Current Knowledge on Epizootic Haemorrhagic Disease in China
Abstract
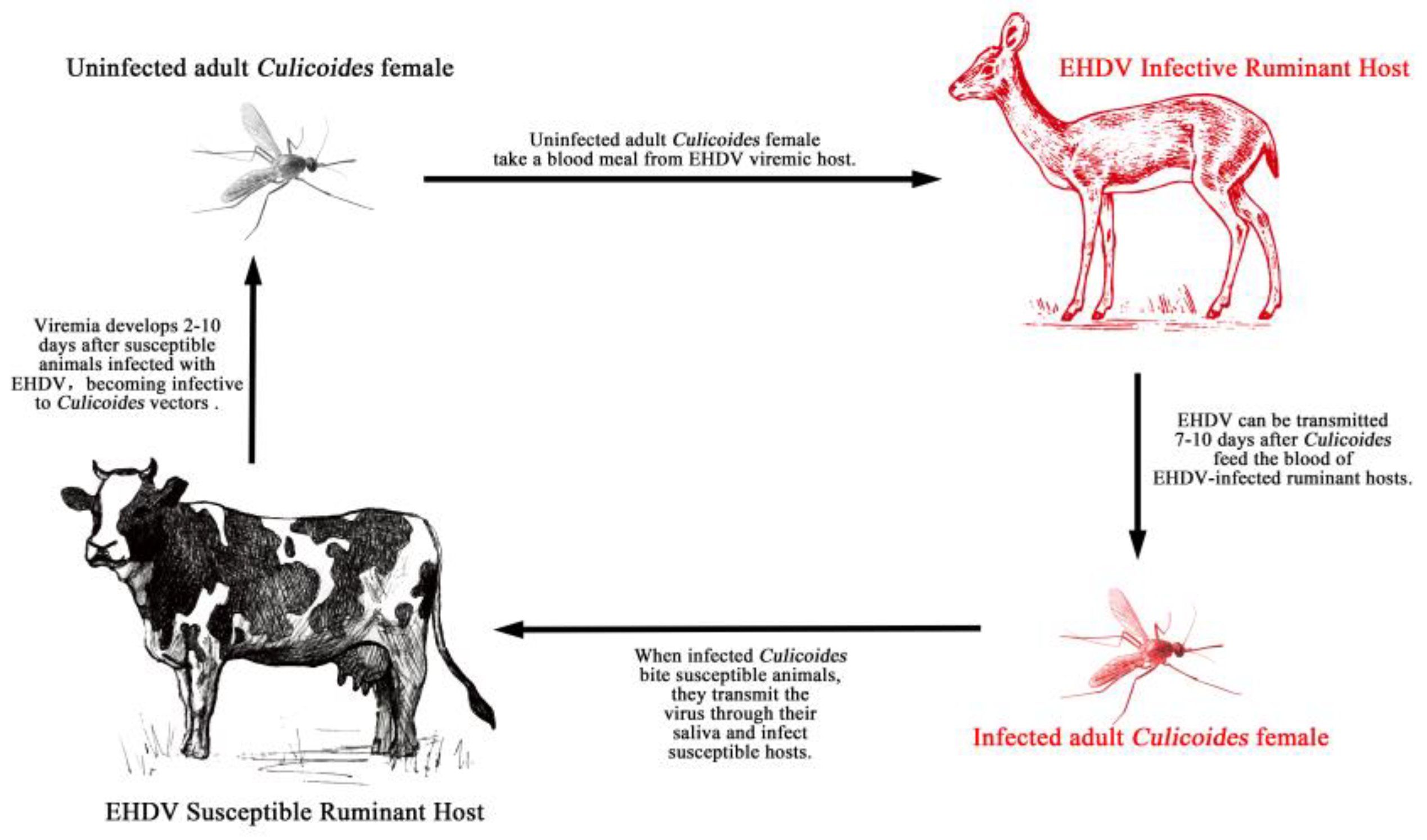
1. Introduction
2. Serological Investigation, Isolation and Identification of EHDV
3. Study on Detection Methods of EHDV
4. Recommendations for EHD Prevention and Control
4.1. Control the Number of Culicoides and Reduce Contact between Culicoides and Hosts
4.2. Long-Term Surveillance of EHDV and Culicoides in Different Areas and Risk Assessment
4.3. Basic Research and Applications Related to EHD Prevention and Control Should Be Further Carried Out
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kedmi, M.; Straten, V.M.; Ezra, E.; Galon, N.; Klement, E. Assessment of the productivity effects associated with epizootic hemorrhagic disease in dairy herds. J. Dairy Sci. 2010, 93, 2486–2495. [Google Scholar] [CrossRef]
- McGregor, B.L.; Erram, D.; Acevedo, C.; Alto, B.W.; Burkett-Cadena, N.D. Vector competence of Culicoides sonorensis (diptera: Ceratopogonidae) for epizootic hemorrhagic disease virus serotype 2 strains from Canada and Florida. Viruses 2019, 11, 367. [Google Scholar] [CrossRef]
- Lv, M.N.; Zhu, J.B.; Li, J.; Yang, Z.X.; Lin, X.H.; Chen, Q.L.; Zhang, J.F.; Liao, S.Q.; Qi, N.S.; Wu, C.Y.; et al. Isolation and identification of the epizootic hemorrhagic disease virus from cattle in Guangdong. Chin. J. Prev. Vet. Med. 2017, 39, 67–70. [Google Scholar] [CrossRef]
- Rivera, N.A.; Varga, C.; Ruder, M.G.; Dorak, S.J.; Roca, A.L.; Novakofski, J.E.; Mateus-Pinilla, N.E. Bluetongue and epizootic hemorrhagic disease in the United States of America at the wildlife-livestock interface. Pathogens 2021, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Office International Epizooties (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 11th ed.; OIE Standards Commission, Ed.; Office International Epizooties (OIE): Paris, France, 2021. [Google Scholar]
- Anthony, S.J.; Maan, N.; Maan, S.; Sutton, G.; Attoui, H.; Mertens, P.P. Genetic and phylogenetic analysis of the core proteins VP1, VP3, VP4, VP6 and VP7 of epizootic haemorrhagic disease virus (EHDV). Virus Res. 2009, 145, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Chuma, T.; Roy, P. Characterization of the genes encoding two of the major capsid proteins of epizootic haemorrhagic disease virus indicates a close genetic relationship to bluetongue virus. J. Gen. Virol. 1992, 73, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Meng, J.X.; Xiao, L.; Zhu, J.B.; Liao, D.F.; Gao, L.; Li, Z.H.; Yang, H.; Li, H.C. Isolation of epizootic haemorrhagic disease virus serotype 7 strain in China for the first time. Acta. Vet. Zootech. Sin. 2019, 50, 602–610. [Google Scholar]
- Yamamoto, K.; Hiromatsu, R.; Kaida, M.; Kato, T.; Yanase, T.; Shirafuji, H. Isolation of epizootic hemorrhagic disease virus serotype 7 from cattle showing fever in 5 Japan in 2016 and improvement of a reverse transcription-polymerase chain reaction 6 assay to detect epizootic hemorrhagic disease virus. J. Vet. Med. Sci. 2021, 83, 1378–1388. [Google Scholar] [CrossRef]
- Li, Z.H.; Yang, Z.X.; Lin, X.H.; Lv, M.N.; Xiao, L.; Kou, M.L.; Liao, D.F.; Zhu, J.B.; Yang, H.; Li, H.C. Isolation and genetic characteristics of epidemic hemorrhagic disease virus serotype 6 (EHDV-6) strains from Yunnan and Guangdong provinces. J. South Chin. Agric. Univ. 2020, 41, 8–15. [Google Scholar] [CrossRef]
- Shirafuji, H.; Kato, T.; Yamakawa, M.; Tanaka, T.; Minemori, Y.; Yanase, T. Characterization of genome segments 2, 3 and 6 of epizootic hemorrhagic disease virus strains isolated in Japan in 1985-2013: Identification of their serotypes and geographical genetic types. Infect Genet Evol. 2017, 53, 38–46. [Google Scholar] [CrossRef]
- Dorak, S.J.; Varga, C.; Ruder, M.G.; Gronemeyer, P.; Rivera, N.A.; Dufford, D.R.; Skinner, D.J.; Roca, A.L.; Novakofski, J.; Mateus-Pinilla, N.E. Spatial epidemiology of hemorrhagic disease in Illinois wild white-tailed deer. Sci. Rep. 2022, 12, 6888. [Google Scholar] [CrossRef] [PubMed]
- Noronha, L.E.; Cohnstaedt, L.W.; Richt, J.A.; Wilson, W.C. Perspectives on the changing landscape of epizootic hemorrhagic disease virus control. Viruses 2021, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.L.; Han, D.G.; Dong, J.; Yang, Y.Q.; Zhu, H.; Ye, L.L.; Zhang, C.; Yin, S.L.; Li, Y.Y.; He, C.L.; et al. Research progress of epidemic hemorrhagic disease. Shanghai J. Anim. Husb. Vet. Med. 2021, 4, 37–41. [Google Scholar] [CrossRef]
- Luo, Q.M.; Wei, L.; Han, D.G.; Ye, L.L.; Yang, Y.Q.; Dong, J.; Zhang, C.; Li, J.; Yin, S.L.; Li, Y.Y.; et al. Discussion on global prevalence and distribution of epizootic haemorrhagic disease. China Anim. Health Insp. 2022, 39, 96–104. [Google Scholar]
- Cao, Y.Y.; Zhong, H.; Wu, J.M. Research progress of deer epidemic hemorrhagic fever virus. Chin. J. Vet. Sci. 2017, 37, 571–576. [Google Scholar] [CrossRef]
- Mecham, J.O.; Nunamaker, R.A. Complex interactions between vectors and pathogens: Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) infection rates with bluetongue viruses. J. Med. Entomol. 1994, 31, 903–907. [Google Scholar] [CrossRef]
- Wittmann, E.J.; Mellor, P.S.; Baylis, M. Effect of temperature on the transmission of orbiviruses by the biting midge, Culicoides sonorensis. Med. Vet. Entomol. 2002, 16, 147–156. [Google Scholar] [CrossRef]
- Yang, Z.X.; Li, Z.H.; Song, Z.A.; Zhu, J.B.; Li, Z.R.; Li, H.C.; Xie, J.R.; Liao, D.F.; Yang, H. Development and application of a real-time quantitative RT-PCR method for detection and serotyping of the epizootic haemorrhagic disease virus (EHDV). Chin. J. Virol. 2020, 36, 897–906. [Google Scholar] [CrossRef]
- Yang, Z.X.; Li, Z.H.; Wu, J.M.; Wang, J.P.; Xiao, L.; Kou, M.L.; Zhu, J.B.; Liao, D.F.; Li, H.C.; Yang, H. Characterization of strains of epizootic haemorrhagic disease virus serotype 5 isolated in China from 2013 to 2016. Chin. J. Virol. 2020, 36, 475–483. [Google Scholar] [CrossRef]
- Duan, Y.L.; Yang, Z.X.; Zhu, P.; Xiao, L.; Li, Z.H.; Li, Z.R.; Li, L.; Zhu, J.B. A serologic investigation of epizootic hemorrhagic disease virus in China between 2014 and 2019. Virol. Sin. 2022, 37, 513–520. [Google Scholar] [CrossRef]
- Kou, M.L.; Xie, J.R.; Yang, Z.X.; Miao, H.S. Serological investigation on epizootic hemorrhagic disease in Yunnan province. Chin. J. Anim. Health Insp. 2021, 38, 14–17. [Google Scholar]
- Cao, Y.Y. Guangxi Domestic Ruminant EHD Serology and Media Inbestigation and Pathogen Isolation and Identification. Master’s Thesis, Guangxi University, Nanning, China, 2015; p. 80. [Google Scholar]
- Li, Z.H.; Xiao, L.; Yang, Z.X.; Meng, J.X.; Liao, D.F.; Gao, L.; Li, H.C.; Yang, H. Isolation and identification of the epidemic hemorrhagic fever virus (EHDV) serotype 10 strain from cattle in China. Chin. J. Virol. 2019, 35, 112–120. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kou, M.L.; Li, Z.H.; Liao, D.F.; Xiao, L.; Zhu, J.B.; Gao, L.; Li, H.C.; Yang, H. The first isolation and identification of serotype 6 epizootic haemorrhagic disease virus in Yunan province of China. Chin. J. Prev. Vet. Med. 2019, 41, 1113–1119. [Google Scholar]
- Li, Z.R.; Wu, J.M.; Zhu, J.B.; Wang, J.P.; Lv, M.N.; Xiao, L.; Yang, Z.X.; Liao, D.F.; Li, H.C.; Yang, H. Isolation and genetic characterization of epizootic hemorrhagic disease virus serotype 1 strains prevalent in China from 2013 to 2019. J. South. Agric. 2021, 52, 2043–2052. [Google Scholar]
- Nomikou, K.; Hughes, J.; Wash, R.; Kellam, P.; Breard, E.; Zientara, S.; Palmarini, M.; Biek, R.; Mertens, P. Widespread reassortment shapes the evolution and epidemiology of bluetongue virus following European invasion. PLoS Pathog. 2015, 11, e1005056. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, J.P.; Yang, Z.X.; Li, Z.H.; Liao, D.F.; Li, H.C.; Yang, H. Isolation and identification of a novel type of epizootic haemorrhagic disease virus. Chin. J. Prev. Vet. Med. 2021, 43, 363–369. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.; Wang, J.; Li, Z.; Yang, Z.; Liao, D.; Zhu, J.; Li, H. Novel serotype of epizootic hemorrhagic disease virus, China. Emerg. Infect. Dis. 2020, 26, 3081–3083. [Google Scholar] [CrossRef]
- Forzan, M.; Pizzurro, F.; Zaccaria, G.; Mazzei, M.; Spedicato, M.; Carmine, I.; Salini, R.; Tolari, F.; Cerri, D.; Savini, G.; et al. Competitive enzyme-linked immunosorbent assay using baculovirus-expressed VP7 for detection of epizootic haemorrhagic disease virus (EHDV) antibodies. J. Virol. Methods 2017, 248, 212–216. [Google Scholar] [CrossRef]
- Yang, J.X.; Hua, Q.Y.; Chen, H.C.; Chen, B.; Lv, J.Q.; Cao, C.F.; Ruan, Z.X.; Zhang, C.H. Cloning and prokaryotic expression of VP7 gene of epizootic hemorrhagic disease virus of deer in E. coli. Prog. Vet. Med. 2009, 30, 5–8. [Google Scholar] [CrossRef]
- Li, Z.H.; Song, Z.A.; Xiao, L.; Zhu, J.B.; Yang, Z.X.; Li, Z.R.; Liao, D.F.; Li, H.C.; Yang, H. Prokaryotic expression and polyclonal antibody preparation of epizootic haemorrhagic disease virus VP7 protein. Chin. Anim. Husb. Vet. Med. 2021, 48, 1405–1413. [Google Scholar] [CrossRef]
- Guo, Y.J.; Yang, J.X.; Hua, Q.Y.; Xu, C.; Lin, Q.Y.; Ruan, Z.X.; Chen, B.; Lu, T.K.; Zhan, A.J. Preparation and characterization of monoclonal antibodies against epidemic hemorrhagic disease virus. Prog. Vet. Med. 2009, 30, 14–17. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.B.; Yang, J.X.; Ruan, Z.X.; Sun, J.; Zhang, C.H.; Liu, J.L.; Hua, Q.Y.; Zhou, X.L. Establishment of sandwich ELISA for epidemic hemorrhagic disease of deer virus. Prog. Vet. Med. 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Dong, X.L.; Wang, Q.; Han, D.G.; Dong, J.; Yang, Y.Q.; Zhu, H.; Ye, L.L.; Zhang, C.; Yin, S.L.; Li, Y.Y.; et al. Development on blocking ELISA kit for antibodies against epizootic hemorrhagic disease virus. Chin. J. Anim. Health Insp. 2021, 38, 92–97. [Google Scholar]
- Hua, Q.Y.; Yang, J.X.; Guo, Y.J.; Lv, J.Q.; Zeng, S.L.; Qin, Z.F.; Chen, B.; Ruan, Z.X.; Dong, J. Establishment of an indirect ELISA based on recombinant VP7 protein for detection of antibody against epidemic hemorrhagic disease virus of deer. Chin. Vet. Sci. 2009, 39, 527–530. [Google Scholar] [CrossRef]
- Yang, Z.X.; Zhu, J.B.; Xiao, L.; Gao, L.; Miao, H.S.; He, Y.W.; Meng, J.X.; Yang, H.; Li, H.C. Establishment of antigen-captured ELISA for detecting epidemic hemorrhagic disease virus. Chin. J. Vet. Sci. 2019, 39, 14–17. [Google Scholar] [CrossRef]
- Zhu, J.B.; Yang, Z.X.; Xiao, L.; Gao, L.; Miao, H.S.; He, Y.W.; Meng, J.X.; Yang, H.; Li, H.C. Establishment of a rapid competitive ELISA for detecting antibodies against epidemic hemorrhagic disease virus. Acta. Vet. Zootech. Sin. 2018, 49, 1440–1450. [Google Scholar] [CrossRef]
- Yang, Z.X.; Li, Z.H.; Song, Z.A.; Li, Z.R.; Zhu, J.B.; Liao, D.F.; Yang, H. Development and utilization of RT-PCR assay for determining Serotypes of epizootic haemorrhagic disease virus. J. Huazhong Agric. Univ. 2020, 39, 85–92. [Google Scholar] [CrossRef]
- Yang, Z.X.; Li, Z.H.; Song, Z.A.; Li, Z.R.; Li, H.C.; Zhu, J.B.; Liao, D.F.; Yang, H. Development and application of a real-time quantitative RT-PCR and RT-PCR method for detection a novel serotype of epizootic haemorrhagic disease virus. Chin. Vet. Sci. 2021, 51, 446–454. [Google Scholar] [CrossRef]
- Yang, Z.X.; Zhu, J.B.; Liao, D.F.; Li, Z.H.; Xiao, L.; Gao, L.; Li, N.; Yang, H.; Li, H.C. Development and application of one-step RT-PCR method for the detection of epizootic haemorrhagic disease virus. Chin. Vet. Sci. 2019, 49, 280–286. [Google Scholar] [CrossRef]
- Jiang, H.T.; Yang, Y.Q.; Ai, J.; Zhu, H.; Ye, L.L.; Wang, Q.; Hua, Q.Y.; Yang, J.X.; Lv, J.Q.; Dong, J.; et al. Establishment of real-time PCR method for detection of deer epidemic hemorrhagic disease virus. Yunnan J. Anim. Sci. Vet. Med. 2014, 5, 1–6. [Google Scholar]
- Yang, Z.X.; Zhu, J.B.; Li, Z.H.; Li, Z.R.; He, Y.W.; Xie, J.R.; Li, H.C.; Liao, D.F.; Yang, H. Establishment and application of a duplex real-time RT-PCR assay for simultaneous detection of bluetongue virus and epidemic haemorrhagic disease virus. Chin. Vet. Sci. 2019, 49, 1104–1111. [Google Scholar] [CrossRef]
- Yang, Z.X.; Li, Z.H.; Xiao, L.; Xie, J.R.; Liao, D.F.; Li, Z.R.; Li, H.C.; Zhu, J.B.; Yang, H. Establishment and application of triplex RT-qPCR for detection of bluetongue virus, epizootic hemorrhagic disease virus and Palyam serogroup virus. J. S. Chin. Agric. Univ. 2021, 42, 17–25. [Google Scholar] [CrossRef]
- Jiang, H.T.; Yang, Y.Q.; Zhu, H.; Ye, L.L.; Wang, Q.; Hua, Q.Y.; Yang, J.X.; Lv, J.Q.; Dong, J.; Zhou, X.L. Preliminary establishment of RT-LAMP assay for the detection of epidemic hemorrhagic disease. Yunnan J. Anim. Sci. Vet. Med. 2014, 6, 4–7. [Google Scholar]
- Li, F.X.; Zhao, W.H.; Yang, S.B. Development of visual reverse transcription loop-mediated isothermal amplification assay for field detection of epizootic haemorrhagic disease virus. Chin. Vet. Sci. 2020, 50, 1481–1485. [Google Scholar] [CrossRef]
- Zhan, A.J.; Wang, X.W.; Chen, S.K.; Sun, J.; Chen, B.; Ruan, Z.X.; Hua, Q.Y. Establishmentofa high-through put liquid chip method for the detection of deer epidemic hemorrhagic disease virus. Pro-Vet. Med. 2010, 31, 36–40. [Google Scholar] [CrossRef]
- Calvo, J.H.; Berzal, B.; Calvete, C.; Miranda, M.A.; Estrada, R.; Lucientes, J. Host feeding patterns of Culicoides species (Diptera: Ceratopogonidae) within the Picos de Europa National Park in northern Spain. Bull. Entomol. Res. 2012, 102, 692–697. [Google Scholar] [CrossRef]
- Wiemers, D.W.; Fulbright, T.E.; Wester, D.B.; Ortega-S, J.A.; Rasmussen, G.A.; Hewitt, D.G.; Hellickson, M.W. Role of thermal environment in habitat selection by male white-tailed deer during summer in Texas, USA. Wildl. Biol. 2014, 20, 47–56. [Google Scholar] [CrossRef]
- Yanase, T.; Murota, K.; Hayama, Y. Endemic and emerging arboviruses in domestic ruminants in East Asia. Front. Vet. Sci. 2020, 7, 168. [Google Scholar] [CrossRef]
- Zheng, F.; Qiu, C. Phylogenetic relationships of the glycoprotein gene of bovine ephemeral fever virus isolated from mainland China, Taiwan, Japan, Turkey, Israel and Australia. Virol. J. 2012, 9, 268. [Google Scholar] [CrossRef]
- Piet, A. Prospects of next-generation vaccines for bluetongue. Front. Vet. Sci. 2019, 6, 407. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, J.; Dong, J.; Li, J.; Ye, L.; Zhang, C.; Nie, F.; Gu, Y.; Ji, X.; Song, Z.; Luo, Q.; et al. Current Knowledge on Epizootic Haemorrhagic Disease in China. Vaccines 2023, 11, 1123. https://doi.org/10.3390/vaccines11061123
Xin J, Dong J, Li J, Ye L, Zhang C, Nie F, Gu Y, Ji X, Song Z, Luo Q, et al. Current Knowledge on Epizootic Haemorrhagic Disease in China. Vaccines. 2023; 11(6):1123. https://doi.org/10.3390/vaccines11061123
Chicago/Turabian StyleXin, Jige, Jun Dong, Jing Li, Lingling Ye, Chong Zhang, Fuping Nie, Yeqing Gu, Xincheng Ji, Zhigang Song, Qianmin Luo, and et al. 2023. "Current Knowledge on Epizootic Haemorrhagic Disease in China" Vaccines 11, no. 6: 1123. https://doi.org/10.3390/vaccines11061123
APA StyleXin, J., Dong, J., Li, J., Ye, L., Zhang, C., Nie, F., Gu, Y., Ji, X., Song, Z., Luo, Q., Ai, J., & Han, D. (2023). Current Knowledge on Epizootic Haemorrhagic Disease in China. Vaccines, 11(6), 1123. https://doi.org/10.3390/vaccines11061123





