399, 2351–2380. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Osman, S.; Wright, J.; Richard-Greenblatt, M.; Buchan, S.A.; Sadarangani, M.; Bolotin, S. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS ONE 2022, 17, e0266852. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Marconato, M.; Abela, I.A.; Hauser, A.; Schwarzmüller, M.; Katzensteiner, R.; Braun, D.L.; Epp, S.; Audigé, A.; Weber, J.; Rusert, P.; et al. Antibodies from convalescent plasma promote SARS-CoV-2 clearance in individuals with and without endogenous antibody response. J. Clin. Investig. 2022, 132, e158190. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, J.; Boeckh, M.; Waghmare, A. Monoclonal antibodies for prophylaxis and treatment of respiratory viral infections. Curr. Opin. Infect. Dis. 2022, 35, 280–287. [Google Scholar] [CrossRef]
- Phad, G.E.; Pinto, D.; Foglierini, M.; Akhmedov, M.; Rossi, R.L.; Malvicini, E.; Cassotta, A.; Fregni, C.S.; Bruno, L.; Sallusto, F.; et al. Clonal structure, stability and dynamics of human memory B cells and circulating plasmablasts. Nat. Immunol. 2022, 23, 1076–1085. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef]
- McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005, 23, 487–513. [Google Scholar] [CrossRef] [PubMed]
- Wishnie, A.J.; Chwat-Edelstein, T.; Attaway, M.; Vuong, B.Q. BCR Affinity Influences T-B Interactions and B Cell Development in Secondary Lymphoid Organs. Front. Immunol. 2021, 12, 703918. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef]
- Dugan, H.L.; Henry, C.; Wilson, P.C. Aging and influenza vaccine-induced immunity. Cell. Immunol. 2020, 348, 103998. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Burns, E.A. Age-related decline in immunity: Implications for vaccine responsiveness. Expert Rev. Vaccines 2008, 7, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Versteegen, P.; Barkoff, A.-M.; Pinto, M.V.; van de Kasteele, J.; Knuutila, A.; Bibi, S.; de Rond, L.; Teräsjärvi, J.; Sanders, K.; de Zeeuw-Brouwer, M.-L.; et al. Memory B Cell Activation Induced by Pertussis Booster Vaccination in Four Age Groups of Three Countries. Front. Immunol. 2022, 13, 864674. [Google Scholar] [CrossRef]
- Carr, E.; Dooley, J.; Garcia-Perez, J.E.; Lagou, V.; Lee, J.; Wouters, C.; Meyts, I.; Goris, V.L.A.; Boeckxstaens, G.; Linterman, E.J.C.M.A.; et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nat. Immunol. 2016, 17, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Humblet-Baron, S.; Duffy, D.; Goris, A. Human immune diversity: From evolution to modernity. Nat. Immunol. 2021, 22, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Kissling, E.; Hooiveld, M.; Martínez-Baz, I.; Mazagatos, C.; William, N.; Vilcu, A.-M.; Kooijman, M.N.; Ilić, M.; Domegan, L.; Machado, A.; et al. Effectiveness of complete primary vaccination against COVID-19 at primary care and community level during predominant Delta circulation in Europe: Multicentre analysis, I-MOVE-COVID-19 and ECDC networks, July to August 2021. Euro Surveill 2022, 27, 2101104. [Google Scholar] [CrossRef]
- Stowe, J.; Andrews, N.; Kirsebom, F.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat. Commun. 2022, 13, 5736. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.; Blokstra, A.; Picavet, H.; Smit, H. Cohort profile: The Doetinchem Cohort Study. Int. J. Epidemiol. 2008, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Picavet, H.S.J.; Blokstra, A.; Spijkerman, A.M.W.; Verschuren, W.M.M. Cohort Profile Update: The Doetinchem Cohort Study 1987–2017: Lifestyle, health and chronic diseases in a life course and ageing perspective. Int. J. Epidemiol. 2017, 46, 1751–1751g. [Google Scholar] [CrossRef] [PubMed]
- Hoogen, L.L.V.D.; Boer, M.; Postema, A.; de Rond, L.; de Zeeuw-Brouwer, M.-L.; Pronk, I.; Wijmenga-Monsuur, A.J.; Bijvank, E.; Kruiper, C.; Beckers, L.; et al. Reduced Antibody Acquisition with Increasing Age following Vaccination with BNT162b2: Results from Two Longitudinal Cohort Studies in The Netherlands. Vaccines 2022, 10, 1480. [Google Scholar] [CrossRef]
- Cevirgel, A.; Shetty, S.A.; Vos, M.; Nanlohy, N.M.; Beckers, L.; Bijvank, E.; Rots, N.; van Beek, J.; Buisman, A.; van Baarle, D. Identification of aging-associated immunotypes and immune stability as indicators of post-vaccination immune activation. Aging Cell 2022, 21, e13703. [Google Scholar] [CrossRef]
- Hartog, G.D.; Schepp, R.M.; Kuijer, M.; GeurtsvanKessel, C.; van Beek, J.; Rots, N.; Koopmans, M.P.G.; van der Klis, F.R.M.; van Binnendijk, R.S. SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J. Infect. Dis. 2020, 222, 1452–1461. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 478–1488. [Google Scholar] [CrossRef]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Ben Tanfous, T.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887.e8. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Goh, Y.S.; Rouers, A.; Fong, S.W.; Zhuo, N.Z.; Hor, P.X.; Loh, C.Y.; Huang, Y.; Neo, V.K.; Kam, I.K.J.; Wang, B.; et al. Waning of specific antibodies against Delta and Omicron variants five months after a third dose of BNT162b2 SARS-CoV-2 vaccine in elderly individuals. Front. Immunol. 2022, 13, 1031852. [Google Scholar] [CrossRef]
- Hansen, L.; Brokstad, K.A.; Bansal, A.; Zhou, F.; Bredholt, G.; Onyango, T.B.; Sandnes, H.H.; Elyanow, R.; Madsen, A.; Trieu, M.-C.; et al. Durable immune responses after BNT162b2 vaccination in home-dwelling old adults. Vaccine X 2023, 13, 100262. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.R.; Guillaume, S.M.; Foster, W.S.; Wheatley, A.K.; Hill, D.L.; Carr, E.J.; Linterman, M.A. The memory B cell response to influenza vaccination is impaired in older persons. Cell Rep. 2022, 41, 111613. [Google Scholar] [CrossRef] [PubMed]
- Colonna-Romano, G.; Bulati, M.; Aquino, A.; Pellicanò, M.; Vitello, S.; Lio, D.; Candore, G.; Caruso, C. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech. Ageing Dev. 2009, 130, 681–690. [Google Scholar] [CrossRef]
- Ciocca, M.; Zaffina, S.; Salinas, A.F.; Bocci, C.; Palomba, P.; Conti, M.G.; Terreri, S.; Frisullo, G.; Giorda, E.; Scarsella, M.; et al. Evolution of Human Memory B Cells From Childhood to Old Age. Front. Immunol. 2021, 12, 690534. [Google Scholar] [CrossRef] [PubMed]
- Pritz, T.; Lair, J.; Ban, M.; Keller, M.; Weinberger, B.; Krismer, M.; Grubeck-Loebenstein, B. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 2015, 45, 738–746. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.; Lim, E.; Touizer, E.; Meng, B.; Abdullahi, A. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Jo, N.; Hidaka, Y.; Kikuchi, O.; Fukahori, M.; Sawada, T.; Aoki, M.; Yamamoto, M.; Nagao, M.; Morita, S.; Nakajima, T.E.; et al. Impaired CD4(+) T cell response in older adults is associated with reduced immunogenicity and reactogenicity of mRNA COVID-19 vaccination. Nat. Aging 2023, 3, 82–92. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Stefanski, A.-L.; Potekhin, A.; Staub-Hohenbleicher, H.; Choi, M.; Bachmann, F.; Proβ, V.; Hammett, C.; Schrezenmeier, H.; et al. B and T Cell Responses after a Third Dose of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 3027–3033. [Google Scholar] [CrossRef]
- Schulz, A.R.; Mälzer, J.N.; Domingo, C.; Jürchott, K.; Grützkau, A.; Babel, N.; Nienen, M.; Jelinek, T.; Niedrig, M.; Thiel, A. Low Thymic Activity and Dendritic Cell Numbers Are Associated with the Immune Response to Primary Viral Infection in Elderly Humans. J. Immunol. 2015, 195, 4699–4711. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.H.; Liu, X.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: Exploratory analyses of Com-COV, a randomised control trial. Lancet Respir. Med. 2022, 10, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Grunau, B.; Asamoah-Boaheng, M.; Lavoie, P.M.; Karim, M.E.; Kirkham, T.L.; Demers, P.A.; Barakauskas, V.; Marquez, A.C.; Jassem, A.N.; O’Brien, S.F.; et al. A Higher Antibody Response Is Generated with a 6- to 7-Week (vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval. Clin. Infect. Dis. 2022, 75, e888–e891. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Ayodele, R.; Sylla, P.; McIlroy, G.; Logan, N.; Scott, S.; Nicol, S.; Verma, K.; Stephens, C.; et al. Vaccine subtype and dose interval determine immunogenicity of primary series COVID-19 vaccines in older people. Cell Rep. Med. 2022, 3, 100739. [Google Scholar] [CrossRef]
- Quandt, J.; Muik, A.; Salisch, N.; Lui, B.G.; Lutz, S.; Krüger, K.; Wallisch, A.-K.; Adams-Quack, P.; Bacher, M.; Finlayson, A.; et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci. Immunol. 2022, 7, eabq2427. [Google Scholar] [CrossRef] [PubMed]

| All Participants | Adults (18–50) | Older Adults (50+) | |
|---|---|---|---|
| size (N | 88 | 26 | 62 |
| age (median (range)) | 66 (20–99) | 32.5 (20–49) | 71 (52–99) |
| sex (N(% female)) | 52 (59) | 16 (62) | 36 (58) |
| Primary immunization | |||
| Comirnaty (N (%)) | 59 (68) | 17 (65) | 43 (69) |
| Vaccine combination | |||
| CCC (N (%)) | 15 (26) | 8 (67) | 7 (15) |
| CCS (N ( %)) | 28 (48) | 1 (8) | 27 (59) |
| SSC (N (%)) | 15 (26) | 3 (25) | 12 (26) |
| Sample availability | |||
| P28 (N (%)) | 87 (99) | 26 (100) | 61 (98) |
| B0 (N (%)) | 44 (50) | 6 (23) | 38 (61) |
| B28 (N (%)) | 58 (66) | 12 (46) | 46 (74) |
| Vaccine Interval (days) | |||
| Primary (median (range)) | 35 (28–48) | 35 (28–48) | 35 (28–42) |
| Booster (median (range)) | 215 (135–271) | 157 (140–225) | 218 (135–271) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verheul, M.K.; Nijhof, K.H.; de Zeeuw-Brouwer, M.-l.; Duijm, G.; ten Hulscher, H.; de Rond, L.; Beckers, L.; Eggink, D.; van Tol, S.; Reimerink, J.; et al. Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization. Vaccines 2023, 11, 1196. https://doi.org/10.3390/vaccines11071196
Verheul MK, Nijhof KH, de Zeeuw-Brouwer M-l, Duijm G, ten Hulscher H, de Rond L, Beckers L, Eggink D, van Tol S, Reimerink J, et al. Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization. Vaccines. 2023; 11(7):1196. https://doi.org/10.3390/vaccines11071196
Chicago/Turabian StyleVerheul, Marije K., Kim H. Nijhof, Mary-lène de Zeeuw-Brouwer, Geraly Duijm, Hinke ten Hulscher, Lia de Rond, Lisa Beckers, Dirk Eggink, Sophie van Tol, Johan Reimerink, and et al. 2023. "Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization" Vaccines 11, no. 7: 1196. https://doi.org/10.3390/vaccines11071196
APA StyleVerheul, M. K., Nijhof, K. H., de Zeeuw-Brouwer, M.-l., Duijm, G., ten Hulscher, H., de Rond, L., Beckers, L., Eggink, D., van Tol, S., Reimerink, J., Boer, M., van Beek, J., Rots, N., van Binnendijk, R., & Buisman, A.-M. (2023). Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization. Vaccines, 11(7), 1196. https://doi.org/10.3390/vaccines11071196








