Vaccine-Preventable Hospitalisations from Seasonal Respiratory Diseases: What Is Their True Value?
Abstract
1. Introduction
2. Methods
2.1. Vaccine-Preventable Hospitalisations
| Disease | Age Groups | Vaccine Modelled | Effectiveness | Coverage |
|---|---|---|---|---|
| Flu | 65 years and above | Influenza Vaccine | 50% [11] 1 | 72% [11] |
| PD | Pneumococcal Polysaccharide Vaccine (PPV23) | 27% against IPD [16]; 0% against pneumococcal CAP [17] | 69% [12] 2 | |
| RSV | Hypothetical vaccine | 50% [14,18] 1 | 69% 3 | |
| COVID-19 | 50 years and above | COVID booster vaccine | 90% [19] | 66.7–91.5% 4 [15] |
2.2. Reference Costing versus Opportunity Costing
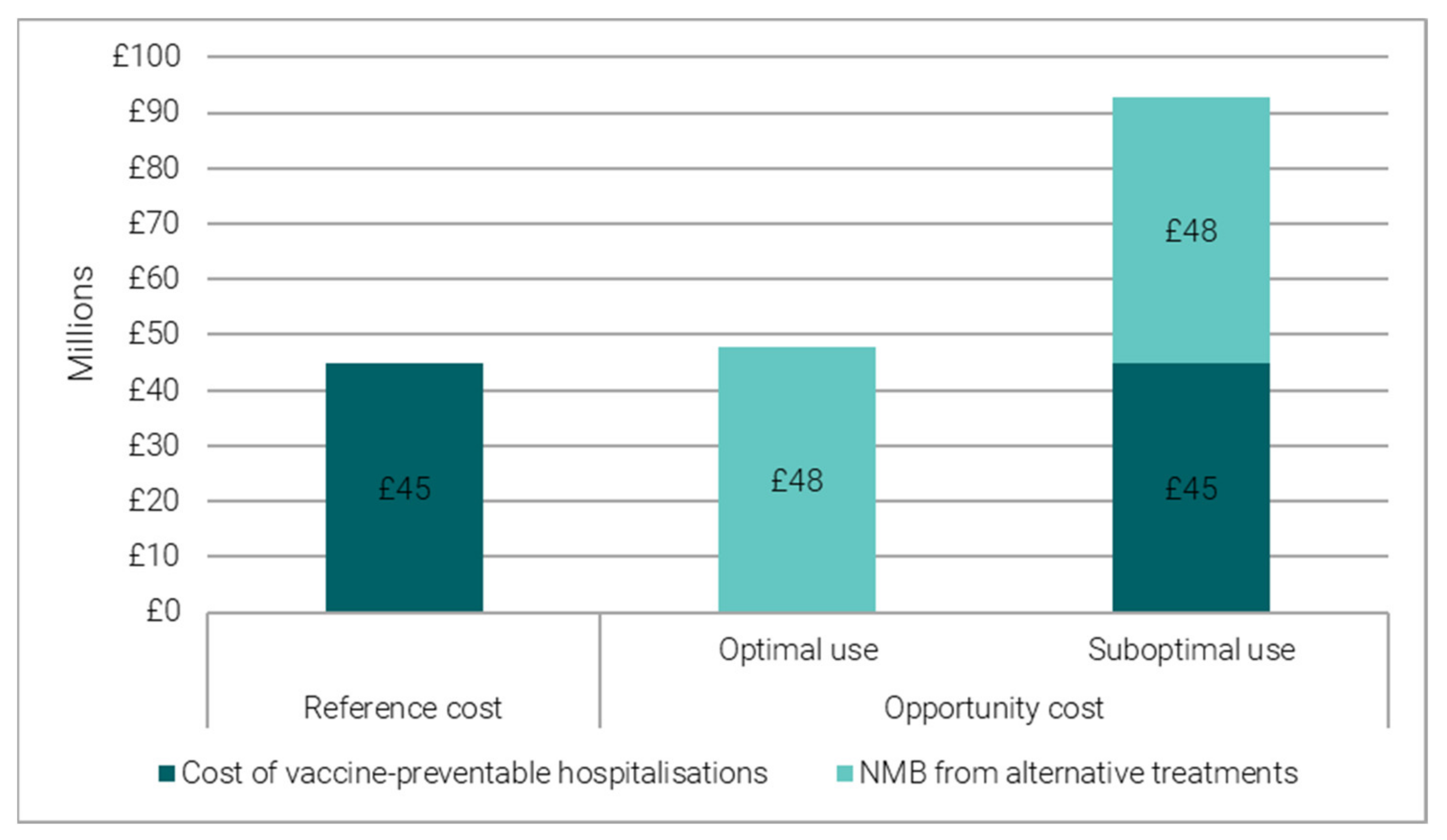
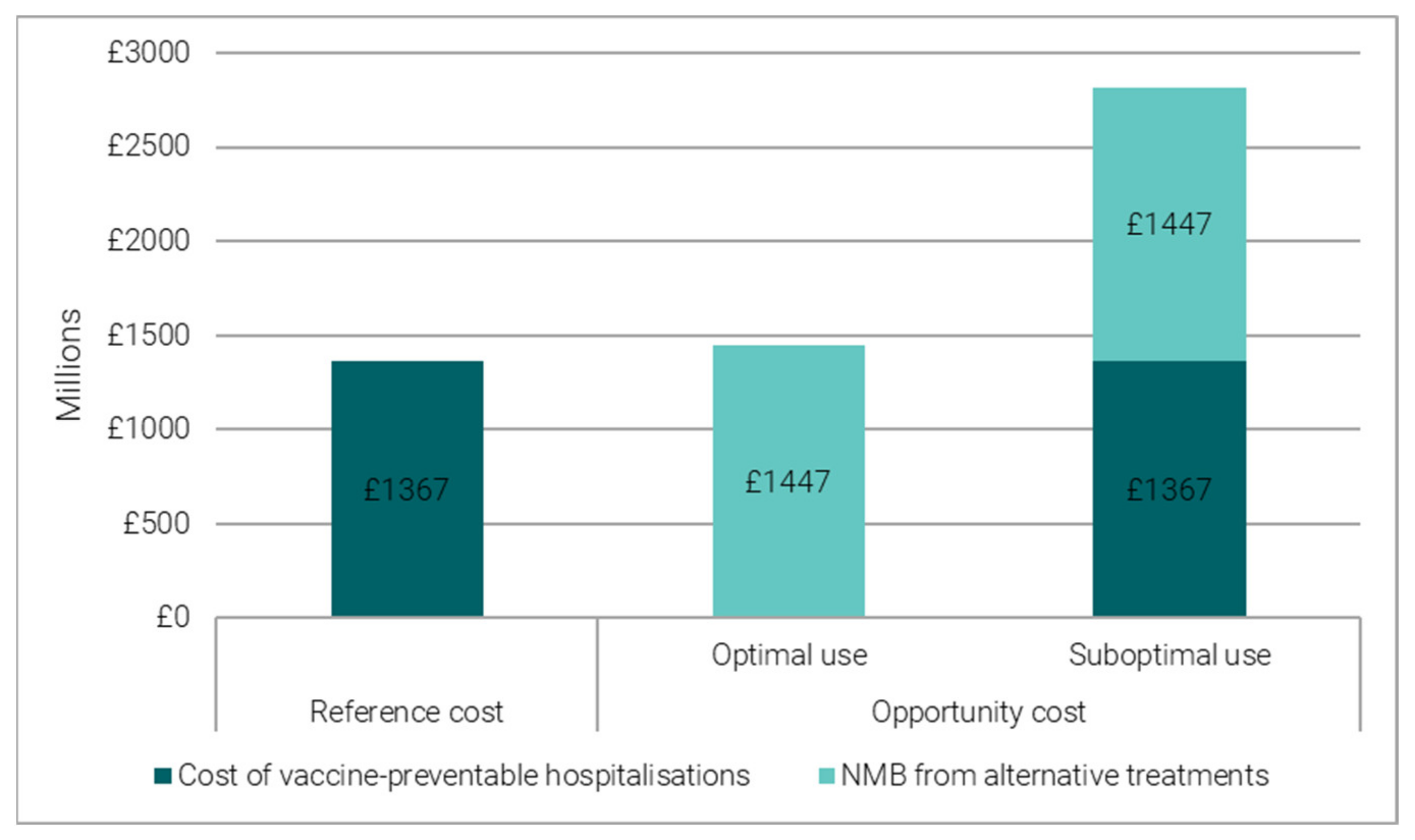
- When vaccine-preventable hospitalisations are an optimal use of hospital beds, their opportunity cost is proxied by the forgone benefit from alternative treatment opportunities, which can be quantified in Net Monetary Benefit (NMB) terms. We converted the bed days freed up by vaccination into the number of alternative treatments and valued them in Net Monetary Benefit (NMB) terms. The NMB is estimated using average health gains and average costs from an alternative treatment [6] (see Table S4 in Supplementary Materials).
- When vaccine-preventable hospitalisations are a suboptimal use of hospital beds, their opportunity cost is proxied by the total economic cost, given by the sum of the forgone benefit (NMB) from alternative treatments and the cost of vaccine-preventable hospitalisations.
2.3. What-If Analyses
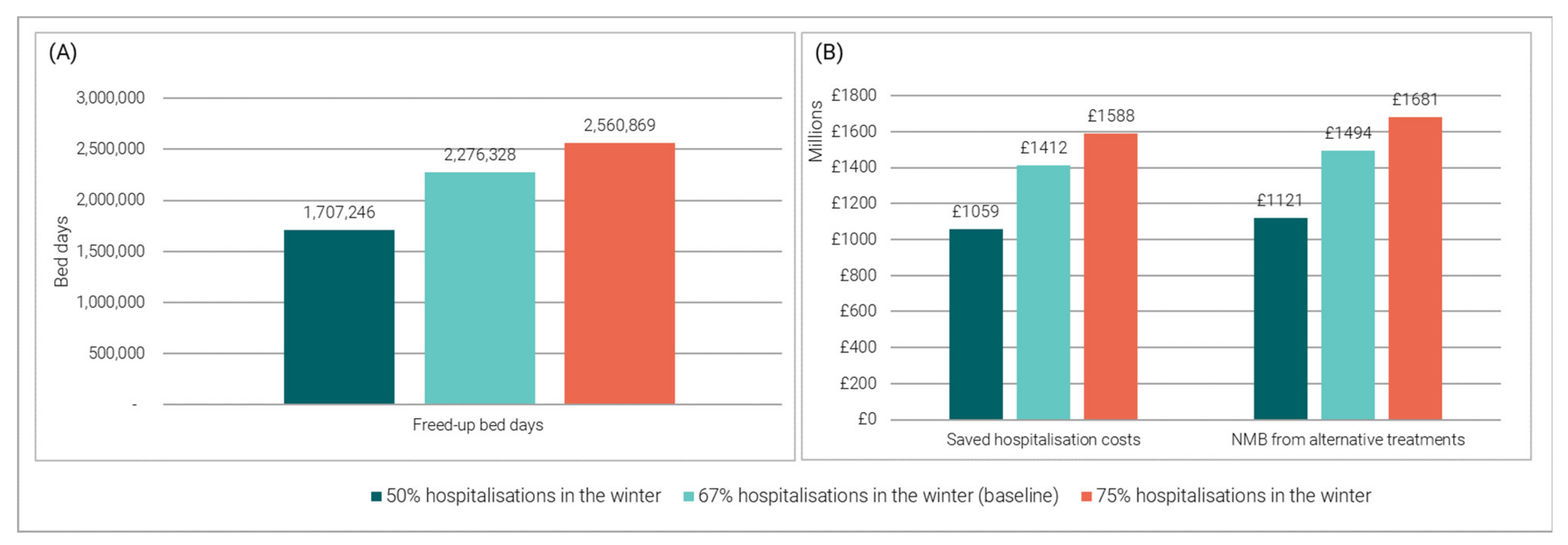
2.3.1. Impact of Varying the Proportion of Hospitalisations Occurring in the Winter
2.3.2. Impact of Replacing the Current PPV23 Programme for PD with PCV20 Data
3. Results
3.1. Winter Flu, PD and RSV Impact
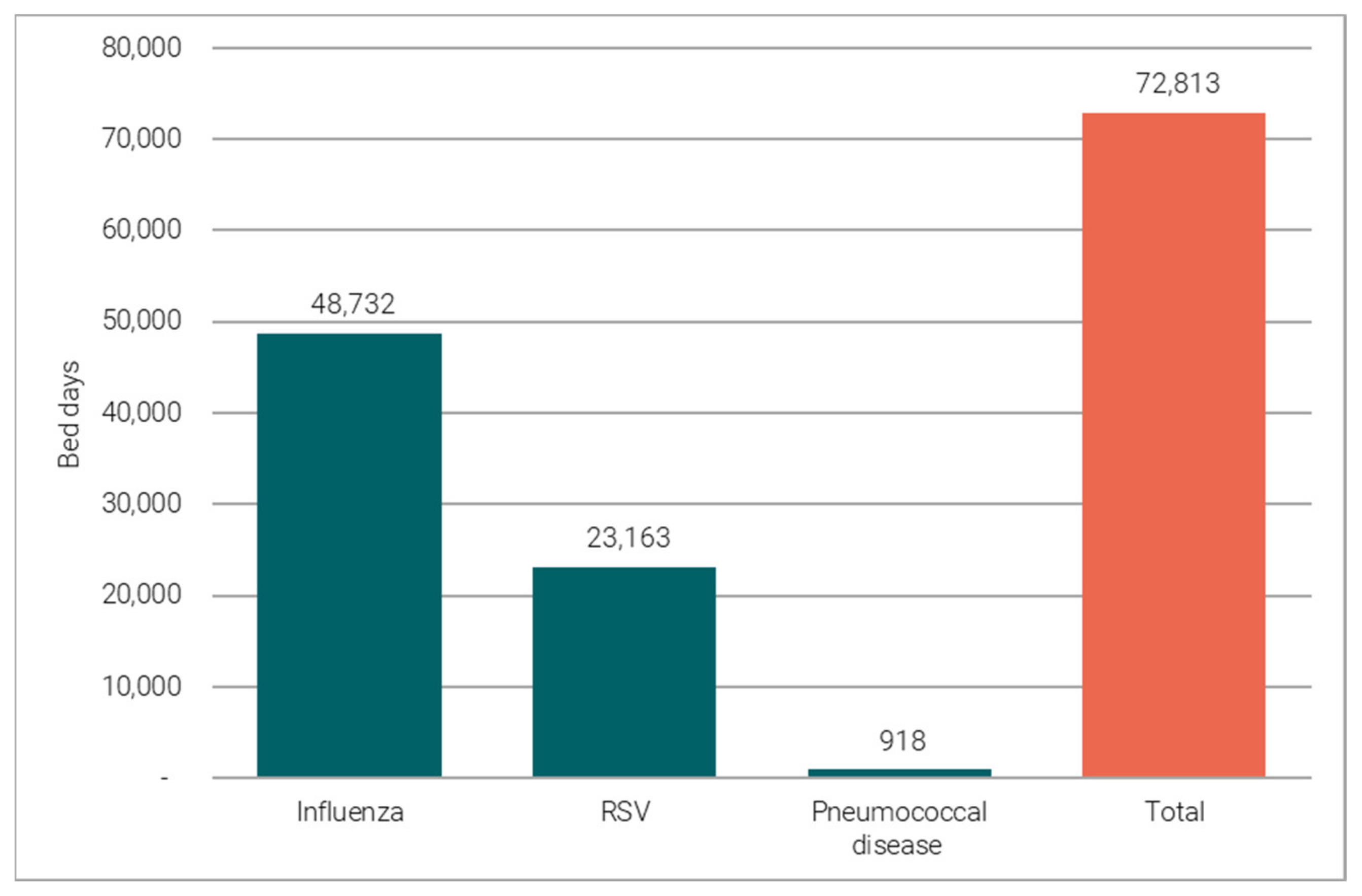
3.1.1. Vaccine-Preventable Hospitalisations
3.1.2. Reference Costing versus Opportunity Costing
3.2. Winter COVID-19 Impact
3.2.1. Vaccine Preventable Hospitalisations
3.2.2. Reference Costing versus Opportunity Costing
3.3. What-If Analyses
3.3.1. Impact of Varying the Proportion of Hospitalisations Occurring in the Winter
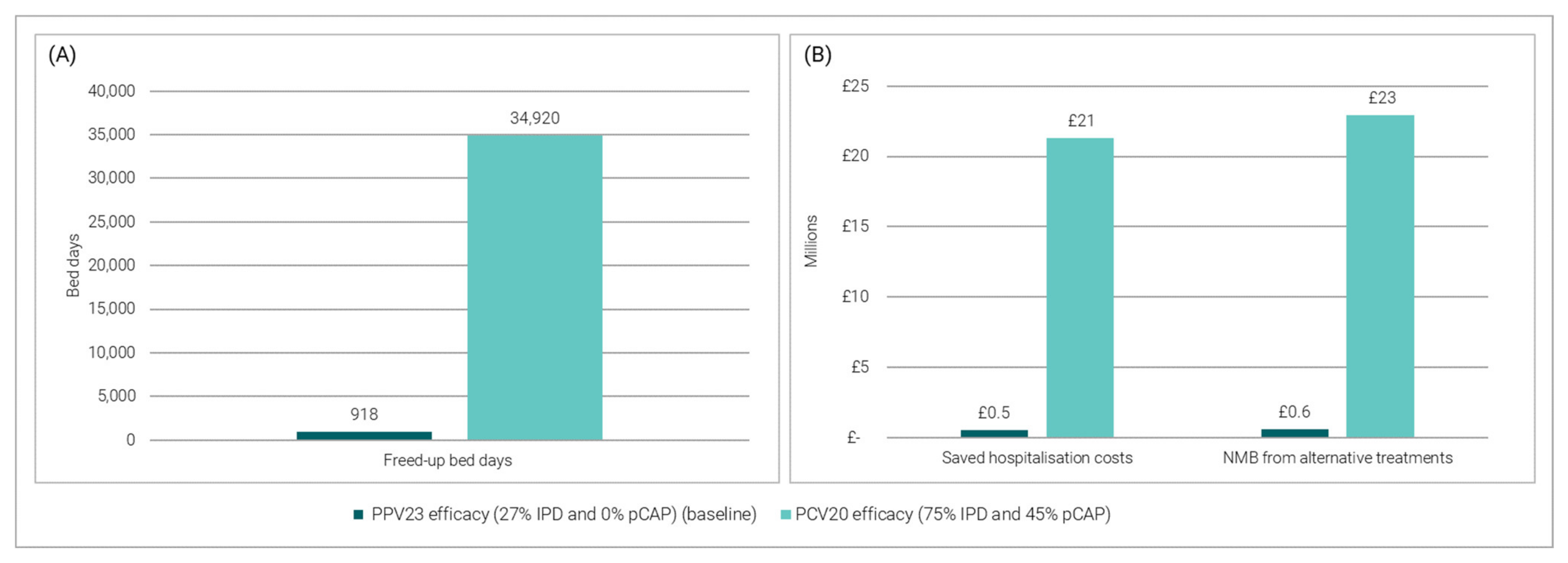
3.3.2. Impact of Replacing the Current PPV23 Programme for PD with PCV20 Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorlby, R.; Gardner, T.; Turton, C. NHS Performance and Waiting Times; The Health Foundation: London, UK, 2019; Available online: https://www.health.org.uk/publications/long-reads/nhs-performance-and-waiting-times (accessed on 5 October 2021).
- NHS England. Statistical Press Notice NHS Referral to Treatment (RTT) Waiting Times Data, October 2022; NHS England: London, UK, 2022; Available online: https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2022/12/Oct22-RTT-SPN-publication-version-PDF-438K-39982.pdf (accessed on 9 December 2022).
- NHS England Statistics. Consultant-Led Referral to Treatment Waiting Times Data 2021–22; NHS England: London, UK, 2022; Available online: https://www.england.nhs.uk/statistics/statistical-work-areas/rtt-waiting-times/rtt-data-2021-22/ (accessed on 26 October 2022).
- NHS Providers. NHS Winter Watch 2022/23. Week 6, 2 January–8 January; NHS Providers: London, UK, 2023; Available online: https://nhsproviders.org/nhs-winter-watch-202223/week-6 (accessed on 22 February 2023).
- Sandmann, F.G.; Robotham, J.V.; Deeny, S.R.; Edmunds, W.J.; Jit, M. Estimating the opportunity costs of bed-days. Health Econ. 2018, 27, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, F.G.; Shallcross, L.; Adams, N.; Allen, D.J.; Coen, P.G.; Jeanes, A.; Kozlakidis, Z.; Larkin, L.; Wurie, F.; Robotham, J.V.; et al. Estimating the Hospital Burden of Norovirus-Associated Gastroenteritis in England and Its Opportunity Costs for Nonadmitted Patients. Clin. Infect. Dis. 2018, 67, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, F. The True Cost of Epidemic and Outbreak Diseases in Hospitals. Ph.D. Thesis, London School of Hygiene & Tropical Medicine, London, UK, 2018. Available online: http://researchonline.lshtm.ac.uk/id/eprint/4648208 (accessed on 3 March 2021).
- Iacobucci, G. Covid-19: Hospitals forced to suspend routine care amid second surge. BMJ 2020, 371, m4339. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Minaji, M.; Panagiotopoulos, N.; Reeves, R.; Charlett, A.; Pebody, R. Estimating the burden of adult hospital admissions due to RSV and other respiratory pathogens in England. Influenza Other Respir. Viruses 2022, 16, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Brassel, S.; Neri, M.; Schirrmacher, H.; Steuten, L. The Value of Vaccines in Maintaining Health System Capacity in England. Value Health 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- PHE. Surveillance of Influenza and Other Respiratory Viruses in the UK Winter 2018 to 2019; Public Health England: London, UK, 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/839350/Surveillance_of_influenza_and_other_respiratory_viruses_in_the_UK_2018_to_2019-FINAL.pdf (accessed on 6 July 2021).
- PHE. Pneumococcal Polysaccharide Vaccine (PPV) Coverage Report, England, April 2018 to March 2019; Public Health England: London, UK, 2019. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20211106003307/https:/www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-vaccine-coverage-estimates (accessed on 7 October 2022).
- UKHSA. COVID-19 Vaccine Surveillance Report Week 12, 24 March 2022; UK Health Security Agency: London, UK, 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1063023/Vaccine-surveillance-report-week-12.pdf (accessed on 6 July 2021).
- Zeevat, F.; Luttjeboer, J.; Paulissen, J.; van der Schans, J.; Beutels, P.; Boersma, C.; Postma, M. Exploratory analysis of the economically justifiable price of a hypothetical RSV vaccine for older adults in the Netherlands and the United Kingdom. J. Infect. Dis. 2022, 226, S102–S109. [Google Scholar] [CrossRef] [PubMed]
- UKHSA. Weekly National Influenza and COVID-19 Surveillance Report Week 14 Report (up to Week 13 Data) 7 April 2022; UK Health Security Agency: London, UK, 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1067092/Weekly_Flu_and_COVID-19_report_w14.pdf (accessed on 6 July 2021).
- Djennad, A.; Ramsay, M.E.; Pebody, R.; Fry, N.K.; Sheppard, C.; Ladhani, S.N.; Andrews, N.J. Effectiveness of 23-Valent Polysaccharide Pneumococcal Vaccine and Changes in Invasive Pneumococcal Disease Incidence from 2000 to 2017 in Those Aged 65 and over in England and Wales. eClinicalMedicine 2018, 6, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, H.; Pick, H.; Baskaran, V.; Daniel, P.; Rodrigo, C.; Ashton, D.; Edwards-Pritchard, R.C.; Sheppard, C.; Eletu, S.D.; Litt, D.; et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: A case-control test-negative design study. PLoS Med. 2020, 17, e1003326. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.; Pebody, R.; Panovska-Griffiths, J.; Baguelin, M.; Atkins, K.E. Evaluating the next generation of RSV intervention strategies: A mathematical modelling study and cost-effectiveness analysis. BMC Med. 2020, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Chapman, R.; Aruffo, E.; Gal, P.; Nguyen, J.L.; Hamson, L.; Di Fusco, M.; Czudek, C.; Yang, J. Public health impact of UK COVID-19 booster vaccination programs during Omicron predominance. Expert Rev. Vaccines 2023, 22, 90–103. [Google Scholar] [CrossRef] [PubMed]
- NHS Digital. Hospital Admitted Patient Care Activity. 2022. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity (accessed on 11 March 2022).
- NHS Digital. HRG4+ 2020/21 Local Payment Grouper V2 (COVID-19). 2021. Available online: https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/hrg4-2020-21-local-payment-grouper-v2-covid-19 (accessed on 7 October 2022).
- NHS England. National Schedule of NHS Costs. 2021. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.england.nhs.uk%2Fwp-content%2Fuploads%2F2022%2F07%2F2_National_schedule_of_NHS_costs_FY20-21.xlsx&wdOrigin=BROWSELINK (accessed on 6 December 2022).
- Hussain, F.; Kotecha, S.; Edwards, M.O. RSV bronchiolitis season 2021 has arrived, so be prepared! Arch. Dis. Child. 2021, 106, e51. [Google Scholar] [CrossRef] [PubMed]
- Bertran, M.; Amin-Chowdhury, Z.; Sheppard, C.; Eletu, S.; Zamarreño, D.V.; Ramsay, M.E.; Litt, D.; Fry, N.; Ladhani, S.N. Increased Incidence of Invasive Pneumococcal Disease in Children in England: July to December 2021, Compared to Pre-Pandemic Years (2017–2019). Emerg. Infect. Dis. 2022, 28, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- E.M.A Apexxnar Pneumococcal Polysaccharide Conjugate Vaccine (20-Valent, Adsorbed). 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/apexxnar#authorisation-details-section (accessed on 8 December 2022).
- Mugwagwa, T.; Averin, A.; Atwood, M.; Sato, R.; Vyse, A.; Campling, J.; Weycker, D.; Slack, M.; Ellsbury, G.; Mendes, D. Public health and budgetary impact of 20-valent pneumococcal conjugate vaccine for adults in England. Expert Rev. Vaccines 2022, 21, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Essink, B.; Sabharwal, C.; Cannon, K.; Frenck, R.; Lal, H.; Xu, X.; Sundaraiyer, V.; Peng, Y.; Moyer, L.; Pride, M.W. Pivotal phase 3 randomized clinical trial of the safety, tolerability, and immunogenicity of 20-valent pneumococcal conjugate vaccine in adults aged ≥ 18 years. Clin. Infect. Dis. 2022, 75, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- ONS. Healthcare Expenditure, UK Health Accounts: 2020; Office for National Statistics: London, UK, 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthcaresystem/bulletins/ukhealthaccounts/2020#:~:text=Government%20preventive%20care%20expenditure%20in%202020&text=This%20makes%20up%2033.3%25%20of,the%20financial%20year%20ending%202021 (accessed on 23 February 2023).
- Iacobucci, G. England and Wales see rise in excess deaths amid flu surge. BMJ 2023, 380, p40. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 17 April 2023).
- Pick, H.; Daniel, P.; Rodrigo, C.; Bewick, T.; Ashton, D.; Lawrence, H.; Baskaran, V.; Edwards-Pritchard, R.C.; Sheppard, C.; Eletu, S.D.; et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013-18. Thorax. 2020, 75, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; van Hoek, A.J.; Newall, A.T.; Pollard, A.J.; Jit, M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: A modelling and cost-effectiveness analysis for England. Lancet Public Health 2017, 2, e367–e374. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.; Hochlaf, D. An Economic Analysis of Flu Vaccination; The International Longevity Centre: London, UK, 2018; Available online: https://ilcuk.org.uk/wp-content/uploads/2018/07/An_economic_analysis_of_flu_vaccination_-_ILC-UK.pdf (accessed on 7 October 2022).
- Delgleize, E.; Leeuwenkamp, O.; Theodorou, E.; Van de Velde, N. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: A comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open 2016, 6, e010776. [Google Scholar] [CrossRef] [PubMed]
- NICE. Assessing Cost-Effectiveness | The Guidelines Manual. National Institute for Health and Care Excellence. National Institute for Health and Care Excellence. 2012. Available online: https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness (accessed on 6 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, M.; Brassel, S.; Schirrmacher, H.; Mendes, D.; Vyse, A.; Steuten, L.; Hamson, E. Vaccine-Preventable Hospitalisations from Seasonal Respiratory Diseases: What Is Their True Value? Vaccines 2023, 11, 945. https://doi.org/10.3390/vaccines11050945
Neri M, Brassel S, Schirrmacher H, Mendes D, Vyse A, Steuten L, Hamson E. Vaccine-Preventable Hospitalisations from Seasonal Respiratory Diseases: What Is Their True Value? Vaccines. 2023; 11(5):945. https://doi.org/10.3390/vaccines11050945
Chicago/Turabian StyleNeri, Margherita, Simon Brassel, Hannah Schirrmacher, Diana Mendes, Andrew Vyse, Lotte Steuten, and Elizabeth Hamson. 2023. "Vaccine-Preventable Hospitalisations from Seasonal Respiratory Diseases: What Is Their True Value?" Vaccines 11, no. 5: 945. https://doi.org/10.3390/vaccines11050945
APA StyleNeri, M., Brassel, S., Schirrmacher, H., Mendes, D., Vyse, A., Steuten, L., & Hamson, E. (2023). Vaccine-Preventable Hospitalisations from Seasonal Respiratory Diseases: What Is Their True Value? Vaccines, 11(5), 945. https://doi.org/10.3390/vaccines11050945






