Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave
Abstract
1. Introduction
2. Patients and Methods
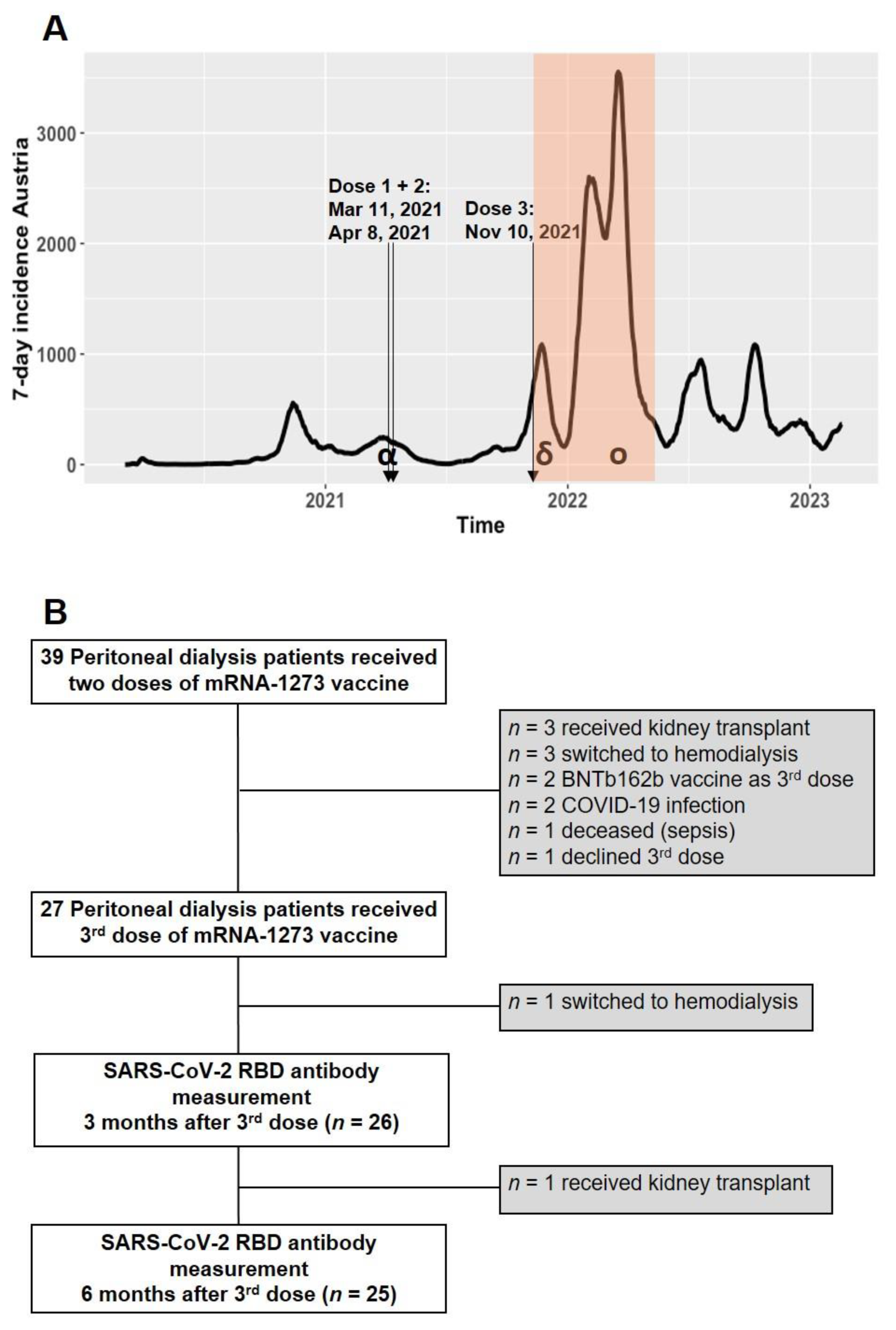
2.1. Study Population
2.2. Peritoneal Dialysis Modalities
2.3. Serological Assessment
2.4. Statistical Analysis
3. Results
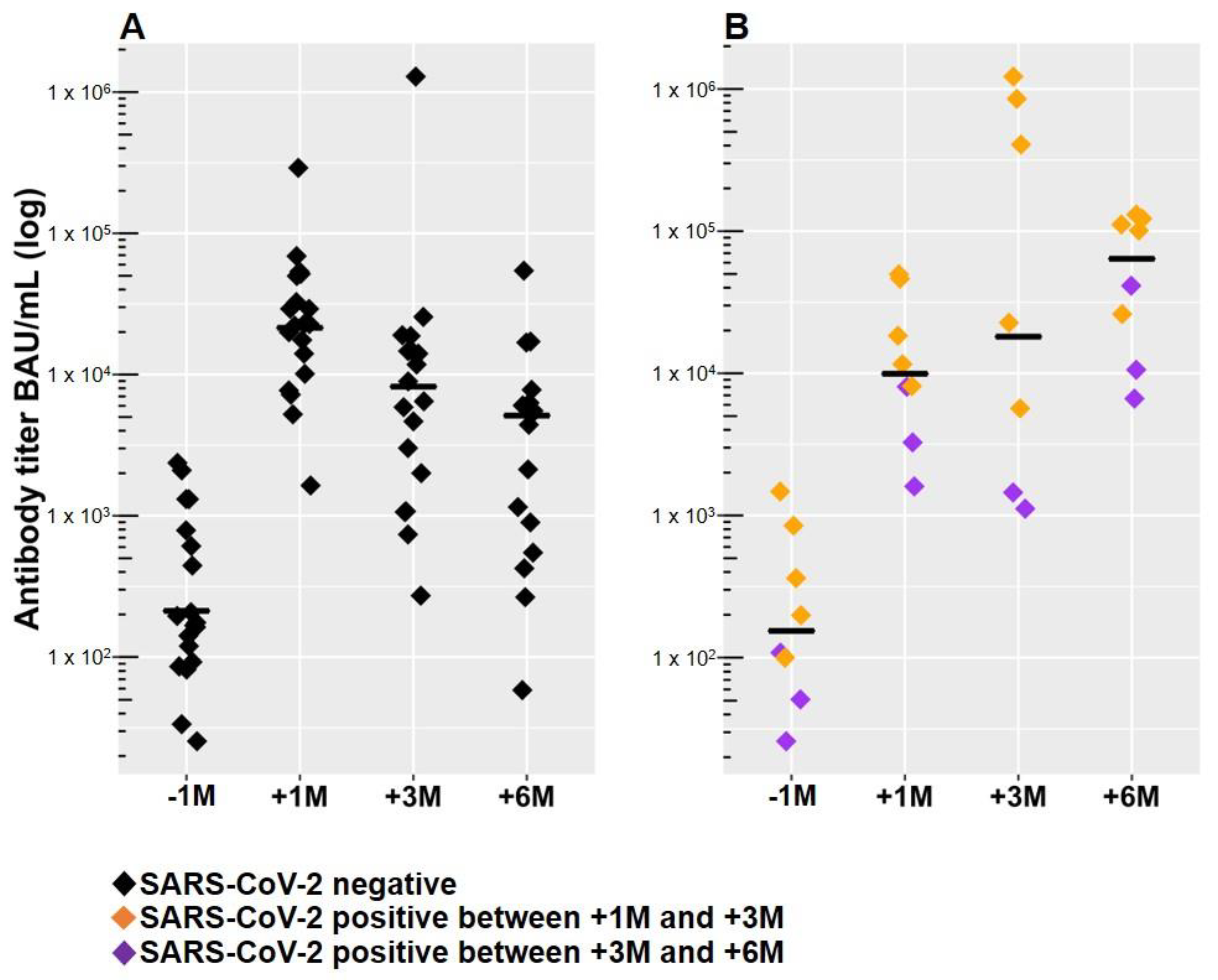
3.1. Anti-SARS-CoV-2 RBD Antibodies Declined within Six Months after the Third Dose of mRNA-1273 in SARS-CoV-2 Naive Patients
3.2. Breakthrough Infections in PD Patients within Six Months after the Third Dose of mRNA-1273
3.3. Previous Antibody Levels, High GFR, and Low Davies Comorbidity Score Are Predictors of a Robust Anti-SARS-CoV-2 Antibody Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Mahalingasivam, V.; Su, G.; Iwagami, M.; Davids, M.R.; Wetmore, J.B.; Nitsch, D. COVID-19 and kidney disease: Insights from epidemiology to inform clinical practice. Nat. Rev. Nephrol. 2022, 18, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Babel, N.; Hugo, C.; Westhoff, T.H. Vaccination in patients with kidney failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022, 18, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.M.; Yap, D.Y.H.; Yip, T.P.S.; Hung, I.F.N.; Tang, S.C.W.; Chan, T.M. Vaccination in patients with chronic kidney disease-Review of current recommendations and recent advances. Nephrology 2021, 26, 5–11. [Google Scholar] [CrossRef]
- Eiselt, J.; Kielberger, L.; Rajdl, D.; Racek, J.; Pazdiora, P.; Malanova, L. Previous Vaccination and Age are More Important Predictors of Immune Response to Influenza Vaccine than Inflammation and Iron Status in Dialysis Patients. Kidney Blood Press. Res. 2016, 41, 139–147. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Aghamohammadi, A.; Rezaei, N.; Lessan-Pezeshki, M.; Pourmand, G.; Mohagheghi, M.A.; Abdollahzade, S.; Mousavi-Jarrahi, A. Antibody response to pneumococcal capsular polysaccharide vaccination in patients with chronic kidney disease. Eur. Cytokine Netw. 2009, 20, 69–74. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Coates, P.T.; Rovin, B.H.; Ronco, P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021, 99, 1275–1279. [Google Scholar] [CrossRef]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Outcome and effect of vaccination in SARS-CoV-2 Omicron infection in hemodialysis patients: A cohort study. Nephrol. Dial. Transplant. 2022, 37, 1944–1950. [Google Scholar] [CrossRef]
- Moore, L.R.; Al-Jaddou, N.; Wodeyar, H.; Sharma, A.; Schulz, M.; Rao, A.; Abraham, K. SARS-CoV-2 in dialysis patients and the impact of vaccination. BMC Nephrol. 2022, 23, 317. [Google Scholar] [CrossRef]
- Sibbel, S.; McKeon, K.; Luo, J.; Wendt, K.; Walker, A.G.; Kelley, T.; Lazar, R.; Zywno, M.L.; Connaire, J.J.; Tentori, F.; et al. Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2022, 33, 49–57. [Google Scholar] [CrossRef]
- Torres, R.; Toro, L.; Sanhueza, M.E.; Lorca, E.; Ortiz, M.; Pefaur, J.; Clavero, R.; Machuca, E.; Gonzalez, F.; Herrera, P.; et al. Clinical Efficacy of SARS-CoV-2 Vaccination in Hemodialysis Patients. Kidney Int. Rep. 2022, 7, 2176–2185. [Google Scholar] [CrossRef]
- Agur, T.; Zingerman, B.; Ben-Dor, N.; Alkeesh, W.; Steinmetz, T.; Rachamimov, R.; Korzets, A.; Rozen-Zvi, B.; Herman-Edelstein, M. Humoral Response to the Third Dose of BNT162b2 COVID-19 Vaccine among Hemodialysis Patients. Nephron 2022, 147, 185–192. [Google Scholar] [CrossRef]
- Attias, P.; Azzaoui, I.; El Karoui, K.; de La Selle, A.; Sokal, A.; Chappert, P.; Grimbert, P.; Fernandez, I.; Bouvier, M.; Samson, C.; et al. Immune Responses after a Third Dose of mRNA Vaccine Differ in Virus-Naive versus SARS-CoV-2-Recovered Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2022, 17, 1008–1016. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.; Housset, P. SARS-CoV-2 Antibody Response after a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e181. [Google Scholar] [CrossRef]
- Berar-Yanay, N.; Freiman, S.; Shapira, M.; Saffoury, A.; Elemy, A.; Hamze, M.; Elhaj, M.; Zaher, M.; Matanis, L.; Armaly, Z.A. Waning Humoral Response 3 to 6 Months after Vaccination with the SARS-CoV-2 BNT162b2 mRNA Vaccine in Dialysis Patients. J. Clin. Med. 2021, 11, 64. [Google Scholar] [CrossRef]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mahr, H.; Lhotta, K.; Zitt, E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: Time for a boost. Kidney Int. 2021, 100, 1334–1335. [Google Scholar] [CrossRef]
- Einbinder, Y.; Perl, J.; Nacasch, N.; Bnaya, A.; Shavit, L.; Erez, D.; Shashar, M.; Halperin, T.; Grupper, A.; Benchetrit, S.; et al. Humoral Response and SARS-CoV-2 Infection Risk following the Third and Fourth Doses of the BNT162b2 Vaccine in Dialysis Patients. Am. J. Nephrol. 2022, 53, 586–590. [Google Scholar] [CrossRef]
- Garcia, P.; Han, J.; Montez-Rath, M.E.; Sun, S.; Shang, T.; Parsonnet, J.; Chertow, G.M.; Anand, S.; Schiller, B.; Abra, G. SARS-CoV-2 Booster Vaccine Response among Patients Receiving Dialysis. Clin. J. Am. Soc. Nephrol. 2022, 17, 1036–1038. [Google Scholar] [CrossRef]
- Hsu, C.M.; Weiner, D.E.; Manley, H.J.; Aweh, G.N.; Ladik, V.; Frament, J.; Miskulin, D.; Argyropoulos, C.; Abreo, K.; Chin, A.; et al. Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months. Clin. J. Am. Soc. Nephrol. 2022, 17, 403–413. [Google Scholar] [CrossRef]
- Murt, A.; Dinc, H.O.; Altiparmak, M.R.; Yalin, S.F.; Yadigar, S.; Parmaksiz, E.; Kocazeybek, B.; Pekpak, M.; Ataman, M.R. Waning of SARS-CoV-2 Vaccine-Induced Immune Response over 6 Months in Peritoneal Dialysis Patients and the Role of a Booster Dose in Maintaining Seropositivity. Nephron 2022, 146, 559–563. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodriguez-Espinosa, D.; Cuadrado, E.; Rodriguez, N.; Bedini, J.L.; Maduell, F. Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients. Vaccines 2022, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Beilhack, G.; Monteforte, R.; Frommlet, F.; Reindl-Schwaighofer, R.; Strassl, R.; Vychytil, A. Humoral Response to mRNA-1273 SARS-CoV-2 Vaccine in Peritoneal Dialysis Patients: Is Boostering after Six Months Adequate? Front. Med. 2022, 9, 905798. [Google Scholar] [CrossRef] [PubMed]
- Biedunkiewicz, B.; Tylicki, L.; Slizien, W.; Lichodziejewska-Niemierko, M.; Dabrowska, M.; Kubanek, A.; Rodak, S.; Polewska, K.; Tylicki, P.; Renke, M.; et al. Waning Humoral Response after COVID-19 mRNA Vaccination in Maintenance Dialysis Patients and Recovery after a Complementary Third Dose. Vaccines 2022, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Housset, P.; Kubab, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Caudwell, V.; Faucon, A.L. Waning but persistent humoral response 6 months after the third dose of the mRNA BNT162b2 vaccine in hemodialysis and peritoneal dialysis patients. J. Nephrol. 2022, 35, 783–785. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Gansevoort, R.T.; Leyva, A.; Rojas, J.; de Sequera, P.; Network, S.C. Long-Term Dynamic Humoral Response to SARS-CoV-2 mRNA Vaccines in Patients on Peritoneal Dialysis. Vaccines 2022, 10, 1738. [Google Scholar] [CrossRef]
- Manley, H.J.; Li, N.C.; Aweh, G.N.; Hsu, C.M.; Weiner, D.E.; Miskulin, D.; Harford, A.M.; Johnson, D.; Lacson, E., Jr. SARS-CoV-2 Vaccine Effectiveness and Breakthrough Infections Among Patients Receiving Maintenance Dialysis. Am. J. Kidney Dis. 2022, 81, 406–415. [Google Scholar] [CrossRef]
- Montez-Rath, M.E.; Garcia, P.; Han, J.; Cadden, L.; Hunsader, P.; Morgan, C.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A.; et al. SARS-CoV-2 Infection during the Omicron Surge among Patients Receiving Dialysis: The Role of Circulating Receptor-Binding Domain Antibodies and Vaccine Doses. J. Am. Soc. Nephrol. 2022, 33, 1832–1839. [Google Scholar] [CrossRef]
- Beilhack, G.; Monteforte, R.; Frommlet, F.; Gaggl, M.; Strassl, R.; Vychytil, A. Antibody Response and Safety After mRNA-1273 SARS-CoV-2 Vaccination in Peritoneal Dialysis Patients—The Vienna Cohort. Front. Immunol. 2021, 12, 780594. [Google Scholar] [CrossRef]
- Chen, C.H.; Perl, J.; Teitelbaum, I. Prescribing high-quality peritoneal dialysis: The role of preserving residual kidney function. Perit. Dial. Int. 2020, 40, 274–281. [Google Scholar] [CrossRef]
- Davies, S.J.; Phillips, L.; Naish, P.F.; Russell, G.I. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol. Dial. Transplant. 2002, 17, 1085–1092. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Jarava Mantecon, C.J.; Gomes Perez, V.O.; Bordils, A.; Lacueva, J.; Marin Franco, A.J.; Delgado Conde, P.; Munoz Ramos, P.; et al. Humoral response after the fourth dose of the SARS-CoV-2 vaccine in the CKD spectrum: A prespecified analysis of the SENCOVAC study. Nephrol. Dial. Transplant. 2022, 38, 969–981. [Google Scholar] [CrossRef]
- Wand, O.; Nacasch, N.; Fadeela, A.; Shashar, M.; Grupper, A.; Benchetrit, S.; Erez, D.; Shitrit, P.; Cohen-Hagai, K. Humoral response and breakthrough infections with SARS-CoV-2 B.1.617.2 variant in vaccinated maintenance hemodialysis patients. J. Nephrol. 2022, 35, 1479–1487. [Google Scholar] [CrossRef]
- El Haggan, W.; Berdin, B.; El Salhy, M. Antibody titers against SARS-CoV-2 spike protein 6 months after a third BNT162b2 vaccine in chronic hemodialysis patients. Clin. Kidney J. 2022, 15, 1202–1203. [Google Scholar] [CrossRef]
- Tanriover, C.; Ucku, D.; Basile, C.; Tuttle, K.R.; Kanbay, M. On the importance of the interplay of residual renal function with clinical outcomes in end-stage kidney disease. J. Nephrol. 2022, 35, 2191–2204. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef]
- Kanai, D.; Wakui, H.; Haze, T.; Azushima, K.; Kinguchi, S.; Kanaoka, T.; Toya, Y.; Hirawa, N.; Kato, H.; Uneda, K.; et al. Improved Immune Response to the Third COVID-19 mRNA Vaccine Dose in Hemodialysis Patients. Kidney Int. Rep. 2022, 7, 2718–2721. [Google Scholar] [CrossRef]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdorfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Zitt, E.; Hafner-Giessauf, H.; Wimmer, B.; Herr, A.; Horn, S.; Friedl, C.; Sprenger-Mahr, H.; Kramar, R.; Rosenkranz, A.R.; Lhotta, K. Response to active hepatitis B vaccination and mortality in incident dialysis patients. Vaccine 2017, 35, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Dekervel, M.; Henry, N.; Torreggiani, M.; Pouteau, L.M.; Imiela, J.P.; Mellaza, C.; Garnier, A.S.; Dujardin, A.; Asfar, M.; Ducancelle, A.; et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021, 14, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, F.P.; Figiel, L.; Ricken, J.; Still, H.; Korte, C.; Plassmann, G.; von Landenberg, P. Evolution of SARS-CoV-2-Neutralizing Antibodies after Two Standard Dose Vaccinations, Risk Factors for Non-Response and Effect of a Third Dose Booster Vaccination in Non-Responders on Hemodialysis: A Prospective Multi-Centre Cohort Study. J. Clin. Med. 2021, 10, 5113. [Google Scholar] [CrossRef] [PubMed]
- Panizo, N.; Albert, E.; Gimenez-Civera, E.; Puchades, M.J.; D’Marco, L.; Gandia-Salmeron, L.; Gimenez, E.; Torre, I.; Sancho, A.; Gavela, E.; et al. Dynamics of SARS-CoV-2-Spike-reactive antibody and T-cell responses in chronic kidney disease patients within 3 months after COVID-19 full vaccination. Clin. Kidney J. 2022, 15, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Zielinski, M.; Tylicki, L.; Biedunkiewicz, B.; Kubanek, A.; Slizien, Z.; Polewska, K.; Tylicki, P.; Muchlado, M.; Sakowska, J.; et al. Local and Systemic Immunity Are Impaired in End-Stage-Renal-Disease Patients Treated with Hemodialysis, Peritoneal Dialysis and Kidney Transplant Recipients Immunized with BNT162b2 Pfizer-BioNTech SARS-CoV-2 Vaccine. Front. Immunol. 2022, 13, 832924. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Fu, J.; Shen, X.; Anderson, M.; Stec, M.; Petratos, T.; Cloherty, G.; Montefiori, D.C.; Landay, A.; Moy, J.N. Correlation of Binding and Neutralizing Antibodies against SARS-CoV-2 Omicron Variant in Infection-Naive and Convalescent BNT162b2 Recipients. Vaccines 2022, 10, 1904. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
| Patient Demographics | Total (n = 27) |
|---|---|
| Age (years, mean, range) | 54.9 (33–76) |
| Men (%) | 17 (63) |
| Davies Comorbidity Score (%) | |
| 0 | 11 (41) |
| 1 | 9 (33) |
| 2 | 5 (19) |
| 3 | 2 (7) |
| 4 | 0 |
| 5 | 0 |
| Dialysis vintage, months median (IQR) | 21.5 (13.4–38.7) |
| Weekly total (renal + peritoneal) Kt/V median (IQR) | 1.94 (1.76–2.10) |
| GFR (mL/min) median (IQR) | 1.86 (0.65–4.67) |
| Laboratory (mean ± SD) | |
| Hemoglobin (g/dL) | 10.4 ± 1.3 |
| Leukocytes (G/L) | 7.66 ± 2.19 |
| Thrombocytes (G/L) | 230 ± 76.5 |
| Albumin (g/L) | 36.3 ± 4.37 |
| Sodium (mmol/L) | 135 ± 3.65 |
| Potassium (mmol/L) | 4.28 ± 0.51 |
| Calcium (mmol/L) | 2.24 ± 0.21 |
| Phosphate (mmol/L) | 1.93 ± 0.44 |
| Bicarbonate (mmol/L) | 26.6 ± 2.76 |
| C-reactive protein (mg/dL) | 0.78 ± 1.02 |
| Medication n, (%) | |
| Immunosuppressive therapy | 5 (18.5) |
| RAAS-inhibitor medication | 14 (51.8) |
| Vitamin D medication | 18 (66.7) |
| Antibody Titer (BAU/mL) | |||
|---|---|---|---|
| Total Population (n = 27) | SARS-CoV-2 Naive Patients (n = 19) | SARS-CoV-2 Infected Patients (n = 8) * | |
| 6 months after 2nd dose | 185 (88–757) | 212 (99–783) | 156 (83–542) |
| 1 month after 3rd dose | 19,405 (8884–40,650) | 21,424 (11,402–43,650) | 9927 (8222–22,586) |
| 6 months after 3rd dose | 6832 (1839–27,690) | 5120 (768–7159) | 73,500 (23,815–111,825) |
| Age (Years) | Sex | Days between 3rd Dose and Infection | Clinical Symptoms | Outcome | Antibody Titer (Median, BAU/mL) | |
|---|---|---|---|---|---|---|
| 1 Month after 3rd Dose | 6 Months after 3rd Dose | |||||
| 54 | M | 69 | Mild | Favorable | 9927 | 109,800 |
| 33 | F | 82 | Mild | Favorable | 51,900 | 124,200 |
| 59 | M | 86 | Mild | Favorable | 9620 | 116,100 |
| 43 | M | 92 | Mild | Favorable | 16,214 | 110,400 |
| 35 | F | 109 | Mild | Favorable | 41,700 | 27,690 |
| 38 | F | 125 | None | Favorable | 1820 | 37,200 |
| 56 | M | 130 | None | Favorable | 9927 | 12,190 |
| 55 | M | 156 | None | Favorable | 4029 | 6832 |
| Coefficient | F Value | p-Value | |
|---|---|---|---|
| Anti-SARS-CoV-2 RBD levels after 1st dose | 1.67 | 87.20 | 1.95 × 10−11 |
| Davies Comorbidity Score | −0.48 | 4.87 | 0.034 |
| GFR | 0.13 | 4.25 | 0.047 |
| Age | −0.03 | 2.47 | 0.12 |
| Gender (male) | −0.61 | 1.28 | 0.27 |
| Ferritin | 0.0005 | 0.61 | 0.44 |
| Albumin | 0.107 | 2.12 | 0.15 |
| Vitamin D | −0.02 | 3.91 | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beilhack, G.; Monteforte, R.; Frommlet, F.; Reindl-Schwaighofer, R.; Strassl, R.; Vychytil, A. Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave. Vaccines 2023, 11, 1121. https://doi.org/10.3390/vaccines11061121
Beilhack G, Monteforte R, Frommlet F, Reindl-Schwaighofer R, Strassl R, Vychytil A. Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave. Vaccines. 2023; 11(6):1121. https://doi.org/10.3390/vaccines11061121
Chicago/Turabian StyleBeilhack, Georg, Rossella Monteforte, Florian Frommlet, Roman Reindl-Schwaighofer, Robert Strassl, and Andreas Vychytil. 2023. "Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave" Vaccines 11, no. 6: 1121. https://doi.org/10.3390/vaccines11061121
APA StyleBeilhack, G., Monteforte, R., Frommlet, F., Reindl-Schwaighofer, R., Strassl, R., & Vychytil, A. (2023). Durable Anti-SARS-CoV-2 Antibody Response after mRNA-1273 Booster in Peritoneal Dialysis Patients during the Omicron Wave. Vaccines, 11(6), 1121. https://doi.org/10.3390/vaccines11061121





