Is There a Difference in Immune Response to SARS-CoV-2 Vaccination between Liver and Lung Transplant Patients with Cystic Fibrosis?
Abstract
1. Introduction
Cystic Fibrosis and SARS-CoV-2
2. Methods
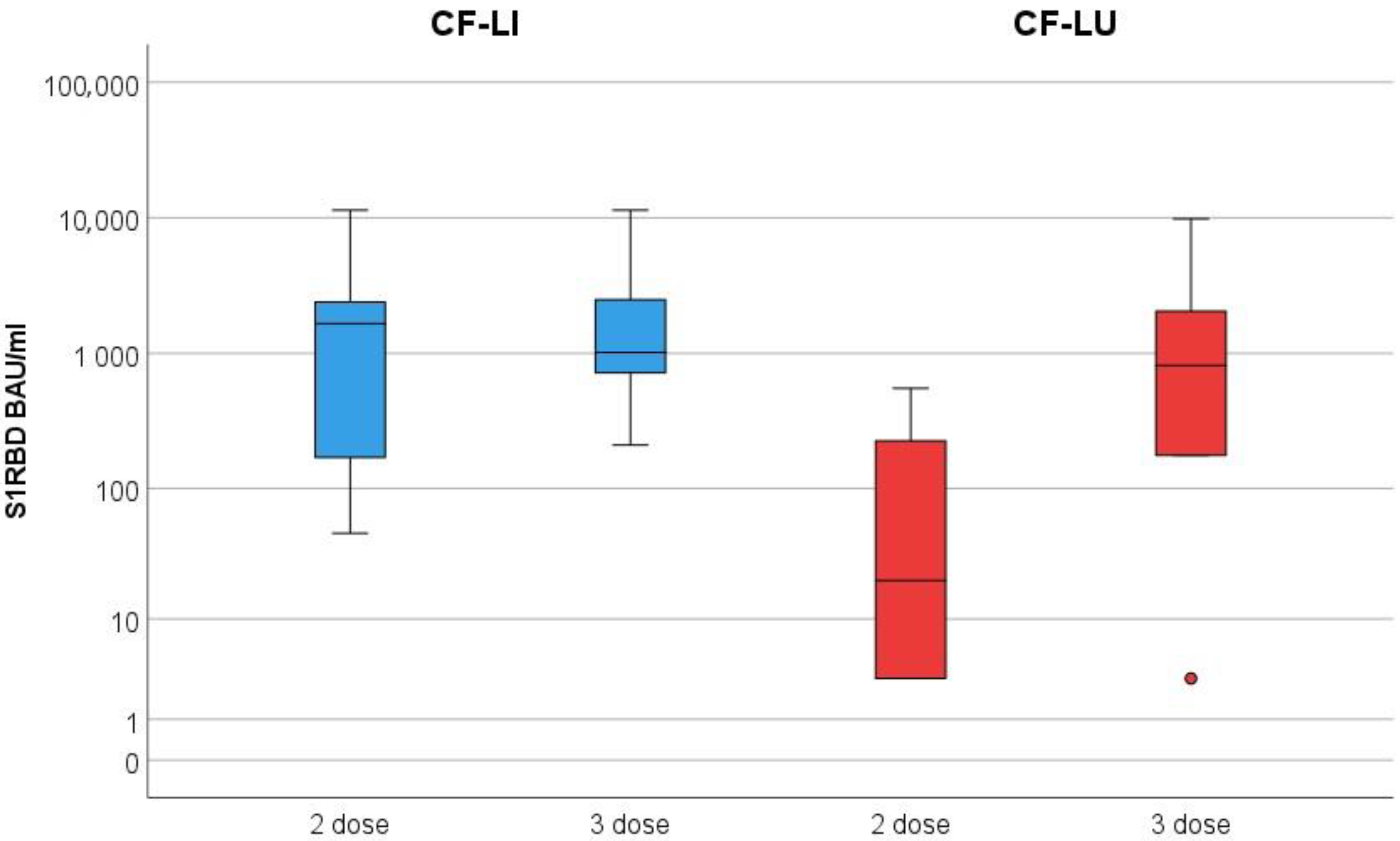
3. Results
3.1. Patient Characteristics
3.2. Monitoring Secreted Antibodies against SARS-CoV-2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| CFTR | CF transmembrane conductance regulator |
| CFLD | CF liver disease |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus type 2 |
| COVID-19 | Coronavirus disease 2019 |
| LCI | Lung Clearance Index |
| WHO | World health organization |
| PA | Pseudomonas aeruginosa |
| S1RBD | receptor-binding domain of S1 subunit of spike protein |
| CF-LI | CF liver transplantation |
| CF-LU | CF lung transplantation |
References
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic Fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R., Jr.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Forest, M.G.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef] [PubMed]
- Benden, C. Lung transplantation as standard of care for advanced cystic fibrosis lung disease. J. Heart Lung Transplant. 2020, 39, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Jones, A.M.; Hanley, K.P.; Athwal, V.S. Review article: Epidemiology, pathogenesis and management of liver disease in adults with cystic fibrosis. Aliment. Pharmacol. Ther. 2022, 55, 389–400. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 17 January 2022).
- Colombo, C.; Burgel, P.-R.; Gartner, S.; van Koningsbruggen-Rietschel, S.; Naehrlich, L.; Sermet-Gaudelus, I.; Southern, K.W. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir. Med. 2020, 8, e35–e36. [Google Scholar] [CrossRef] [PubMed]
- McClenaghan, E.; Cosgriff, R.; Brownlee, K.; Ahern, S.; Burgel, P.R.; Byrnes, C.A.; Colombo, C.; Corvol, H.; Cheng, S.Y.; Daneau, G.; et al. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J. Cyst. Fibros 2020, 19, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; Saiman, L.; Marshall, B. The Impact of COVID-19 in Cystic Fibrosis. Arch. Bronconeumol. 2021, 58, 466–468. [Google Scholar] [CrossRef]
- Jung, A.; Orenti, A.; Dunlevy, F.; Aleksejeva, E.; Bakkeheim, E.; Bobrovnichy, V.; Carr, S.B.; Colombo, C.; Corvol, H.; Cosgriff, R.; et al. Factors for severe outcomes following SARS-CoV-2 infection in people with cystic fibrosis in Europe. ERJ open Res. 2021, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, V.; Motisi, M.A.; Pellegrino, R.; Padoan, R.; Chiappini, E. Risk factors for severe COVID-19 in people with cystic fibrosis: A systematic review. Front Pediatr. 2022, 10, 958658. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz. COVID-19-Impfungen: Anwendungsempfehlungen des Nationalen Impfgremiums. 2022. Available online: https://www.sozialministerium.at/Corona/fachinformationen.html#corona-schutzimpfung (accessed on 16 January 2022).
- Manothummetha, K.; Chuleerarux, N.; Sanguankeo, A.; Kates, O.S.; Hirankarn, N.; Thongkam, A.; Dioverti-Prono, M.V.; Torvorapanit, P.; Langsiri, N.; Worasilchai, N.; et al. Immunogenicity and Risk Factors Associated With Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw Open 2022, 5, e226822. [Google Scholar] [CrossRef] [PubMed]
- Toniutto, P.; Cussigh, A.; Cmet, S.; Bitetto, D.; Fornasiere, E.; Fumolo, E.; Fabris, M.; D’Aurizio, F.; Fabris, C.; Grillone, L.; et al. Immunogenicity and safety of a third dose of anti-SARS-CoV-2 BNT16b2 vaccine in liver transplant recipients. Liver Int. 2022, 43, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.W.; Meek, B.; Rijkers, G.T.; van Kessel, D.A. Serologic response to a third dose of an mRNA-based SARS-CoV-2 vaccine in lung transplant recipients. Transpl. Immunol. 2022, 72, 101599. [Google Scholar] [CrossRef] [PubMed]
- Zentralinstitut für med. u. chem. Labordiagnostik (ZIMCL). SARS-CoV-2, quant. IgG Antikörper gegen S1-RBD nach WHO (Abbott). 2021. Available online: https://zimcl.tirol-kliniken.at/page.cfm?vpath=parameterdetails&genericpageid=952 (accessed on 5 December 2022).
- Cooper, B.G.; Stocks, J.; Hall, G.; Culver, B.; Steenbruggen, I.; Carter, K.W.; Thompson, B.R.; Graham, B.L.; Miller, M.R.; Ruppel, G.; et al. The Global Lung Function Initiative (GLI) Network: Bringing the world’s respiratory reference values together. Breathe 2017, 13, e56–e64. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Abravanel, F.; Marion, O.; Couat, C.; Esposito, L.; Lavayssière, L.; Izopet, J.; Kamar, N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am. J. Transplant. 2022, 22, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Michos, A.; Filippatos, F.; Tatsi, E.-B.; Dellis, C.; Efthymiou, V.; Zarkada, I.; Troupi, E.; Syriopoulou, V.; Loukou, I. Immunogenicity of the COVID-19 BNT162b2 vaccine in adolescents and young adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, e184–e187. [Google Scholar] [CrossRef] [PubMed]
| Item | Total | Liver | Lung |
|---|---|---|---|
| (N) | 13 | 5 | 8 |
| Sex n (%) | |||
| male | 8 (62%) | 4 (80%) | 4 (50%) |
| female | 5 (38%) | 1 (20%) | 4 (50%) |
| Age (y) median (range) | 34 (20, 61) | 21 (20, 46) | 41 (32, 61) |
| time since Tx (y) median (range) | 11 (6, 24) | 11 (9, 22) | 10 (6, 24) |
| FEV1% median (range) | 78 (42, 82) | 81 (49, 118) | |
| LCI median (range) | 12.6 (8.8–16.6) | 9 (7.2–12) | |
| BMI/kg/m2) median (range) | 20 (19, 22) | 22 (16, 26) | |
| CFRD n (%) | 10 (77%) | 4 (80%) | 6 (75%) |
| Insulin therapy | 9 (69%) | 3 (60%) | 6 (75%) |
| Mycophenolic acid therapy n (%) | 11 (84%) | 3 (60%) | 8 (100%) |
| Azithromycin therapy n (%) | 1 (7%) | 1 (20%) | 0 (0%) |
| Chron. PA colonisation n (%) | 7 (54%) | 3 (60%) | 4 (50%) |
| Serologic Response n (%) | |||
| positive 2. Vaccine | 9 (69%) | 5 (100%) | 4 (50%) |
| (N) * | 12 | 5 | 7 |
| positive 3. vaccine | 10 (83%) | 5 (100%) | 5 (71%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, T.; Appelt, D.; Ellemunter, H. Is There a Difference in Immune Response to SARS-CoV-2 Vaccination between Liver and Lung Transplant Patients with Cystic Fibrosis? Vaccines 2023, 11, 657. https://doi.org/10.3390/vaccines11030657
Fuchs T, Appelt D, Ellemunter H. Is There a Difference in Immune Response to SARS-CoV-2 Vaccination between Liver and Lung Transplant Patients with Cystic Fibrosis? Vaccines. 2023; 11(3):657. https://doi.org/10.3390/vaccines11030657
Chicago/Turabian StyleFuchs, Teresa, Dorothea Appelt, and Helmut Ellemunter. 2023. "Is There a Difference in Immune Response to SARS-CoV-2 Vaccination between Liver and Lung Transplant Patients with Cystic Fibrosis?" Vaccines 11, no. 3: 657. https://doi.org/10.3390/vaccines11030657
APA StyleFuchs, T., Appelt, D., & Ellemunter, H. (2023). Is There a Difference in Immune Response to SARS-CoV-2 Vaccination between Liver and Lung Transplant Patients with Cystic Fibrosis? Vaccines, 11(3), 657. https://doi.org/10.3390/vaccines11030657






