COVID-19 Infection and Vaccination and Its Relation to Amyloidosis: What Do We Know Currently?
Abstract
1. Introduction
2. Search Process for Scoping Review
2.1. Eligibility Criteria
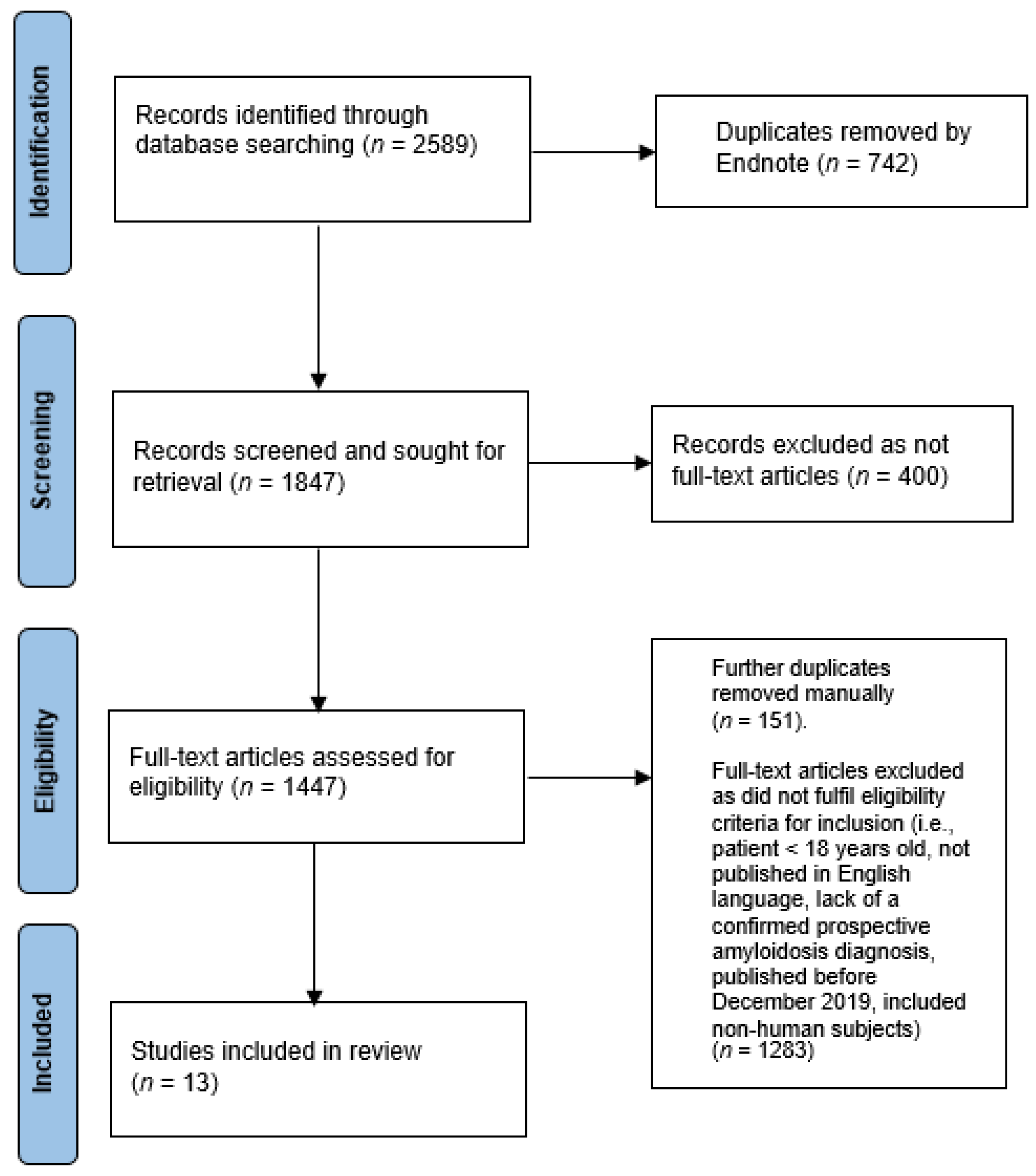
2.2. Search Strategy and Study Selection
2.3. Summary of Search Outcome
3. Clinical Outcomes of COVID-19 Infection in Patients with Amyloidosis
4. Impact of COVID-19 Pandemic on the Management of Amyloidosis
5. Reported Cases of COVID-19 Infection-Induced Amyloidosis
6. Proposed Pathophysiological Associations of COVID-19 Infection-Induced Amyloidosis
7. Reported Cases of COVID-19 Vaccination Induced Amyloidosis
| Author and Year | Age (years) | Sex | New Onset or Relapse | Comorbidities | Primary Management | Organ Involved | Presentation | Biopsy | Treatment Received | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 Infection | ||||||||||
| Djafari et al., (2021) [42] | 68 | F | Relapse | Rheumatoid arthritis | Methotrexate Prednisolone Etanercept | Urinary Bladder | Gross hematuria | Bladder mucosa: AA Amyloidosis | Conservative management (bladder irrigation, platelet, and packed cell transfusion) | Died due to respiratory failure |
| Mir et al., (2023) [43] | 55 | M | New onset | None | - | Kidney | Acute kidney injury (unexplained) | Kidney: Renal AA Amyloidosis | Prednisolone and Colchicine | Recovering following treatment initiation |
| COVID-19 Vaccination | ||||||||||
| Shahandeh et al., (2023) [62] | 54 | F | New onset | Non-ischemic cardiomyopathy to COVID-19 vaccine associated myocarditis | - | Heart | Cardiogenic shock | Heart: AL Amyloidosis | Heart transplant Daratumumab | Recovered following treatment |
8. Proposed Pathophysiological Associations of COVID-19 Vaccination-Induced Amyloidosis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bellotti, V.; Mangione, P.; Stoppini, M. Biological activity and pathological implications of misfolded proteins. Cell. Mol. Life Sci. 1999, 55, 977–991. [Google Scholar] [CrossRef]
- Bellotti, V.; Nuvolone, M.; Giorgetti, S.; Obici, L.; Palladini, G.; Russo, P.; Lavatelli, F.; Perfetti, V.; Merlini, G. The workings of the amyloid diseases. Ann. Med. 2007, 39, 200–207. [Google Scholar] [CrossRef]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.N.; Dispenzieri, A.; Eisenberg, D.S.; Fändrich, M.; Merlini, G.; Saraiva, M.J.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2022: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA). Nomencl. Committee. Amyloid. 2022, 29, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Lachmann, H.J.; Wechalekar, A.D. Epidemiologic and survival trends in amyloidosis, 1987–2019. New Engl. J. Med. 2020, 382, 1567–1568. [Google Scholar] [CrossRef]
- Westermark, G.T.; Fändrich, M.; Westermark, P. AA amyloidosis: Pathogenesis and targeted therapy. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 321–344. [Google Scholar] [CrossRef]
- World Health Organization. Pneumonia of Unknown Cause. 2020. Available online: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (accessed on 12 April 2023).
- World Health Organization. WHO Coronavirus Disease (COVID−19) Dashboard. Available online: https://covid19.who.int/?gclid=CjwKCAiAqJn9BRB0EiwAJ1SztaDZX6XhnL9tmEp0weSVA_KvmX3mJ8nAxXXR0jS7dSWfo813v3PYURoCVcEQAvD_BwE (accessed on 12 April 2023).
- Akhmerov, A.; Marbán, E. COVID−19 and the heart. Circ. Res. 2020, 126, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Alrashed, F.; Shuaibi, S.; Alajmi, D.; Barkun, A. Gastroenterological and hepatic manifestations of patients with COVID−19, prevalence, mortality by country, and intensive care admission rate: Systematic review and meta-analysis. BMJ Open Gastroenterol. 2021, 8, e000571. [Google Scholar] [CrossRef]
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID−19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [CrossRef]
- Jeyalan, V.; Storrar, J.; Wu, H.H.; Ponnusamy, A.; Sinha, S.; Kalra, P.A.; Chinnadurai, R. Native and transplant kidney histopathological manifestations in association with COVID−19 infection: A systematic review. World J. Transplant. 2021, 11, 480–502. [Google Scholar] [CrossRef]
- Niazkar, H.R.; Zibaee, B.; Nasimi, A.; Bahri, N. The neurological manifestations of COVID−19: A review article. Neurol. Sci. 2020, 41, 1667–1671. [Google Scholar] [CrossRef]
- Galkin, A.P. Hypothesis: AA amyloidosis is a factor causing systemic complications after coronavirus disease. Prion 2021, 15, 53–55. [Google Scholar] [CrossRef]
- Wood, W.A.; Neuberg, D.S.; Thompson, J.C.; Tallman, M.S.; Sekeres, M.A.; Sehn, L.H.; Anderson, K.C.; Goldberg, A.D.; Pennell, N.A.; Niemeyer, C.M.; et al. Outcomes of patients with hematologic malignancies and COVID−19: A report from the ASH Research Collaborative Data Hub. Blood Adv. 2020, 4, 5966–5975. [Google Scholar] [CrossRef]
- Lewis, E.; Fine, N.; Miller, R.J.; Hahn, C.; Chhibber, S.; Mahe, E.; Tay, J.; Duggan, P.; McCulloch, S.; Bahlis, N.; et al. Amyloidosis and COVID−19: Experience from an amyloid program in Canada. Ann. Hematol. 2022, 101, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Zanwar, S.; Buadi, F.K.; Ailawadhi, S.; Larsen, J.; Bergsagel, L.; Binder, M.; Chanan-Khan, A.; Dingli, D.; Dispenzieri, A.; et al. Risk factors for severe infection and mortality In patients with COVID−19 in patients with multiple myeloma and AL amyloidosis. Am. J. Hematol. 2023, 98, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Brannagan, T.H.; Auer-Grumbach, M.; Berk, J.L.; Briani, C.; Bril, V.; Coelho, T.; Damy, T.; Dispenzieri, A.; Drachman, B.M.; Fine, N.; et al. ATTR amyloidosis during the COVID−19 pandemic: Insights from a global medical roundtable. Orphanet J. Rare Dis. 2021, 16, 1–3. [Google Scholar] [CrossRef]
- Nussbaumer-Streit, B.; Mayr, V.; Dobrescu, A.I.; Chapman, A.; Persad, E.; Klerings, I.; Wagner, G.; Siebert, U.; Ledinger, D.; Zachariah, C.; et al. Quarantine alone or in combination with other public health measures to control COVID−19: A rapid review. Cochrane Database Syst. Rev. 2020, 4, CD013574. [Google Scholar]
- Chu, I.Y.; Alam, P.; Larson, H.J.; Lin, L. Social consequences of mass quarantine during epidemics: A systematic review with implications for the COVID−19 response. J. Travel Med. 2020, 27, taaa192. [Google Scholar] [CrossRef]
- Ferreira, L.N.; Pereira, L.N.; da Fé Brás, M.; Ilchuk, K. Quality of life under the COVID−19 quarantine. Qual. Life Res. 2021, 30, 1389–1405. [Google Scholar] [CrossRef]
- Kastritis, E.; Wechalekar, A.; Schoenland, S.; Sanchorawala, V.; Merlini, G.; Palladini, G.; Minnema, M.; Roussel, M.; Jaccard, A.; Hegenbart, U.; et al. Challenges in the management of patients with systemic light chain (AL) amyloidosis during the COVID−19 pandemic. Br. J. Haematol. 2020, 190, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Tay, J.; Duggan, P.; McCulloch, S.; Neri, P.; Bahlis, N.J.; Jimenez-Zepeda, V.H. The impact of COVID−19 in the management of AL amyloidosis and Immunoglobulin Deposition Disease: A single-center experience. Eur. J. Haematol. 2021, 106, 340–345. [Google Scholar] [CrossRef]
- International Society of Amyloidosis Recommendations on the Management of Patients with Systemic Amyloidosis during the COVID−19 Pandemic (v 2.6, April 6, 2020). Available online: https://cms.cws.net/content/isaamyloidosis.org/files/ISA%20recommendations%20Covid−19%20v_%202_6%20final.pdf (accessed on 14 April 2023).
- Jain, A. Comments on COVID−19 and AL Amyloidosis, the Missing Links. Am J Med. 2022, 135, e137–e138. [Google Scholar] [CrossRef]
- Crees, Z.D.; Stockerl-Goldstein, K. COVID−19 and Light Chain Amyloidosis, Adding Insult to Injury. Am J Med. 2022, 135, S49–S52. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID−19 vaccines and decreased transmission of SARS-CoV−2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Branagan, A.R.; Duffy, E.; Albrecht, R.A.; Cooper, D.L.; Seropian, S.; Parker, T.L.; Gan, G.; Li, F.; Zelterman, D.; Boddupalli, C.S.; et al. Clinical and Serologic Responses After a Two-dose Series of High-dose Influenza Vaccine in Plasma Cell Disorders: A Prospective, Single-arm Trial. Clin Lymphoma Myeloma Leuk. 2017, 17, 296–304.e2. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Boccadoro, M.; Moreau, P.; San-Miguel, J.; Cavo, M.; Pawlyn, C.; Zweegman, S.; Facon, T.; Driessen, C.; Hajek, R.; et al. Recommendations for vaccination in multiple myeloma: A consensus of the European Myeloma Network. Leukemia 2021, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Liebers, N.; Schönland, S.O.; Speer, C.; Edelmann, D.; Schnitzler, P.; Kräusslich, H.G.; Mueller-Tidow, C.; Hegenbart, U.; Dietrich, S. Seroconversion Rates After the Second COVID−19 Vaccination in Patients With Systemic Light Chain (AL) amyloidosis. Hemasphere 2022, 6, e688. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Wang, C.C.; Ong, C.M.; Lynch, K.L. Adequate antibody response to COVID−19 vaccine in patients with monoclonal gammopathies and light chain amyloidosis. Lab. Med. 2022, 53, 314–319. [Google Scholar] [CrossRef]
- Mahil, S.K.; Bechman, K.; Raharja, A.; Domingo-Vila, C.; Baudry, D.; Brown, M.A.; Cope, A.P.; Dasandi, T.; Graham, C.; Lechmere, T.; et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID−19 vaccine BNT162b2: A cohort study. Lancet Rheumatol. 2021, 3, e627–e637. [Google Scholar] [CrossRef]
- Avouac, J.; Miceli-Richard, C.; Combier, A.; Steelandt, A.; Fogel, O.; Mariaggi, A.A.; Meritet, J.F.; Rozenberg, F.; Molto, A.; Allanore, Y. Risk factors of impaired humoral response to COVID−19 vaccination in rituximab-treated patients. Rheumatology 2022, 68, SI163–SI168. [Google Scholar] [CrossRef]
- Kastritis, E.; Terpos, E.; Evangelakou, Z.; Theodorakakou, F.; Fotiou, D.; Manola, M.S.; Gianniou, D.D.; Bagratuni, T.; Kanellias, N.; Migkou, M.; et al. Kinetics of anti-SARS-CoV-2 neutralizing antibodies development after BNT162b2 vaccination in patients with amyloidosis and the impact of therapy. Am. J. Hematol. 2022, 97, E27. [Google Scholar] [CrossRef]
- Shapiro, L.C.; Thakkar, A.; Campbell, S.T.; Forest, S.K.; Pradhan, K.; Gonzalez-Lugo, J.D.; Quinn, R.; Bhagat, T.D.; Choudhary, G.S.; McCort, M.; et al. Efficacy of booster doses in augmenting waning immune responses to COVID−19 vaccine in patients with cancer. Cancer Cell. 2022, 40, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID syndrome: An insight on its pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Mungmunpuntipantip, R.; Wiwanitkit, V. COVID−19 and Light Chain Amyloidosis: Correspondence. Am J Med. 2022, 135, e136. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.P.; Das, S.S.; Yadav, S.; Khan, W.; Afzal, M.; Alarifi, A.; Ansari, M.T.; Hasnain, M.S.; Nayak, A.K. Global impacts of pre-and post-COVID−19 pandemic: Focus on socio-economic consequences. Sens. Int. 2020, 1, 100042. [Google Scholar] [CrossRef]
- Zoumpourlis, V.; Goulielmaki, M.; Rizos, E.; Baliou, S.; Spandidos, D.A. The COVID−19 pandemic as a scientific and social challenge in the 21st century. Mol. Med. Rep. 2020, 22, 3035–3048. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Charest, O.; Lui, L.M.; Eskander, S.; Ceban, F.; Ho, R.; Di Vincenzo, J.D.; Rosenblat, J.D.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Onset and frequency of depression in post-COVID−19 syndrome: A systematic review. J. Psychiatr. Res. 2021, 144, 129–137. [Google Scholar] [CrossRef]
- Lakhan, R.; Agrawal, A.; Sharma, M. Prevalence of depression, anxiety, and stress during COVID−19 pandemic. J. Neurosci. Rural. Pract. 2020, 11, 519–525. [Google Scholar] [CrossRef]
- Djafari, A.A.; Hasanzadeh, K.; Masrour, H.; Ahadi, M.; Dargahi, M.; Rahavian, A. Is corona virus infection a risk factor for hematuria in secondary bladder amyloidosis? The first case report. Urol. Case Rep. 2021, 38, 101642. [Google Scholar] [CrossRef]
- Mir, T.H.; Zargar, P.A.; Sharma, A.; Jabeen, B.; Sharma, S.; Parvaiz, M.O.; Bashir, S.; Javeed, R. Post COVID−19 AA amyloidosis of the kidneys with rapidly progressive renal failure. Prion 2023, 17, 111–115. [Google Scholar] [CrossRef]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID−19–associated nonocclusive fibrin microthrombi in the heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef]
- Dal Ferro, M.; Bussani, R.; Paldino, A.; Nuzzi, V.; Collesi, C.; Zentilin, L.; Schneider, E.; Correa, R.; Silvestri, F.; Zacchigna, S.; et al. SARS-CoV-2, myocardial injury and inflammation: Insights from a large clinical and autopsy study. Clin. Res. Cardiology. 2021, 110, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID−19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Almamlouk, R.; Kashour, T.; Obeidat, S.; Bois, M.C.; Maleszewski, J.J.; Omrani, O.A.; Tleyjeh, R.; Berbari, E.; Chakhachiro, Z.; Zein-Sabatto, B.; et al. COVID−19-associated cardiac pathology at post-mortem evaluation: A Collaborative systematic Review. Clin. Microbiol. Infect. 2022, 28, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Serum amyloid A concentrations, COVID−19 severity and mortality: An updated systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Ciotti, M.; Nuccetelli, M.; Perrone, M.A.; Calio, M.T.; Lia, M.S.; Minieri, M.; Bernardini, S. Serum Amyloid A Protein as a useful biomarker to predict COVID−19 patients severity and prognosis. Int. Immunopharmacol. 2021, 95, 107512. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Y.; Zhu, I.; Cheng, Y.; Sun, P.D. Structural mechanism of serum amyloid A-mediated inflammatory amyloidosis. Proc. Natl. Acad. Sci. USA 2014, 111, 5189–5194. [Google Scholar] [CrossRef]
- Li, Y.; Xiaojing, H.; Zhuanyun, L.; Li, D.; Yang, J. Prognostic value of serum amyloid A in COVID−19: A meta-analysis. Medicine. 2022, 101, e28880. [Google Scholar] [CrossRef]
- Wang, C.M.; Deng, J.H.; Mao, G.F.; He, Y.L.; Shi, X. Serum amyloid a: A potential biomarker assessing disease activity in systemic lupus erythematosus. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923290–e923291. [Google Scholar] [CrossRef]
- Abbas, A.A.; Alghamdi, A.; Mezghani, S.; Ben Ayed, M.; Alamori, A.M.; Alghamdi, G.A.; Bajhmom, W.; Wajeeh, H.; Almutairi, S.S.; Radwan, W.M. Role of Serum Amyloid A as a Biomarker for Predicting the Severity and Prognosis of COVID−19. J. Immunol. Res. 2022, 2022, 6336556. [Google Scholar] [CrossRef]
- Sinha, N.; Thakur, A.K. Likelihood of amyloid formation in COVID−19-induced ARDS. Trends Microbiol. 2021, 29, 967–969. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, S.; Hammarstrom, P. Amyloidogenesis of SARS-CoV-2 spike protein. J. Am. Chem. Soc. 2022, 144, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, E.M.; Ivanova, E.; Grechko, A.V.; Wu, W.K.; Starodubova, A.V.; Orekhov, A.N. Involvement of oxidative stress and the innate immune system in SARS-CoV-2 infection. Diseases 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.G.; De Brito, C.A.; Dos Reis, V.M.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and other respiratory viruses: What does oxidative stress have to do with it? Oxidative Med. Cell. Longev. 2020, 2020, 8844280. [Google Scholar] [CrossRef]
- Oliveira, T.L.; Melo, I.S.; Cardoso-Sousa, L.; Santos, I.A.; El Zoghbi, M.B.; Shimoura, C.G.; Georjutti, R.P.; Castro, O.W.; Goulart, L.R.; Jardim, A.C.; et al. Pathophysiology of SARS-CoV-2 in lung of diabetic patients. Front. Physiol. 2020, 11, 1506. [Google Scholar] [CrossRef]
- Shahandeh, N.; David, S.; King, M.; Smith, J.; Fishbein, M.; Biniwale, R.; Nsair, A.; Kamath, M. An Alarming Surprise. J. Heart Lung Transplant. 2023, 42, S202. [Google Scholar] [CrossRef]
- Hansen, T.; Titze, U.; Kulamadayil-Heidenreich, N.S.; Glombitza, S.; Tebbe, J.J.; Röcken, C.; Schulz, B.; Weise, M.; Wilkens, L. First case of postmortem study in a patient vaccinated against SARS-CoV-2. Int. J. Infect. Dis. 2021, 107, 172–175. [Google Scholar] [CrossRef]
- Jahan, S.; Rajandran, A.; Baharuddin, N.F.; Rao, N.; Tan, B.Q. Amyloid Post Covid-19 Vaccine-Any Relation? Nephrology. 2022, 27, S88. [Google Scholar]
- Obeid, M.; Fenwick, C.; Pantaleo, G. Reactivation of IgA vasculitis after COVID−19 vaccination. Lancet Rheumatol. 2021, 3, e617. [Google Scholar] [CrossRef]
- Mohseni Afshar, A.; Liang, J.J.; Sharma, A.; Pirzadeh, M.; Babazadeh, A.; Hashemi, E.; Deravi, N.; Abdi, S.; Allahgholipour, A.; Hosseinzadeh, R.; et al. Do we miss rare adverse events induced by COVID−19 vaccination? Front. Med. 2022, 9, 933914. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Raggiunti, M.; Magnanimi, L.M.; Ginaldi, L.; De Martinis, M. Henoch-Schönlein purpura following the first dose of COVID−19 viral vector vaccine: A case report. Vaccines 2021, 9, 1078. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID−19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid−19 vaccine in a nationwide setting. New Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID−19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Parhiz, H.; Brenner, J.S.; Patel, P.N.; Papp, T.E.; Shahnawaz, H.; Li, Q.; Shi, R.; Zamora, M.E.; Yadegari, A.; Marcos-Contreras, O.A.; et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE). J. Control. Release 2022, 344, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T cell therapy beyond oncology: Autoimmune diseases and viral infections. Biomedicines 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Study Period | Cohort and Subgroups | Location of Study | Vaccination Rate | COVID-19 Infection Rate | Clinical Outcomes |
|---|---|---|---|---|---|---|
| Wood et al., 2020 [15] | April–July 2020 | Hematological malignancy with COVID-19 250 pts), of which 40 pts (16%) had MM or AL | Worldwide (65% in North America) | N/A | N/A | 30/37 (81.1%) with moderate to severe infection, 11/39 (28% mortality) |
| Lewis et al., 2022 [16] | January 2020–April 2022 | ATTR (152 pts) and AL (103 pts) | Alberta, Canada | ATTR 137/152 (90.6%) vaccinated. AL 84/103 (81.6%) vaccinated. | ATTR 78/131 (59.5%) tested 4/78 (5.1%) PCR +ve AL 42/60 (70%) tested 11/42 (26.2%) PCR +ve 6/15 (40%) PCR +ve patients were unvaccinated | 4 patients required hospital admission (2 AL patients both vaccinated; 2 ATTR patients both unvaccinated) 1 death caused directly by COVID-19 infection (ATTR patient, not vaccinated) |
| Ho et al., 2023 [17] | January 2020–August 2021 | MM and AL (9225 pts) | United States | 187/9225 (2%) (174 MM.; 13AL) | 187/9225 (2%) (174 MM.; 13 AL) | 3/13 (23.1%) with severe infection, 4/13 (30.8%) required hospital admission, 1/13 (7.8%) required ICU admission, 9 patient deaths (9 MM.; 0 AL) 2 patients (17%) with severe infection; 0 deaths |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, W.-Y.; Wu, H.H.L.; Floyd, L.; Ponnusamy, A.; Chinnadurai, R. COVID-19 Infection and Vaccination and Its Relation to Amyloidosis: What Do We Know Currently? Vaccines 2023, 11, 1139. https://doi.org/10.3390/vaccines11071139
Leung W-Y, Wu HHL, Floyd L, Ponnusamy A, Chinnadurai R. COVID-19 Infection and Vaccination and Its Relation to Amyloidosis: What Do We Know Currently? Vaccines. 2023; 11(7):1139. https://doi.org/10.3390/vaccines11071139
Chicago/Turabian StyleLeung, Wing-Yin, Henry H. L. Wu, Lauren Floyd, Arvind Ponnusamy, and Rajkumar Chinnadurai. 2023. "COVID-19 Infection and Vaccination and Its Relation to Amyloidosis: What Do We Know Currently?" Vaccines 11, no. 7: 1139. https://doi.org/10.3390/vaccines11071139
APA StyleLeung, W.-Y., Wu, H. H. L., Floyd, L., Ponnusamy, A., & Chinnadurai, R. (2023). COVID-19 Infection and Vaccination and Its Relation to Amyloidosis: What Do We Know Currently? Vaccines, 11(7), 1139. https://doi.org/10.3390/vaccines11071139






