The Impact of Educational Intervention on Willingness to Enroll in a Clinical Trial of a Gonorrhea Vaccine
Abstract
1. Introduction
2. Methods
2.1. Study Design
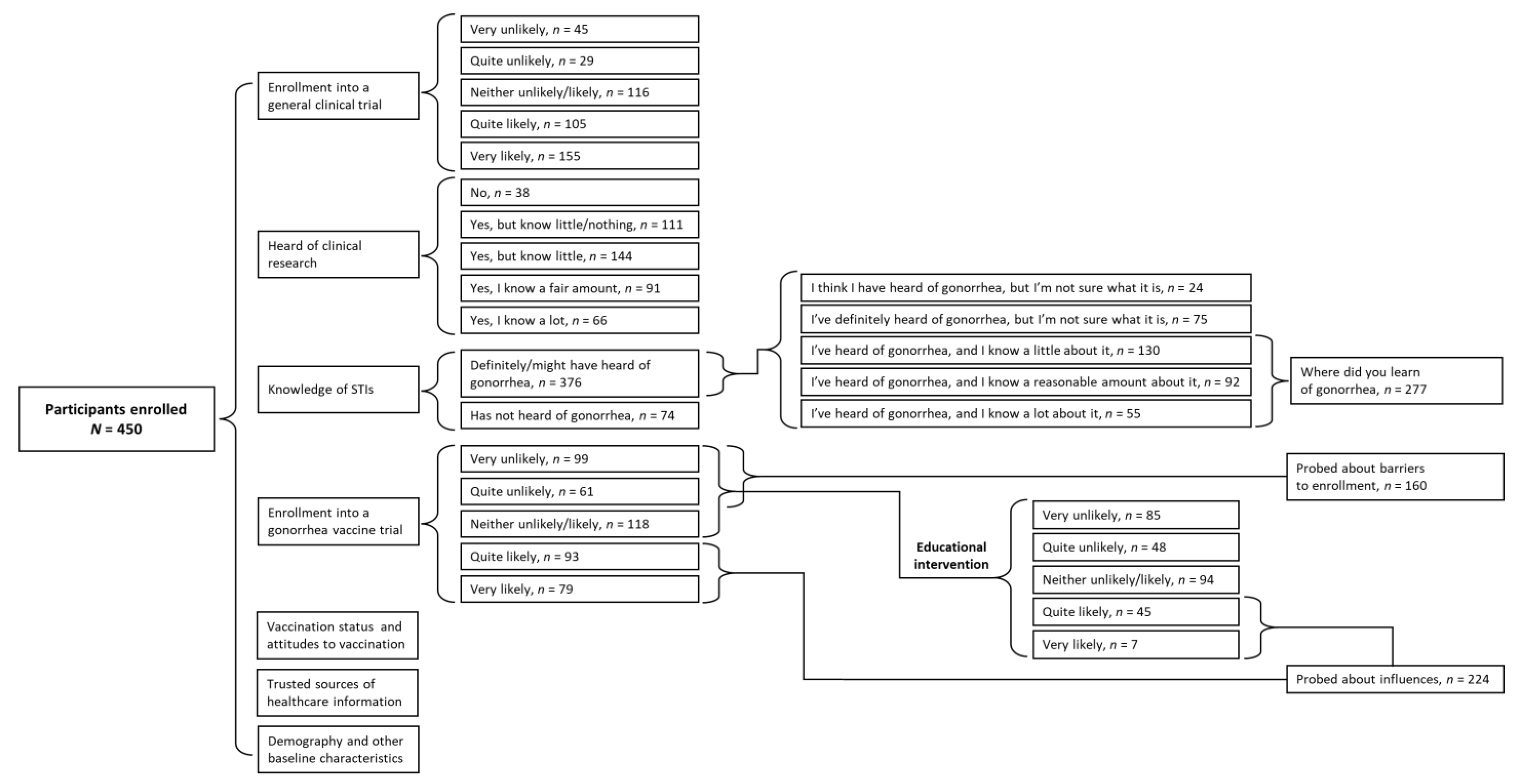
2.2. Participant Enrollment
2.3. Survey Design
2.4. Data Collection and Ethics Approval
2.5. Statistical Analysis
3. Results
3.1. Participants
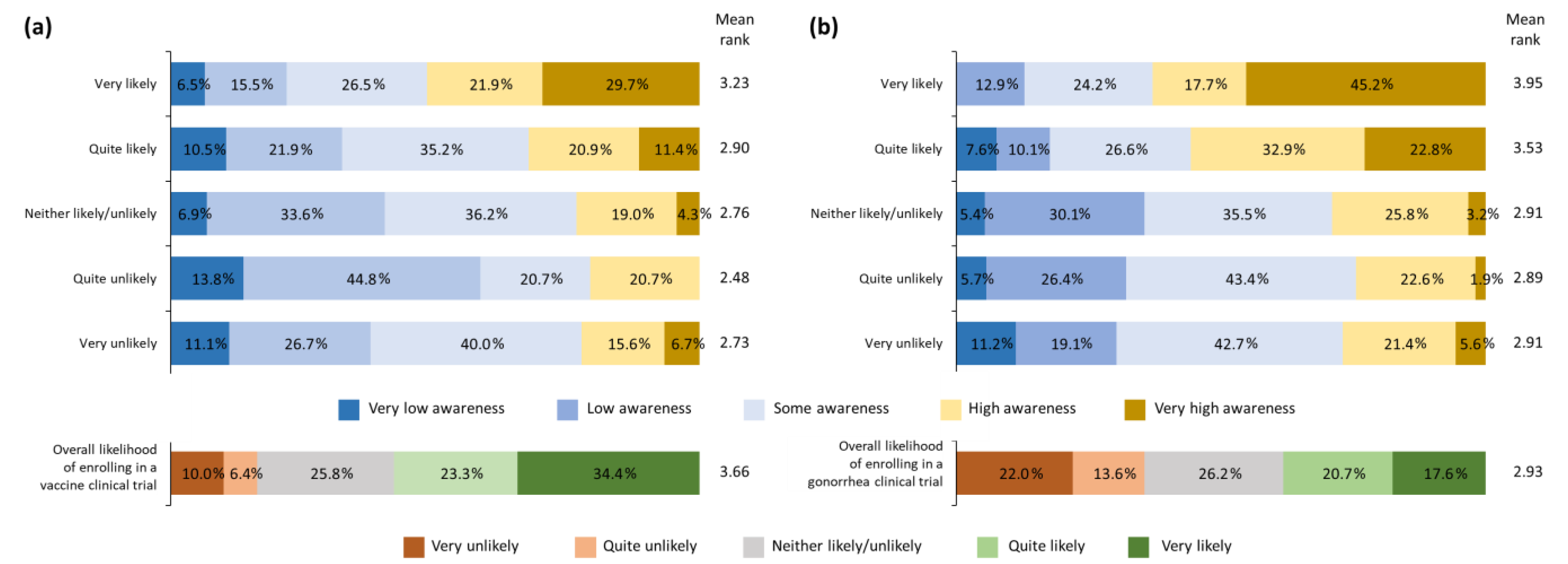
3.2. Willingness to Enroll in General Vaccine Clinical Trials
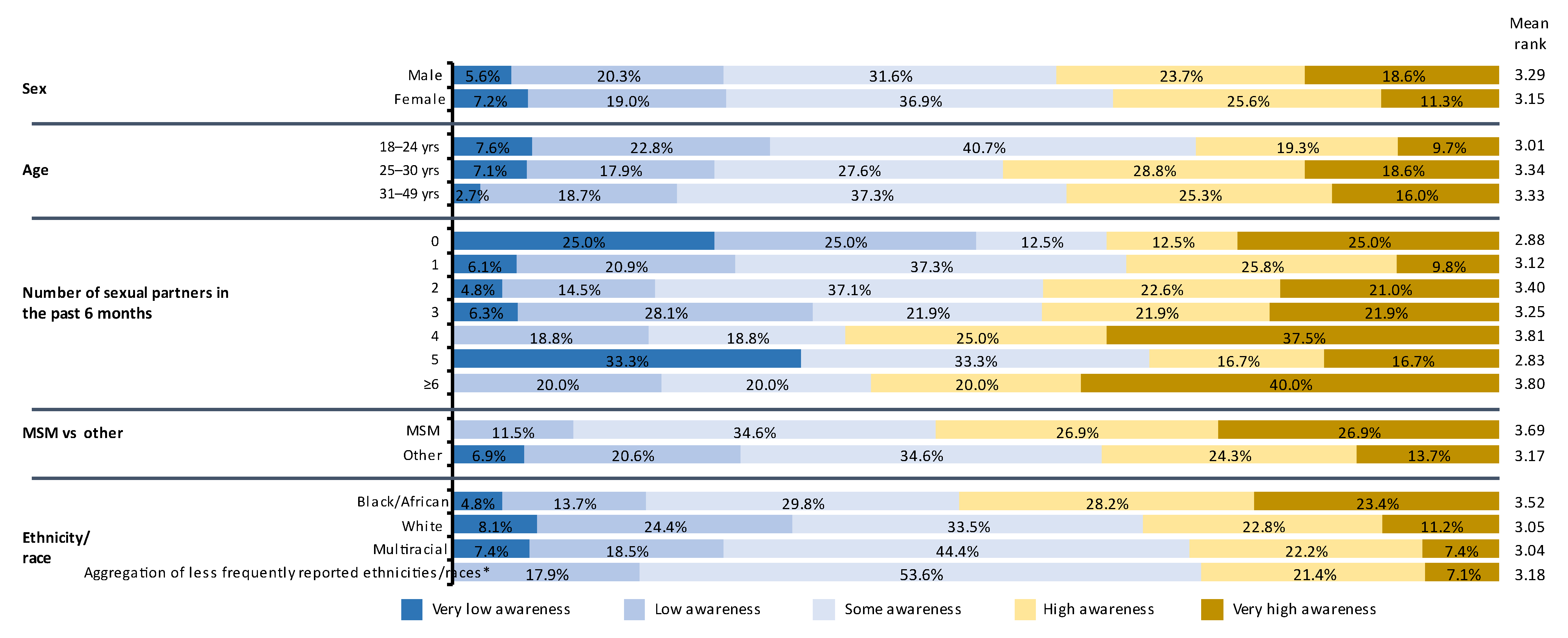
3.3. STD Knowledge and Awareness
3.4. Willingness to Enroll in a Gonorrhea Vaccine Trial
3.5. Change in Willingness to Enroll in a Gonorrhea Vaccine Trial following Basic Educational Intervention
3.6. Factors Influencing Willingness to Get Vaccinated and Enroll in Clinical Trials
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Sexually Transmitted Infections Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 18 January 2023).
- WHO. Multi-Drug Resistant Gonorrhoea Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea (accessed on 18 January 2023).
- CDC. National Overview of Sexually Transmitted Diseases. Available online: https://www.cdc.gov/std/statistics/2020/overview.htm#Disparities (accessed on 18 January 2023).
- Haese, E.C.; Thai, V.C.; Kahler, C.M. Vaccine candidates for the control and prevention of the sexually transmitted disease gonorrhea. Vaccines 2021, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Abara, W.E.; Bernstein, K.T.; Lewis, F.M.T.; Schillinger, J.A.; Feemster, K.; Pathela, P.; Hariri, S.; Islam, A.; Eberhart, M.; Cheng, I.; et al. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: A retrospective observational study. Lancet Infect. Dis. 2022, 22, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Giles, L.; Andraweera, P.; McMillan, M.; Almond, S.; Beazley, R.; Mitchell, J.; Lally, N.; Ahoure, M.; Denehy, E.; et al. Effectiveness and impact of the 4CMenB vaccine against invasive serogroup B meningococcal disease and gonorrhoea in an infant, child, and adolescent programme: An observational cohort and case-control study. Lancet Infect. Dis. 2022, 22, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef]
- McKinsey & Company. Fast-Forward: Will the Speed of COVID-19 Vaccine Development Reset Industry Norms? Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/fast-forward-will-the-speed-of-covid-19-vaccine-development-reset-industry-norms (accessed on 18 January 2023).
- Unemo, M.; Seifert, H.S.; Hook, E.W., III; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. NCT04350138. Available online: https://clinicaltrials.gov/ct2/show/NCT04350138 (accessed on 10 January 2023).
- ClinicalTrials.gov. NCT04415424. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04415424 (accessed on 10 January 2023).
- CDC. Reported STDs in the United States Fact Sheet. Available online: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/STD-Trends-508.pdf (accessed on 18 January 2023).
- Abara, W.E.; Jerse, A.E.; Hariri, S.; Kirkcaldy, R.D. Planning for a gonococcal vaccine: A narrative review of vaccine development and public health implications. Sex Transm. Dis. 2021, 48, 453–457. [Google Scholar] [CrossRef]
- CDC. Sexually Transmitted Disease Surveillance Report. Available online: https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf (accessed on 18 January 2023).
- Wayal, S.; Reid, D.; Weatherburn, P.; Blomquist, P.; Fabiane, S.; Hughes, G.; Mercer, C.H. Association between knowledge, risk behaviours, and testing for sexually transmitted infections among men who have sex with men: Findings from a large online survey in the United Kingdom. HIV Med. 2019, 20, 523–533. [Google Scholar] [CrossRef]
- Balán, I.C.; Lopez-Rios, J.; Dolezal, C.; Rael, C.T.; Lentz, C. Low sexually transmissible infection knowledge, risk perception and concern about infection among men who have sex with men and transgender women at high risk of infection. Sex. Health 2019, 16, 580–586. [Google Scholar] [CrossRef]
- Flores, L.E.; Frontera, W.R.; Andrasik, M.P.; Del Rio, C.; Mondríguez-González, A.; Price, S.A.; Krantz, E.M.; Pergam, S.A.; Silver, J.K. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Netw. Open 2021, 4, e2037640. [Google Scholar] [CrossRef]
- Castillo-Mancilla, J.R.; Cohn, S.E.; Krishnan, S.; Cespedes, M.; Floris-Moore, M.; Schulte, G.; Pavlov, G.; Mildvan, D.; Smith, K.Y.; ACTG Underrepresented Populations Survey Group. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin. Trials 2014, 15, 14–26. [Google Scholar] [CrossRef]
- Dube, E.; MacDonald, N.E. COVID-19 vaccine hesitancy. Nat. Rev. Nephrol. 2022, 18, 409–410. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Moeed, A.; Naeem, U.; Asghar, M.S.; Chughtai, N.U.; Yousaf, Z.; Seboka, B.T.; Ullah, I.; Lin, C.Y.; et al. COVID-19 vaccine hesitancy in the United States: A systematic review. Front. Public Health 2021, 9, 770985. [Google Scholar] [CrossRef]
- Duong, T.V.; Lin, C.Y.; Chen, S.C.; Huang, Y.K.; Okan, O.; Dadaczynski, K.; Lai, C.F. Oxford COVID-19 vaccine hesitancy in school principals: Impacts of gender, well-being, and coronavirus-related health literacy. Vaccines 2021, 9, 985. [Google Scholar] [CrossRef]
- Kahn, B.; Brown, L.; Foege, W.; Gayle, H. (Eds.) National Academies of Sciences, Engineering, and Medicine. In Framework for Equitable Allocation of COVID-19 Vaccine; The National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Zhang, H.; Li, Y.; Peng, S.; Jiang, Y.; Jin, H.; Zhang, F. The effect of health literacy on COVID-19 vaccine hesitancy among community population in China: The moderating role of stress. Vaccine 2022, 40, 4473–4478. [Google Scholar] [CrossRef]
- Fukuda, Y.; Ando, S.; Fukuda, K. Knowledge and preventive actions toward COVID-19, vaccination intent, and health literacy among educators in Japan: An online survey. PLoS ONE 2021, 16, e0257552. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Yu, L.M.; Freeman, J.; Chadwick, A.; Vaccari, C.; Shanyinde, M.; Harris, V.; Waite, F.; Rosebrock, L.; et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): A single-blind, parallel-group, randomised controlled trial. Lancet Public Health 2021, 6, e416–e427. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. Available online: https://www.fda.gov/media/127712/download (accessed on 10 December 2022).
- Chaudhari, N.; Ravi, R.; Gogtay, N.J.; Thatte, U.M. Recruitment and retention of the participants in clinical trials: Challenges and solutions. Perspect. Clin. Res. 2020, 11, 64–69. [Google Scholar]
- Cobb, E.M.; Singer, D.C.; Davis, M.M. Public interest in medical research participation: Differences by volunteer status and study type. Clin. Transl. Sci. 2014, 7, 145–149. [Google Scholar] [CrossRef]
- Kadam, R.A.; Borde, S.U.; Madas, S.A.; Salvi, S.S.; Limaye, S.S. Challenges in recruitment and retention of clinical trial subjects. Perspect. Clin. Res. 2016, 7, 137–143. [Google Scholar] [CrossRef]
- Celum, C.; Barnabas, R.; Cohen, M.S.; Collier, A.; El-Sadr, W.; Holmes, K.K.; Johnston, C.; Piot, P. COVID-19, ebola, and HIV-Leveraging lessons to maximize impact. N. Engl. J. Med. 2020, 383, e106. [Google Scholar] [CrossRef]
- Ashkenazi, S.; Livni, G.; Klein, A.; Kremer, N.; Havlin, A.; Berkowitz, O. The relationship between parental source of information and knowledge about measles/measles vaccine and vaccine hesitancy. Vaccine 2020, 38, 7292–7298. [Google Scholar] [CrossRef] [PubMed]
- Machingaidze, S.; Wiysonge, C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021, 27, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- CDC. Sexually Transmitted Infections Prevalence, Incidence, and Cost Estimates in the United States. Available online: https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm (accessed on 2 March 2023).
- Unemo, M.; Sikora, A.E. Infection: Proof of principle for effectiveness of a gonorrhoea vaccine. Nat. Rev. Urol. 2017, 14, 643–644. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Duran, N.; Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 2014, 104, e16–e31. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Parsons Leigh, J.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 13, 3801. [Google Scholar] [CrossRef]
- Solis Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Gendler, Y.; Ofri, L. Investigating the influence of vaccine literacy, vaccine perception and vaccine hesitancy on Israeli parents’ acceptance of the COVID-19 vaccine for their children: A cross-sectional study. Vaccines 2021, 9, 1391. [Google Scholar] [CrossRef]
- Magon, A.; Arrigoni, C.; Graffigna, G.; Barello, S.; Moia, M.; Palareti, G.; Caruso, R. The effect of health literacy on vaccine hesitancy among Italian anticoagulated population during COVID-19 pandemic: The moderating role of health engagement. Hum. Vaccin. Immunother. 2021, 17, 5007–5012. [Google Scholar] [CrossRef]
- Voo, J.Y.H.; Lean, Q.Y.; Ming, L.C.; Md Hanafiah, N.H.; Al-Worafi, Y.M.; Ibrahim, B. Vaccine knowledge, awareness and hesitancy: A cross sectional survey among parents residing at Sandakan District, Sabah. Vaccines 2021, 9, 1348. [Google Scholar] [CrossRef]
- Khalil, L.; Leary, M.; Rouphael, N.; Ofotokun, I.; Rebolledo, P.A.; Wiley, Z. Racial and ethnic diversity in SARS-CoV-2 vaccine clinical trials conducted in the United States. Vaccines 2022, 10, 290. [Google Scholar] [CrossRef]
- Troiano, G.; Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef]
- U.S. Census Bureau. QuickFacts: Population Estimates: United States, 1 July 2021 (V2021). Available online: https://www.census.gov/quickfacts/fact/table/US/PST045221 (accessed on 18 January 2023).
- Sallam, M. COVID-19 vaccine hesitancy worldwide: A concise systematic review of vaccine acceptance rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Svendsen, M.T.; Bak, C.K.; Sorensen, K.; Pelikan, J.; Riddersholm, S.J.; Skals, R.K.; Mortensen, R.N.; Maindal, H.T.; Boggild, H.; Nielsen, G.; et al. Associations of health literacy with socioeconomic position, health risk behavior, and health status: A large national population-based survey among Danish adults. BMC Public Health 2020, 20, 565. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. Educational Attainment in the United States: 2020. Available online: https://www.census.gov/data/tables/2020/demo/educational-attainment/cps-detailed-tables.html (accessed on 18 January 2023).
- Buchbinder, S.P.; Metch, B.; Holte, S.E.; Scheer, S.; Coletti, A.; Vittinghoff, E. Determinants of enrollment in a preventive HIV vaccine trial: Hypothetical versus actual willingness and barriers to participation. Acquir. Immune Defic. Syndr. 2004, 36, 604–612. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Participants, n (%) |
|---|---|
| Sex | |
| Male | 218 (48.4) |
| Female | 225 (50.0) |
| Non-binary/prefer not to say | 7 (1.6) |
| Age group (years) | |
| 18–24 | 184 (40.9) |
| 25–30 | 176 (39.1) |
| 31–45 | 90 (20.0) |
| Ethnicity/race | |
| White | 236 (52.4) |
| Black/African American | 142 (31.6) |
| Mixed race | 34 (7.6) |
| Aggregation of less frequently reported ethnicities/races * | 38 (8.4) |
| MSM | |
| MSM | 32 (7.1) |
| Other | 418 (92.9) |
| Sexual partners in the past 6 months | |
| 0 | 12 (2.7) |
| 1 | 288 (64.0) |
| 2 | 76 (16.9) |
| ≥3 † | 71 (15.8) |
| Prefer not to say | 3 (0.7) |
| Educational level | |
| College graduate | 308 (68.4) |
| Non-college graduate | 139 (30.9) |
| Prefer not to say | 3 (0.7) |
| Income ($) | |
| Under $15,000 $15,000–$24,999 $25,000–$34,999 $35,000–$49,999 $50,000–$74,999 $75,000–$99,999 $100,000–$149,999 $150,000–$199,999 $200,000 and over Prefer not to say | 54 (12.0) 42 (9.3) 73 (16.2) 57 (12.7) 84 (18.7) 49 (10.9) 57 (12.7) 22 (4.9) 8 (1.8) 4 (0.9) |
| Ethnicity/Race | Open to Vaccination (%) n = 350 | Not open to Vaccination (%) n = 100 | Vaccinated (%) n = 386 | Not Vaccinated (%) n = 64 |
|---|---|---|---|---|
| Black/African American | 69.7 | 30.3 | 79.6 | 20.4 |
| White | 82.2 | 17.8 | 88.6 | 11.4 |
| Multiracial/multi-ethnic | 79.4 | 20.6 | 88.2 | 11.8 |
| Aggregation of less frequently reported ethnicities/races * | 78.9 | 21.1 | 89.5 | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penlington, M.; Nicolay, U.; Galgani, I. The Impact of Educational Intervention on Willingness to Enroll in a Clinical Trial of a Gonorrhea Vaccine. Vaccines 2023, 11, 648. https://doi.org/10.3390/vaccines11030648
Penlington M, Nicolay U, Galgani I. The Impact of Educational Intervention on Willingness to Enroll in a Clinical Trial of a Gonorrhea Vaccine. Vaccines. 2023; 11(3):648. https://doi.org/10.3390/vaccines11030648
Chicago/Turabian StylePenlington, Michael, Uwe Nicolay, and Ilaria Galgani. 2023. "The Impact of Educational Intervention on Willingness to Enroll in a Clinical Trial of a Gonorrhea Vaccine" Vaccines 11, no. 3: 648. https://doi.org/10.3390/vaccines11030648
APA StylePenlington, M., Nicolay, U., & Galgani, I. (2023). The Impact of Educational Intervention on Willingness to Enroll in a Clinical Trial of a Gonorrhea Vaccine. Vaccines, 11(3), 648. https://doi.org/10.3390/vaccines11030648






