Anti-SARS-CoV-2 IgG Antibody Response in Individuals Infected Post Complete Vaccination: A 6-Month Longitudinal Study in Healthcare Professionals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Samples Collection and Analysis
2.3. Statistical Analysis
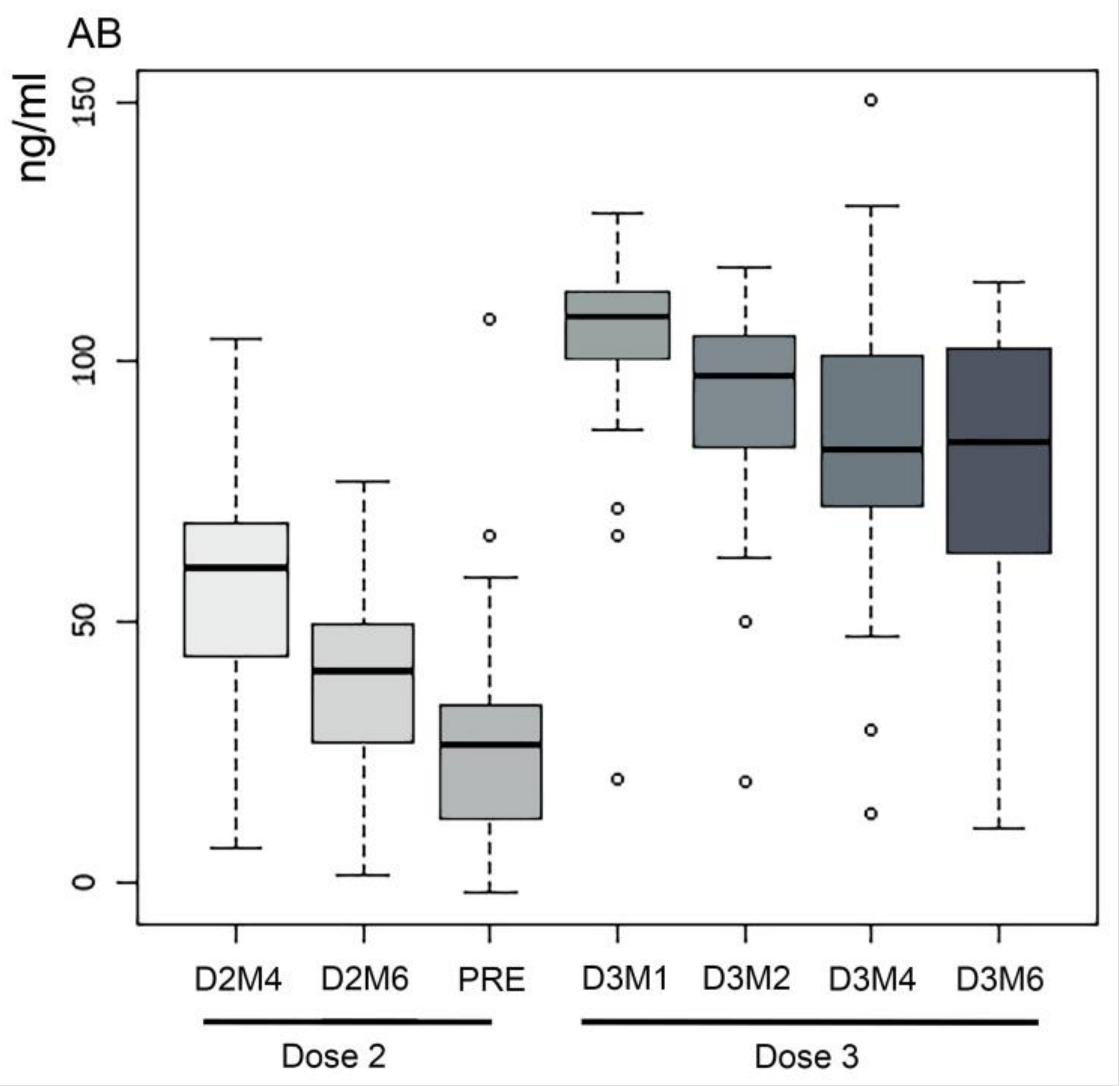
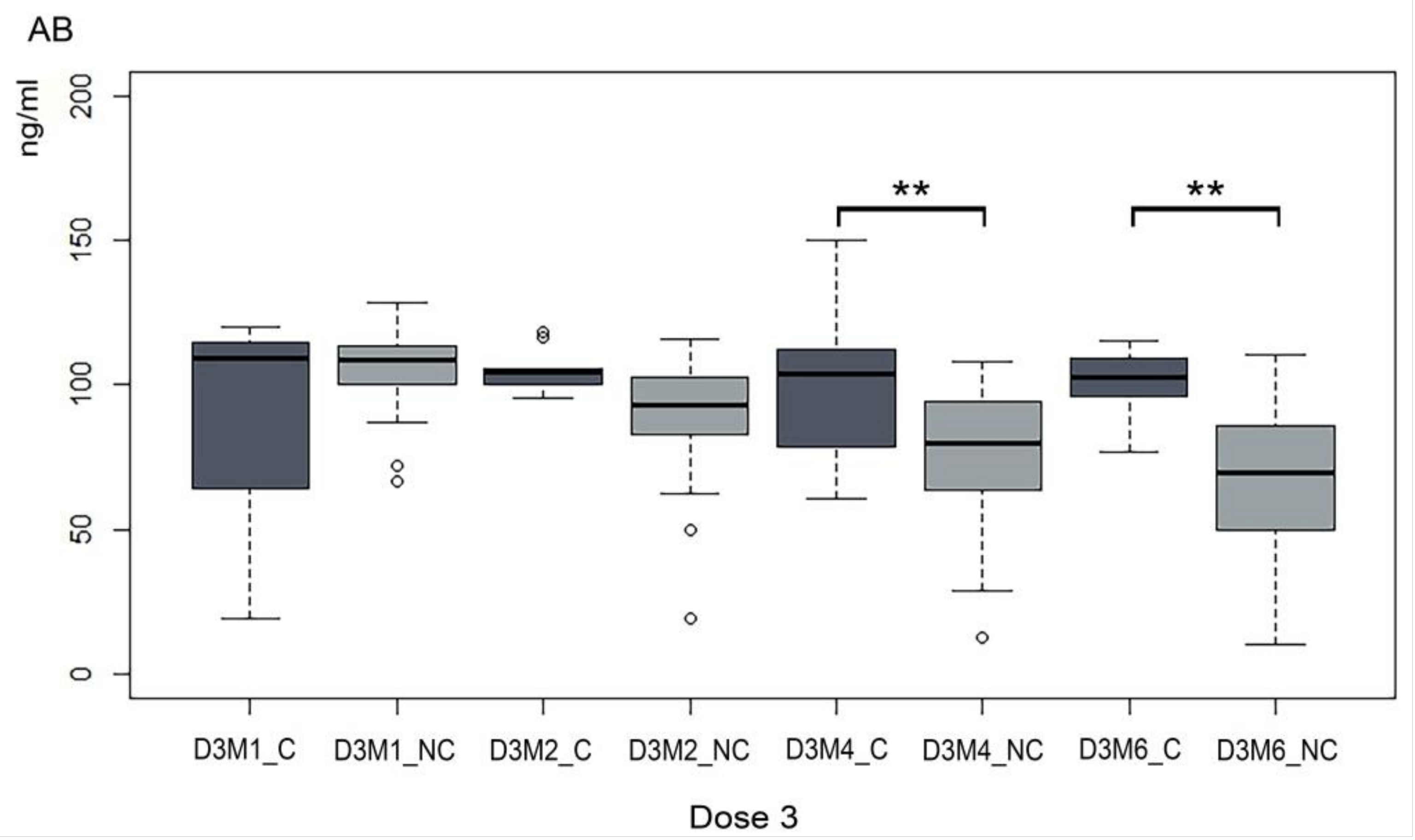
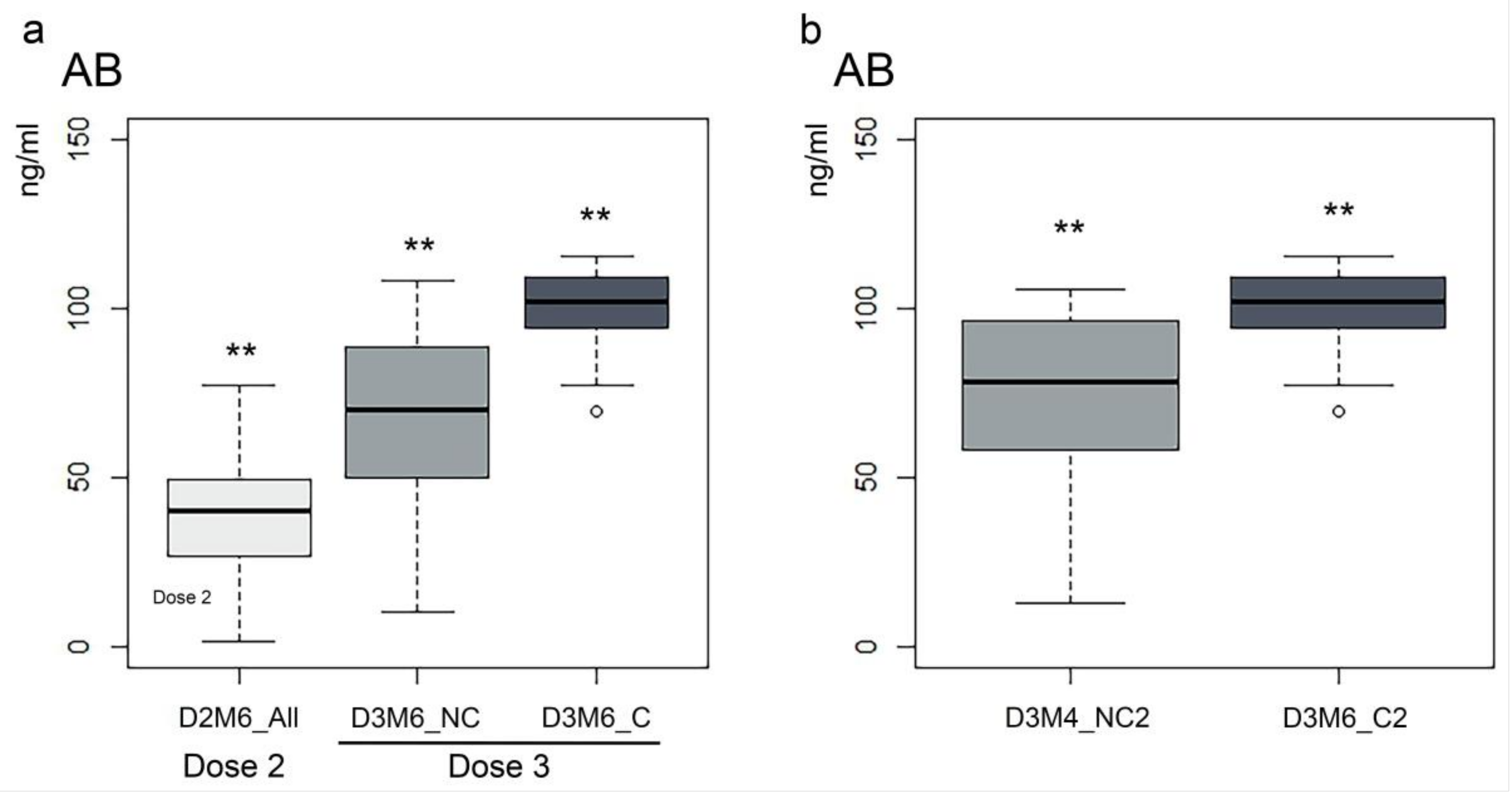
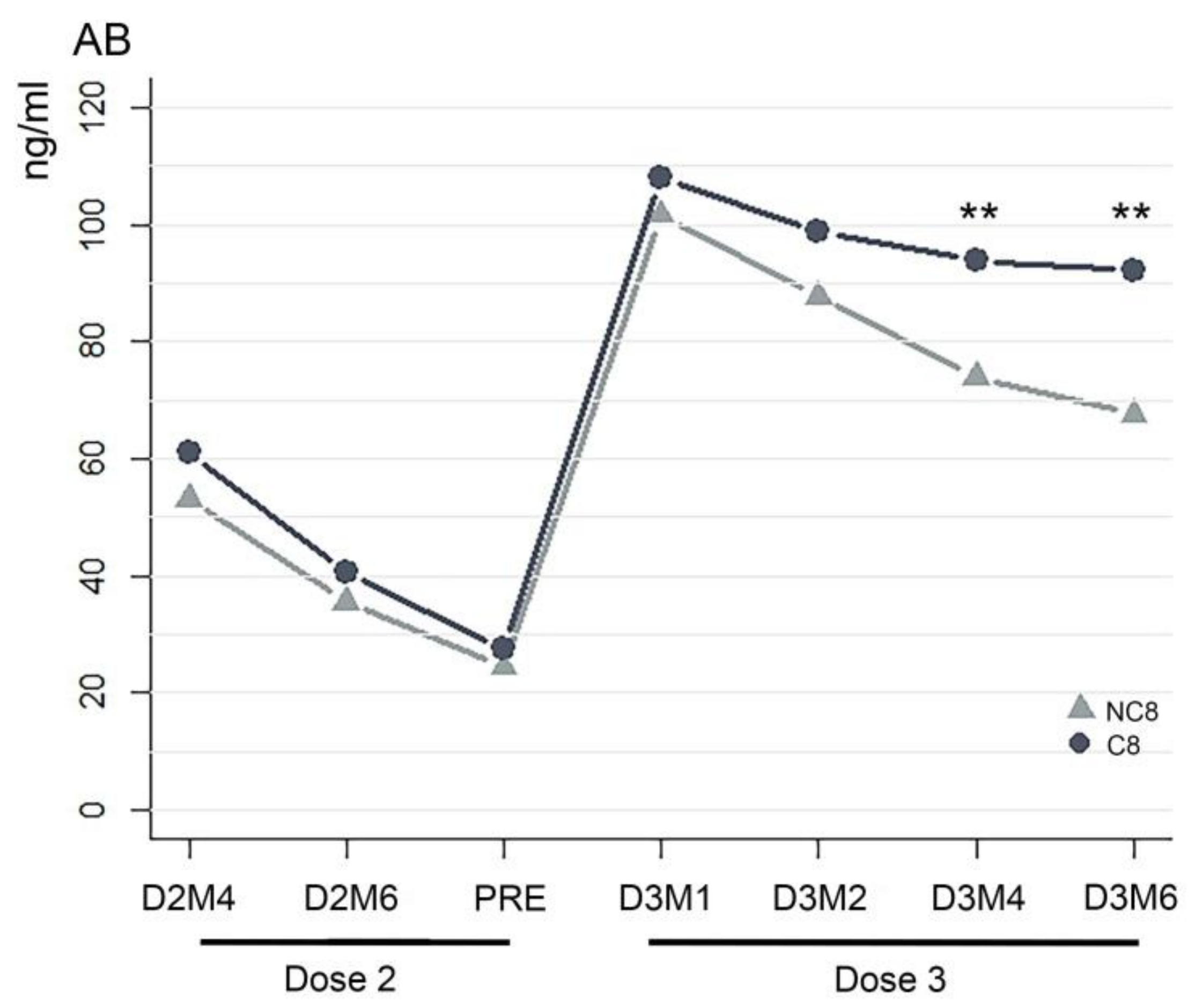
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Aly, Z.; Agarwal, A.; Alwan, N.; Luyckx, V.A. Long COVID: Long-term health outcomes and implications for policy and research. Nat. Rev. Nephrol. 2023, 19, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association between BNT162b2 Vaccination and Long COVID after Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titers six months after Comirnaty vaccination: Kinetics and comparison with SARS-CoV-2 immunoassays. Clin. Chem. Lab. Med. 2022, 60, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Jo, D.H.; Minn, D.; Lim, J.; Lee, K.D.; Kang, Y.M.; Choe, K.W.; Kim, K.N. Rapidly Declining SARS-CoV-2 Antibody Titers within 4 Months after BNT162b2 Vaccination. Vaccines 2021, 9, 1145. [Google Scholar] [CrossRef]
- Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Lustig, Y.; Cohen, C.; Rahav, G.; Asraf, K.; Amit, S.; Jaber, H.; Nemet, I.; et al. Early Immunogenicity and Safety of the Third Dose of BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccine Among Adults Older Than 60 Years: Real-World Experience. J. Infect. Dis. 2022, 225, 785–792. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.; Housset, P. SARS-CoV-2 Antibody Response after a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e181. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef]
- Kontopoulou, K.; Nakas, C.T.; Papazisis, G. Significant Increase in Antibody Titers after the 3rd Booster Dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine in Healthcare Workers in Greece. Vaccines 2022, 10, 876. [Google Scholar] [CrossRef]
- Rastawicki, W.; Juszczyk, G.; Gierczyński, R.; Zasada, A.A. Comparison of anti-SARS-CoV-2 IgG and IgA antibody responses post complete vaccination, 7 months later and after 3rd dose of the BNT162b2 vaccine in healthy adults. J. Clin. Virol. 2022, 152, 105193. [Google Scholar] [CrossRef] [PubMed]
- Uwamino, Y.; Yokoyama, T.; Sato, Y.; Shibata, A.; Kurafuji, T.; Tanabe, A.; Noguchi, M.; Arai, T.; Ohno, A.; Yokota, H.; et al. Humoral and cellular immune response dynamics in Japanese healthcare workers up to six months after receiving a third dose of BNT162b2 monovalent vaccine. Vaccine 2023, 41, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Wang, J.; Zheng, X. The humoral and cellular immune evasion of SARS-CoV-2 Omicron and sub-lineages. Virol. Sin. 2022, 37, 786–795. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Bonanno, M.; Calabrò, R.S. Psychological and Cognitive Effects of Long COVID: A Narrative Review Focusing on the Assessment and Rehabilitative Approach. J. Clin. Med. 2022, 11, 6554. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Sukumaran, A.; Thomas, R.E.; Krishnan, R.A.; Thomas, T.; Thomas, R.; Vijayan, D.K.; Paul, J.K.; Vasudevan, D.M. Sequential Profiling of Anti-SARS CoV-2 IgG Antibody in Post COVID-19 Patients. Indian. J. Clin. Biochem. 2022, 37, 349–355. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 serological tests: Towards harmonization of anti-spike assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef]
- Dashdorj, N.J.; Wirz, O.F.; Röltgen, K.; Haraguchi, E.; Buzzanco, A.S.; Sibai, M.; Wang, H.; Miller, J.A.; Solis, D.; Sahoo, M.K.; et al. Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia. Cell Host Microbe 2021, 29, 1738–1743.e1734. [Google Scholar] [CrossRef]
- Ebrahim, F.; Tabal, S.; Lamami, Y.; Alhudiri, I.M.; El Meshri, S.E.; Al Dwigen, S.; Arfa, R.; Alboeshi, A.; Alemam, H.A.; Abuhtna, F.; et al. Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 or Post-Vaccination in Libyan Population: Comparison of Four Vaccines. Vaccines 2022, 10, 2002. [Google Scholar] [CrossRef]
- Giambra, V.; Piazzolla, A.V.; Cocomazzi, G.; Squillante, M.M.; De Santis, E.; Totti, B.; Cavorsi, C.; Giuliani, F.; Serra, N.; Mangia, A. Effectiveness of Booster Dose of Anti SARS-CoV-2 BNT162b2 in Cirrhosis: Longitudinal Evaluation of Humoral and Cellular Response. Vaccines 2022, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Lobreglio, G.; Bagordo, F.; Zizza, A.; De Donno, A.; Rosato, C.; Lazzari, R.; Chicone, M.; Indino, F.; Recchia, V.; et al. Antibody Response in Healthcare Workers before and after the Third Dose of Anti-SARS-CoV-2 Vaccine: A Pilot Study. Vaccines 2022, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Salerno, G.; Gentile, G.; De Luca, O.; Costanzi, G.; Cirelli, G.; Di Simone Di Giuseppe, B.; Marcellini, L.; Anibaldi, P.; Marcolongo, A.; Simmaco, M.; et al. Age-Related Dynamics of Serum Anti-Spike IgG Ab after the Third Dose of BNT162b2 Vaccine in a Naive Health Care Workers Population. Viral Immunol. 2022, 35, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.R.; Kim, N.; Park, H.; Minn, D.; Park, S.; Roh, E.Y.; Yoon, J.H.; Shin, S. Strong SARS-CoV-2 Antibody Response after Booster Dose of BNT162b2 mRNA Vaccines in Uninfected Healthcare Workers. J. Korean Med. Sci. 2022, 37, e135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; He, F.; Zhang, X.; Li, G.; Wang, X.; Gan, Y.; Zheng, C.; Tang, J.; Xu, L.; Zhao, J.; et al. Antibody response to the third dose of inactivated COVID-19 vaccine in people living with HIV (PLWH): A longitudinal cohort. J. Med. Virol. 2023, 95, e28797. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Kanokudom, S.; Phowatthanasathian, H.; Chansaenroj, J.; Suntronwong, N.; Assawakosri, S.; Yorsaeng, R.; Nilyanimit, P.; Vichaiwattana, P.; Klinfueng, S.; et al. Comparison of the reactogenicity and immunogenicity between two-dose mRNA COVID-19 vaccine and inactivated COVID-19 vaccine followed by an mRNA vaccine in children aged 5–11 years. J. Med. Virol. 2023, 95, e28758. [Google Scholar] [CrossRef]
- Montero, S.; Urrunaga-Pastor, D.; Soto-Becerra, P.; Cvetkovic-Vega, A.; Guillermo-Roman, M.; Figueroa-Montes, L.; Sagástegui, A.A.; Alvizuri-Pastor, S.; Contreras-Macazana, R.M.; Apolaya-Segura, M.; et al. Humoral response after a BNT162b2 heterologous third dose of COVID-19 vaccine following two doses of BBIBP-CorV among healthcare personnel in Peru. Vaccine X 2023, 14, 100311. [Google Scholar] [CrossRef]
- Rose, R.; Neumann, F.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022, 20, 31. [Google Scholar] [CrossRef]
- Hvidt, A.K.; Baerends, E.A.M.; Søgaard, O.S.; Stærke, N.B.; Raben, D.; Reekie, J.; Nielsen, H.; Johansen, I.S.; Wiese, L.; Benfield, T.L.; et al. Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines. Front. Med. 2022, 9, 994160. [Google Scholar] [CrossRef]
- Ogrič, M.; Žigon, P.; Podovšovnik, E.; Lakota, K.; Sodin-Semrl, S.; Rotar, Ž.; Čučnik, S. Differences in SARS-CoV-2-Specific Antibody Responses after the First, Second, and Third Doses of BNT162b2 in Naïve and Previously Infected Individuals: A 1-Year Observational Study in Healthcare Professionals. Front. Immunol. 2022, 13, 876533. [Google Scholar] [CrossRef]
- Terpos, E.; Karalis, V.; Sklirou, A.D.; Apostolakou, F.; Ntanasis-Stathopoulos, I.; Bagratuni, T.; Iconomidou, V.A.; Malandrakis, P.; Korompoki, E.; Papassotiriou, I.; et al. Third dose of the BNT162b2 vaccine results in very high levels of neutralizing antibodies against SARS-CoV-2: Results of a prospective study in 150 health professionals in Greece. Am. J. Hematol. 2022, 97, E147–E150. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Gonen, T.; Meltzer, L.; Gilboa, M.; Indenbaum, V.; Cohen, C.; Amit, S.; Jaber, H.; Doolman, R.; Asraf, K.; et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat. Immunol. 2022, 23, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Piernas, C.; Patone, M.; Astbury, N.M.; Gao, M.; Sheikh, A.; Khunti, K.; Shankar-Hari, M.; Dixon, S.; Coupland, C.; Aveyard, P.; et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2022, 10, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Slotkin, R.; Sheth, A.H.; Pischel, L.; Kyriakides, T.C.; Emu, B.; McNamara, C.; Shi, Q.; Delgobbo, J.; Xu, J.; et al. Clinical Variables Correlate with Serum Neutralizing Antibody Titers after COVID-19 mRNA Vaccination in an Adult, US-based Population. medRxiv 2022. [Google Scholar] [CrossRef]
- Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Choi, J.Y.; Lee, H.W.; Kim, S.S.; Kim, B.; Song, J.Y. Robust neutralizing antibody responses after single-dose BNT162b2 vaccination at long intervals from prior SARS-CoV-2 infection and ceiling effect with repeated vaccination. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Newton, J.N.; Diamond, I.; Studley, R.; Taylor, N.; Bell, J.I.; Farrar, J.; Kolenchery, J.; et al. Protection against SARS-CoV-2 Omicron BA.4/5 variant following booster vaccination or breakthrough infection in the UK. Nat. Commun. 2023, 14, 2799. [Google Scholar] [CrossRef]
- Muik, A.; Lui, B.G.; Bacher, M.; Wallisch, A.K.; Toker, A.; Finlayson, A.; Krüger, K.; Ozhelvaci, O.; Grikscheit, K.; Hoehl, S.; et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci. Immunol. 2022, 7, eade2283. [Google Scholar] [CrossRef]
- Yamamoto, S.; Mizoue, T.; Ohmagari, N. Analysis of Previous Infection, Vaccinations, and Anti-SARS-CoV-2 Antibody Titers and Protection against Infection with the SARS-CoV-2 Omicron BA.5 Variant. JAMA Netw. Open 2023, 6, e233370. [Google Scholar] [CrossRef]
- Cheetham, N.J.; Kibble, M.; Wong, A.; Silverwood, R.J.; Knuppel, A.; Williams, D.M.; Hamilton, O.K.L.; Lee, P.H.; Bridger Staatz, C.; Di Gessa, G.; et al. Antibody levels following vaccination against SARS-CoV-2: Associations with post-vaccination infection and risk factors in two UK longitudinal studies. Elife 2023, 12. [Google Scholar] [CrossRef]
- Cron, R.Q.; Caricchio, R.; Chatham, W.W. Calming the cytokine storm in COVID-19. Nat. Med. 2021, 27, 1674–1675. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long COVID outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef] [PubMed]
- Rolin, S.; Chakales, A.; Verduzco-Gutierrez, M. Rehabilitation Strategies for Cognitive and Neuropsychiatric Manifestations of COVID-19. Curr. Phys. Med. Rehabil. Rep. 2022, 10, 182–187. [Google Scholar] [CrossRef]
- Hagen, B.I.; Lerdal, A.; Søraas, A.; Landrø, N.I.; Bø, R.; Småstuen, M.C.; Becker, J.; Stubberud, J. Cognitive rehabilitation in post-COVID-19 condition: A study protocol for a randomized controlled trial. Contemp. Clin. Trials 2022, 122, 106955. [Google Scholar] [CrossRef] [PubMed]
| Sample (N = 51) | |
|---|---|
| Sex, males/females, n (%) | 16 (31%)/35 (69%) |
| Age, years, mean ± SD | 36.47 ± 9.45 |
| SARS-CoV-2 status, Positive/Negative, n (%) | 26 (51%)/25 (49%) |
| SARS-CoV-2 infection events after 3rd dose (N = 26 + 3 *) | |
| Within 2 months, n (%) | 9/29(31%) |
| Within 2–4 months, n (%) | 3/29 (10%) |
| Within 4–6 months, n (%) | 9/29 (31%) |
| After 6 months, n (%) | 8/29 (28%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratto, N.; Maistrello, L.; Pazienza, E.; Barresi, R. Anti-SARS-CoV-2 IgG Antibody Response in Individuals Infected Post Complete Vaccination: A 6-Month Longitudinal Study in Healthcare Professionals. Vaccines 2023, 11, 1077. https://doi.org/10.3390/vaccines11061077
Baratto N, Maistrello L, Pazienza E, Barresi R. Anti-SARS-CoV-2 IgG Antibody Response in Individuals Infected Post Complete Vaccination: A 6-Month Longitudinal Study in Healthcare Professionals. Vaccines. 2023; 11(6):1077. https://doi.org/10.3390/vaccines11061077
Chicago/Turabian StyleBaratto, Nicole, Lorenza Maistrello, Elena Pazienza, and Rita Barresi. 2023. "Anti-SARS-CoV-2 IgG Antibody Response in Individuals Infected Post Complete Vaccination: A 6-Month Longitudinal Study in Healthcare Professionals" Vaccines 11, no. 6: 1077. https://doi.org/10.3390/vaccines11061077
APA StyleBaratto, N., Maistrello, L., Pazienza, E., & Barresi, R. (2023). Anti-SARS-CoV-2 IgG Antibody Response in Individuals Infected Post Complete Vaccination: A 6-Month Longitudinal Study in Healthcare Professionals. Vaccines, 11(6), 1077. https://doi.org/10.3390/vaccines11061077







