Subsequent COVID-19 Prophylaxis in COVID-19 Associated Glomerulopathies
Abstract
1. Introduction
2. Materials and Methods
3. Results
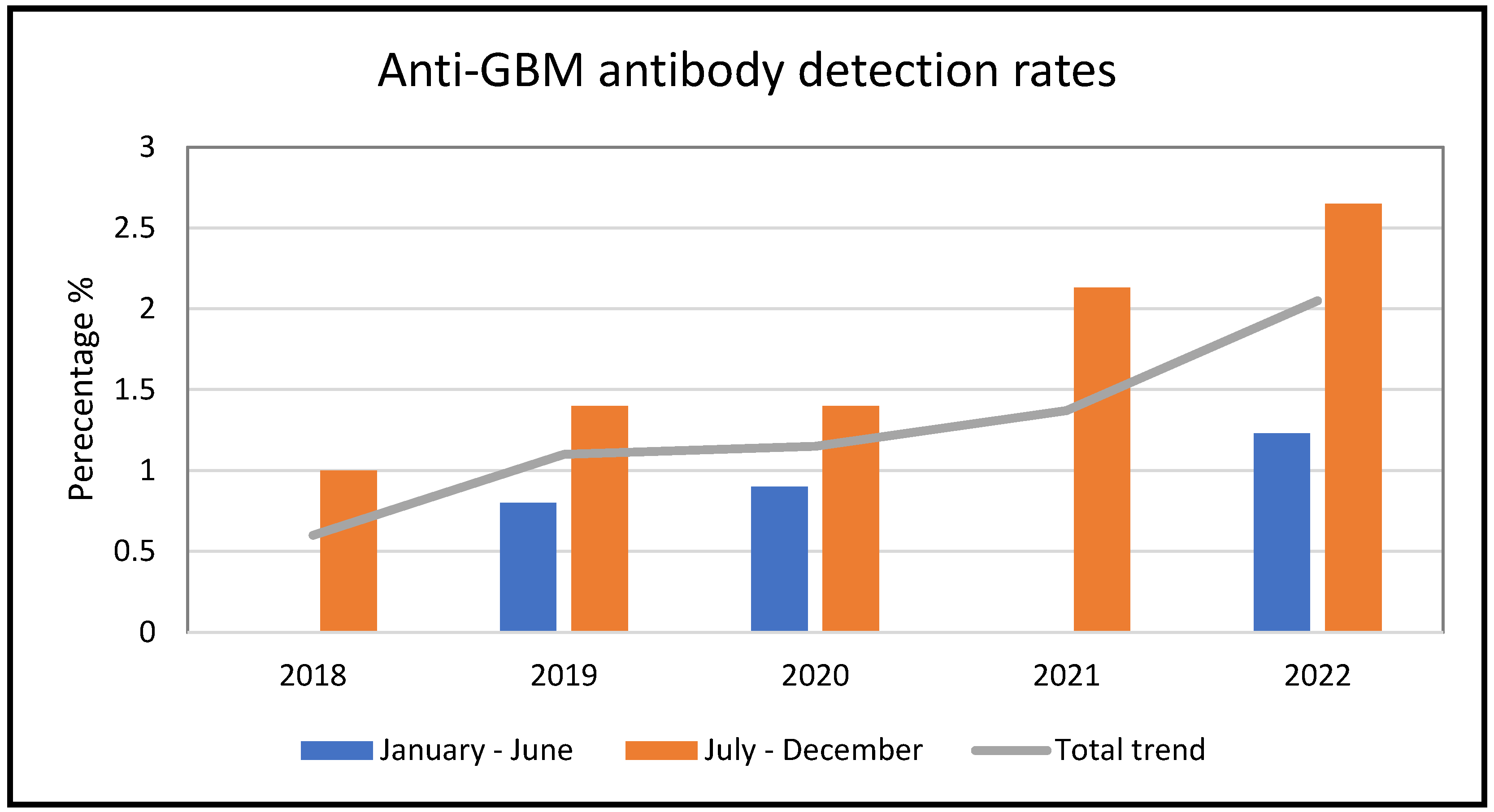
3.1. Anti-GBM Antibody Results
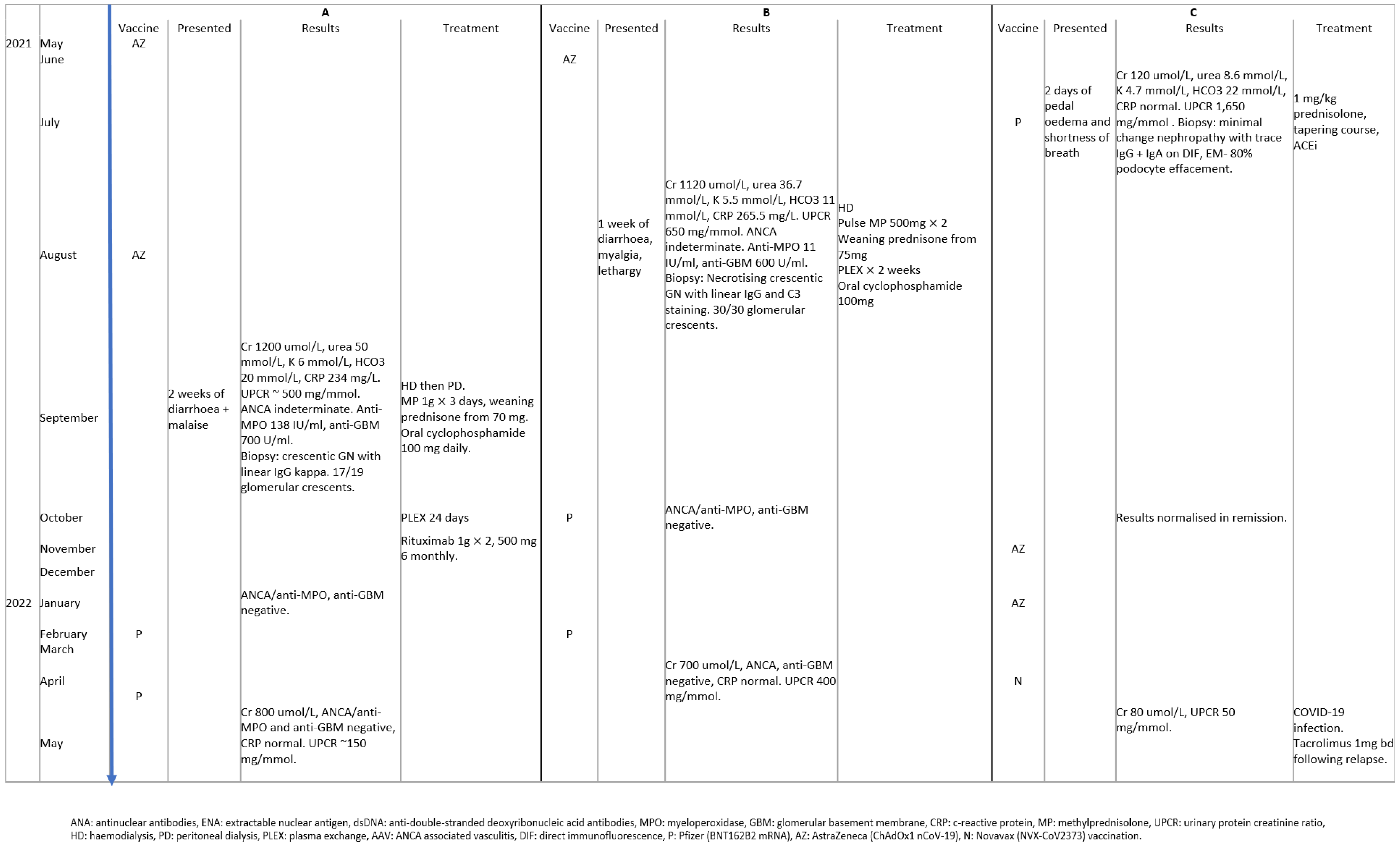
3.2. Subsequent Vaccination Safety in Patients with COVID-19 Vaccine-Associated GN
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethi, S.; Fervenza, F.C. Standardized classification and reporting of glomerulonephritis. Nephrol. Dial. Transpl. 2019, 34, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Cohen, A.H. The primary glomerulopathies. Dis. Mon. 1996, 42, 329–383. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Mohamed, S.; Alhussein, H.; Eltazi, I.; Sibira, R.M.; Abdulhadi, A. COVID-19 Vaccine as a Potential Triggering Factor for Anti-Glomerular Basement Membrane (GBM) Disease: A Case Report and Literature Review. Cureus 2022, 14, e29075. [Google Scholar] [CrossRef] [PubMed]
- Nahhal, S.; Halawi, A.; Basma, H., Sr.; Jibai, A.; Ajami, Z. Anti-Glomerular Basement Membrane Disease as a Potential Complication of COVID-19: A Case Report and Review of Literature. Cureus 2020, 12, e12089. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. [Google Scholar] [CrossRef]
- Christodoulou, M.; Iatridi, F.; Chalkidis, G.; Lioulios, G.; Nikolaidou, C.; Badis, K.; Fylaktou, A.; Papagianni, A.; Stangou, M. ANCA-Associated Vasculitis May Result as a Complication to Both SARS-CoV-2 Infection and Vaccination. Life 2022, 12, 1072. [Google Scholar] [CrossRef]
- Prema, K.S.J.; Kurien, A. Incidence of anti-glomerular basement membrane disease during the COVID-19 pandemic. Clin. Kidney J. 2022, 15, 180–181. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Cairns, T.; Cook, T.; Roufosse, C.; Thomas, D.; Willicombe, M.; Pusey, C.D.; McAdoo, S.P. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020, 98, 780–781. [Google Scholar] [CrossRef]
- Izzedine, H.; Bonilla, M.; Jhaveri, K.D. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: True association or circumstantial? Nephrol. Dial. Transpl. 2021, 36, 1565–1569. [Google Scholar] [CrossRef]
- Foster, M.H.; Ord, J.R. Emerging immunotherapies for autoimmune kidney disease. Hum. Vaccin. Immunother. 2019, 15, 876–890. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, H.Y.; Zhao, M.H. Natural autoantibodies against glomerular basement membrane exist in normal human sera. Kidney Int. 2006, 69, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Kühnl, A.; Hartwig, L.; Dähnrich, C.; Schlumberger, W. Serodiagnosis of Anti-glomerular Basement Membrane Disease Using a Newly Developed Chemiluminescence Immunoassay. Front. Med. 2022, 9, 915754. [Google Scholar] [CrossRef] [PubMed]
- Coorey, C.P.; Phua, E.; Chou, A.; Shen, Y.; Mather, A. Anti-GBM Disease after Oxford-AstraZeneca ChAdOx1 nCoV-19 Vaccination: A Report of Two Cases. Case Rep. Nephrol. Dial. 2022, 12, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Clinical Recommendations for COVID-19 Vaccinations. Available online: https://www.health.gov.au/our-work/covid-19-vaccines/advice-for-providers/clinical-guidance/clinical-recommendations (accessed on 2 February 2023).
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- Nguyen, Y.; Flahault, A.; Chavarot, N.; Melenotte, C.; Cheminant, M.; Deschamps, P.; Carlier, N.; Lafont, E.; Thomas, M.; Flamarion, E.; et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin. Microbiol. Infect. 2022, 28, 1654.e1–1654.e4. [Google Scholar] [CrossRef]
- Windpessl, M.; Bruchfeld, A.; Anders, H.-J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef]
- Corey, K.B.; Koo, G.; Phillips, E.J. Adverse Events and Safety of SARS-CoV-2 Vaccines: What’s New and What’s Next. J. Allergy Clin. Immunol. Pract. 2022, 10, 2254–2266. [Google Scholar] [CrossRef]
- Ting, J.A.; Barbir, E.-B.; McRae, S.A.; Schachter, M.; De Luca, L.; Riazy, M.; Levin, A. Double-Positive Anti–Glomerular Basement Membrane Antibody and Myeloperoxidase Antineutrophil Cytoplasmic Autoantibody–Associated Glomerulonephritis Post COVID-19 mRNA vaccine: A Case Series of 4 Patients. Can. J. Kidney Health Dis. 2023, 10, 20543581231153217. [Google Scholar] [CrossRef]
- Flossmann, O.; Bacon, P.; de Groot, K.; Jayne, D.; Rasmussen, N.; Seo, P.; Westman, K.; Luqmani, R. Development of comprehensive disease assessment in systemic vasculitis. Ann. Rheum. Dis. 2007, 66, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Flossmann, O.; Gross, W.L.; Bacon, P.; Cohen-Tervaert, J.W.; Guillevin, L.; Jayne, D.; Mahr, A.; Merkel, P.A.; Raspe, H.; et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: Focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann. Rheum. Dis. 2007, 66, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Canney, M.; Atiquzzaman, M.; Cunningham, A.M.; Zheng, Y.; Er, L.; Hawken, S.; Zhao, Y.; Barbour, S.J. A Population-Based Analysis of the Risk of Glomerular Disease Relapse after COVID-19 Vaccination. J. Am. Soc. Nephrol. 2022, 33, 2247–2257. [Google Scholar] [CrossRef]
- Klomjit, N.; Alexander, M.P.; Fervenza, F.C.; Zoghby, Z.; Garg, A.; Hogan, M.C.; Nasr, S.H.; Minshar, M.A.; Zand, L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021, 6, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Ast, V.; Costina, V.; Eichner, R.; Bode, A.; Aida, S.; Gerhards, C.; Thiaucourt, M.; Dobler, G.; Geilenkeuser, W.J.; Wolfel, R.; et al. Assessing the Quality of Serological Testing in the COVID-19 Pandemic: Results of a European External Quality Assessment (EQA) Scheme for Anti-SARS-CoV-2 Antibody Detection. J. Clin. Microbiol. 2021, 59, e0055921. [Google Scholar] [CrossRef]
- Arnold, J.; Winthrop, K.; Emery, P. COVID-19 vaccination and antirheumatic therapy. Rheumatology 2021, 60, 3496–3502. [Google Scholar] [CrossRef]
- Paschall, A.V.; Ozdilek, A.; Briner, S.L.; Brindley, M.A.; Avci, F.Y. Modulation of immunosuppressant drug treatment to improve SARS-CoV-2 vaccine efficacy in mice. Vaccine 2021, 40, 854–861. [Google Scholar] [CrossRef]
- Sanders, J.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef]
- Frittoli, M.; Cassia, M.; Barassi, A.; Ciceri, P.; Galassi, A.; Conte, F.; Cozzolino, M.G. Efficacy and Safety of COVID-19 Vaccine in Patients on Renal Replacement Therapy. Vaccines 2022, 10, 1395. [Google Scholar] [CrossRef]
- Carruthers, J.E.; Wells, J.; Gupta, A.; Kallon, D.; Cox, A.; Pina, N.; Yaqoob, M.M.; Rajakariar, R. Response to Vaccination Against SARS-CoV-2 in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis With Renal Involvement. Front. Med. 2022, 8, 817845. [Google Scholar] [CrossRef]
- Prendecki, M.; Willicombe, M.; McAdoo, S.P. COVID-19 vaccination in patients with immunity-mediated kidney disease. Nat. Rev. Nephrol. 2021, 17, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.; Magliulo, D.; Kyttaris, V.C. Seroconversion among rituximab-treated patients following SARS-CoV-2 vaccine supplemental dose. Clin. Immunol. 2022, 245, 109144. [Google Scholar] [CrossRef]
- de Lind van Wijngaarden, R.A.F.; van Rijn, L.; Hagen, E.C.; Watts, R.A.; Gregorini, G.; Tervaert, J.W.C.; Mahr, A.D.; Niles, J.L.; de Heer, E.; Bruijn, J.A.; et al. Hypotheses on the Etiology of Antineutrophil Cytoplasmic Autoantibody–Associated Vasculitis: The Cause Is Hidden, but the Result Is Known. Clin. J. Am. Soc. Nephrol. 2008, 3, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Akhtar, M. Epidemiology, Impact, and Management Strategies of Anti-Glomerular Basement Membrane Disease. Int. J. Nephrol. Renov. Dis. 2022, 15, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.B.; Anders, H.J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Prim. 2020, 6, 68. [Google Scholar] [CrossRef]
- Rizzo, M.; Marino, A.; Sannino, A.; Urciuoli, V.; Capuano, I.; Pisani, A. A possible relationship between anti-SARS-CoV-2 vaccination and glomerular diseases: Food for thought for the nephrologist. G. Ital. Nefrol. 2022, 39, 2022-vol2. [Google Scholar]
- Sacker, A.; Kung, V.; Andeen, N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021, 100, 471–472. [Google Scholar] [CrossRef]
- Uddin, K.; Mohamed, K.H.; Agboola, A.A.; Naqvi, W.A.; Hussaini, H.; Mohamed, A.S.; Haseeb, M.; Nasir, H. Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Renal Vasculitis Following COVID-19 Vaccination: A Case Report and Literature Review. Cureus 2022, 14, e30206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Ye, Q. Renal Side Effects of COVID-19 Vaccination. Vaccines 2022, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, R.; Lalloni, S.; Marchisio, M.; Oddone, V.; De Simone, E.; Del Vecchio, G.; Sciascia, S.; Roccatello, D. New Onset Biopsy-Proven Nephropathies after COVID Vaccination. Am. J. Nephrol. 2022, 53, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Tamborska, A.; Wood, G.K.; Ellul, M.; Thomas, R.H.; Galea, I.; Pett, S.; Singh, B.; Solomon, T.; Pollak, T.A.; et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: From cerebral venous sinus thrombosis to functional neurological disorder. J. Neurol. Neurosurg. Psychiatr. 2021, 92, 1144–1151. [Google Scholar] [CrossRef]
- MacIntyre, C.R. Using the Bradford-Hill criteria to assess causality in the association between CHADOX1 NCOV-19 vaccine and thrombotic immune thrombocytopenia. Glob. Biosecur. 2021, 3, 2. [Google Scholar] [CrossRef]
- Greco, A.; Rizzo, M.I.; De Virgilio, A.; Gallo, A.; Fusconi, M.; Pagliuca, G.; Martellucci, S.; Turchetta, R.; Longo, L.; De Vincentiis, M. Goodpasture’s syndrome: A clinical update. Autoimmun. Rev. 2015, 14, 246–253. [Google Scholar] [CrossRef]
- Kluth, D.C.; Rees, A.J. Anti-Glomerular Basement Membrane Disease. J. Am. Soc. Nephrol. 1999, 10, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, A.; Li, W.; Kanaan, H.D.; Zhang, P.; Blatt, N.B. Minimal Change Disease Secondary to Either COVID-19 Infection or Pfizer-BioNTech COVID-19 Vaccination. Am. J. Clin. Pathol. 2022, 158, S154. [Google Scholar] [CrossRef]
- Pacheco, I.C.R.; Costa, D.M.d.N.; Sousa, D.S.; Salgado Filho, N.; Silva, G.E.B.; Neves, P.D.M.d.M. Kidney injury associated with COVID-19 infection and vaccine: A narrative review. Front. Med. 2022, 9, 3532. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Oth, T.; Vanderlocht, J.; Van Elssen, C.H.M.J.; Bos, G.M.J.; Germeraad, W.T.V. Pathogen-Associated Molecular Patterns Induced Crosstalk between Dendritic Cells, T Helper Cells, and Natural Killer Helper Cells Can Improve Dendritic Cell Vaccination. Mediat. Inflamm. 2016, 2016, 5740373. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Milne, G.; Hames, T.; Scotton, C.; Gent, N.; Johnsen, A.; Anderson, R.M.; Ward, T. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir. Med. 2021, 9, 1450–1466. [Google Scholar] [CrossRef]
- Park, T.; Hwang, H.; Moon, S.; Kang, S.G.; Song, S.; Kim, Y.H.; Kim, H.; Ko, E.J.; Yoon, S.D.; Kang, S.M.; et al. Vaccines against SARS-CoV-2 variants and future pandemics. Expert. Rev. Vaccines 2022, 21, 1363–1376. [Google Scholar] [CrossRef]
- Provine, N.M.; Klenerman, P. Adenovirus vector and mRNA vaccines: Mechanisms regulating their immunogenicity. Eur. J. Immunol. 2022, 53, e2250022. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. Role of infection and molecular mimicry in the pathogenesis of anti-GBM disease. Nat. Rev. Nephrol. 2020, 16, 430. [Google Scholar] [CrossRef]
- Lai, A.S.; Lai, K.N. Viral nephropathy. Nat. Clin. Pract. Nephrol. 2006, 2, 254–262. [Google Scholar] [CrossRef]
- Rodríguez-Iturbe, B.; Burdmann, E.A.; Barsoum, R.S. Glomerular Diseases Associated with Infection. Compr. Clin. Nephrol. 2010, 2010, 662–674. [Google Scholar]
- McAdoo, S.P.; Pusey, C.D. Clustering of Anti-GBM Disease: Clues to an Environmental Trigger? Clin. J. Am. Soc. Nephrol. 2016, 11, 1324–1326. [Google Scholar] [CrossRef]
- Caza, T.N.; Cassol, C.A.; Messias, N.; Hannoudi, A.; Haun, R.S.; Walker, P.D.; May, R.M.; Seipp, R.M.; Betchick, E.J.; Amin, H.; et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney 2021, 2, 1770. [Google Scholar] [CrossRef]
- Krishna, M.T.; Subramanian, A.; Adderley, N.J.; Zemedikun, D.T.; Gkoutos, G.V.; Nirantharakumar, K. Allergic diseases and long-term risk of autoimmune disorders: Longitudinal cohort study and cluster analysis. Eur. Respir. J. 2019, 54, 1900476. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, M.; Shimada, M.; Lee, P.Y.; Johnson, R.J.; Garin, E.H. Idiopathic nephrotic syndrome and atopy: Is there a common link? Am. J. Kidney Dis. 2009, 54, 945–953. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyle, T.; O’Lone, E.; Phua, E.; Anderson, J.; Mather, A.; Fernando, S.L. Subsequent COVID-19 Prophylaxis in COVID-19 Associated Glomerulopathies. Vaccines 2023, 11, 1152. https://doi.org/10.3390/vaccines11071152
Boyle T, O’Lone E, Phua E, Anderson J, Mather A, Fernando SL. Subsequent COVID-19 Prophylaxis in COVID-19 Associated Glomerulopathies. Vaccines. 2023; 11(7):1152. https://doi.org/10.3390/vaccines11071152
Chicago/Turabian StyleBoyle, Therese, Emma O’Lone, Elaine Phua, Janet Anderson, Amanda Mather, and Suran L. Fernando. 2023. "Subsequent COVID-19 Prophylaxis in COVID-19 Associated Glomerulopathies" Vaccines 11, no. 7: 1152. https://doi.org/10.3390/vaccines11071152
APA StyleBoyle, T., O’Lone, E., Phua, E., Anderson, J., Mather, A., & Fernando, S. L. (2023). Subsequent COVID-19 Prophylaxis in COVID-19 Associated Glomerulopathies. Vaccines, 11(7), 1152. https://doi.org/10.3390/vaccines11071152




