Risks and Preventions for Pregnant Women and Their Preterm Infants in a World with COVID-19: A Narrative Review
Abstract
1. Introduction
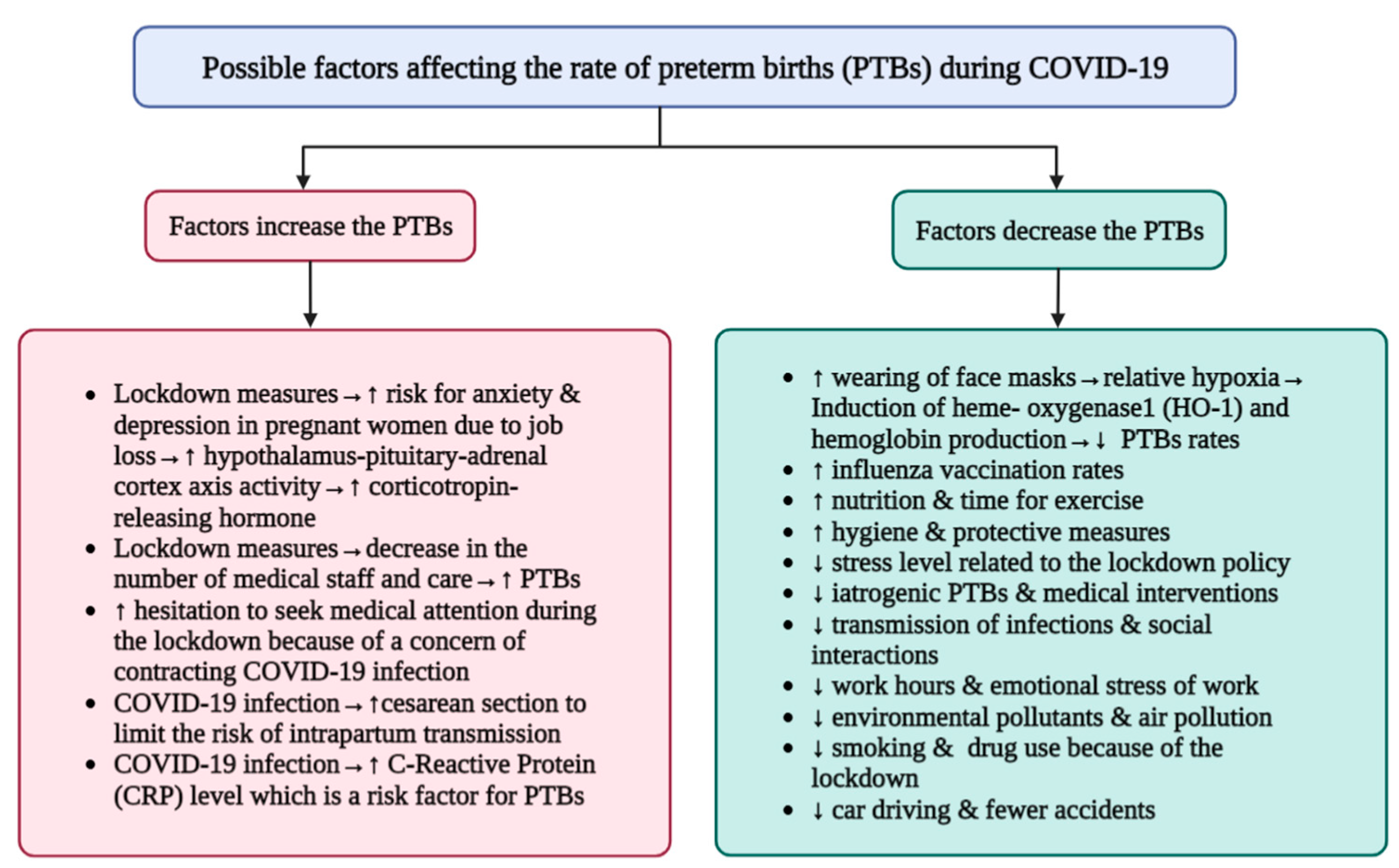
2. Preterm Delivery Rates: Did the COVID-19 Pandemic Cause an Increase or Decrease in Preterm Birth Rates?
| Author(s), Year | Study Type | Country | Study Subject | Outcomes | PTB Incidence | Ref |
|---|---|---|---|---|---|---|
| Allotey J et al., 2020 | A systematic review and Meta-analysis | Global | Pregnant and recently pregnant women with COVID-19 (n = 293,152), and non-pregnant women with COVID-19 (n = 2,903,149) |
| Increased | [9] |
| Woodworth K et al., 2020 | Cohort study | United States | Pregnant women with COVID-19 (n = 5252), Infants born to COVID-19-infected mothers (n = 3912) |
| Increased | [15] |
| Bobei T et al., 2022 | Prospective cohort observational study | Romania | Pregnant women infected with COVID-19 and having preterm birth (n = 34), healthy women having preterm birth (n = 48). |
| Increased | [16] |
| Khalil, A et al., 2020 | A systematic review and Meta-analysis | Global | Pregnant women with COVID-19 (n = 2567) |
| Increased | [17] |
| Singh V et al., 2021 | Retrospective Observational Study | East India | Pregnant women with COVID-19 (n = 132) |
| Increased | [20] |
| Pierce-Williams R et al., 2020 | Cohort Study | United States | Hospitalized pregnant women with COVID-19 (n = 64) |
| Increased | [21] |
| Patil, U.P et al., 2020 | Retrospective cross-sectional study | United States | live births to mothers who had COVID-19 infection testing (n = 118) |
| Increased | [22] |
| Villar, J et al., 2021 | Paired Controlled Study | 18 countries | Paired comparison between pregnant women with (n = 706) and without (n = 1425) COVID-19 |
| Increased | [30] |
| Jafari M et al., 2021 | A systemic review and Meta-analysis | Global | Meta Analysis of 228 studies of nonpregnant patients(n = 128,176) and 121 studies including pregnant patients (n = 10,000) |
| Increased | [31] |
| Berghella V et al., 2020 | Meta-Analysis | United States | Total births during COVID-19 (n = 1197), births before COVID-19 (n = 911) |
| Decreased | [25] |
| Meyer R et al., 2021 | Cohort Study | Israel |
|
| Decreased | [26] |
| Hedermann G. et al., 2021 | Nationwide prevalence proportion study (Observational Study) | Denmark | Live births born between March 12 and April 14, 2015-2020 (n = 31,180) |
| Decreased in extreme PTB | [27] |
| Philip R.K. et al., 2020 | Retrospective descriptive study (Cohort Study) | Ireland | Live births of very low birth weight (VLBW) and extremely low birthweight (ELBW) infants from 1 January 2001 to 30 April 2020 |
| Decreased in VLBW and ELBW infants | [28] |
| Jasper B et al., 2022 | Retrospective cohort study | Australia | Births born between April to July 2018–2020 (n = 64,989) |
| Decreased | [29] |
3. COVID-19-Infected Women Giving Birth during the Pandemic: Characteristics and Consequences
| Author(s), Year | Country | Study Subject | Outcomes | Ref. |
|---|---|---|---|---|
| Zambrano, L.D. et al., 2020 | United States | Symptomatic COVID-19 women (n = 409,462), including pregnant women (n = 23,434) |
| [33] |
| Chinn, J. et al., 2021 | United States | Adult women (n = 869,079) including women with COVID-19 (n = 18,715) |
| [34] |
| Nachega, J.B. et al., 2022 | Six countries in Sub-Saharan Africa | Women at reproductive age (n = 1315) |
| [35] |
| DeSisto, C.L. et al., 2021 | United States | Delivery hospitalizations (n = 1,249,634) including 21,653 deliveries to COVID-19 infected women. |
| [36] |
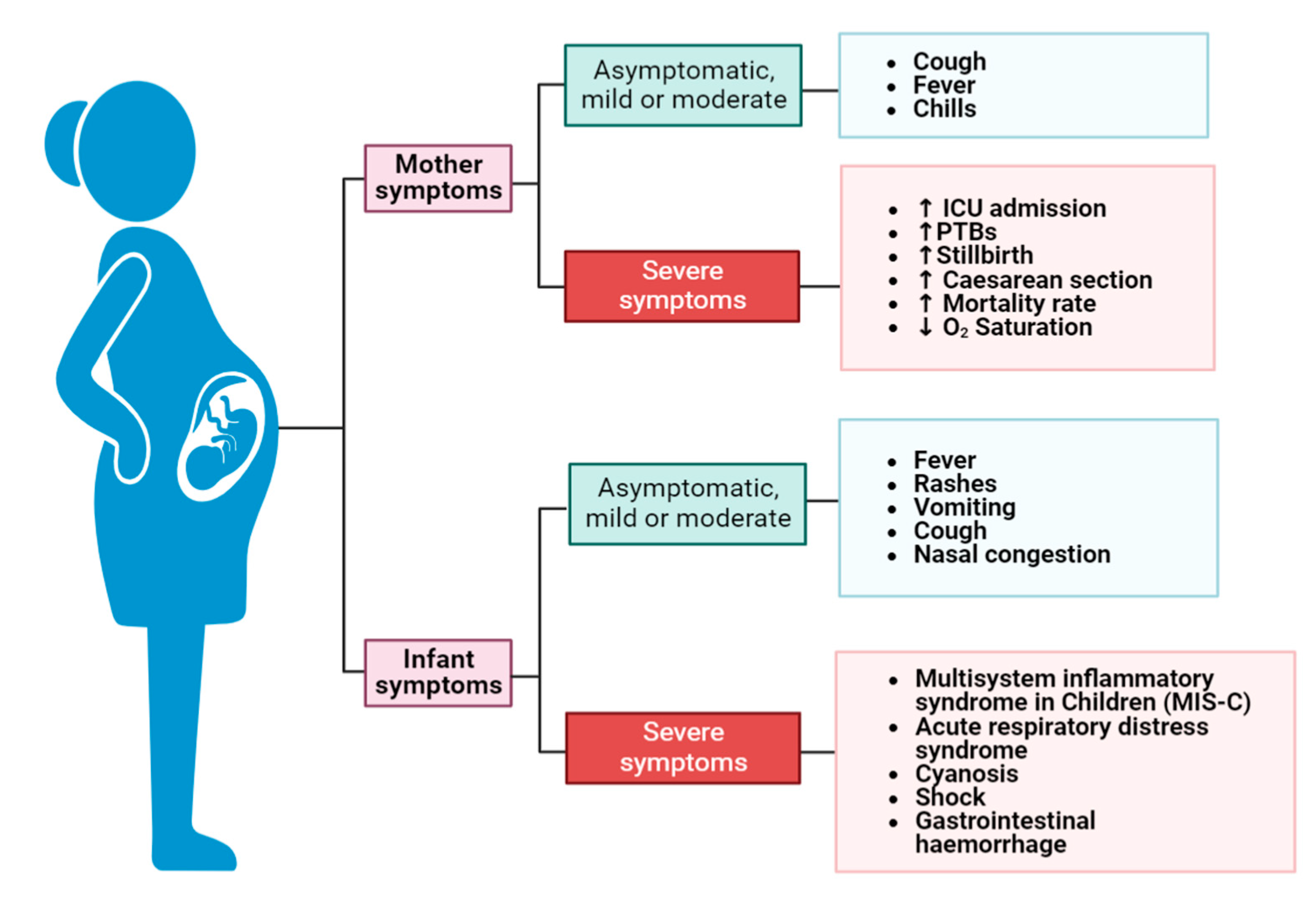
4. Clinical Features and Short-Term Complications of Preterm Infants
5. COVID-19 Vaccines: Safety and Efficacy during Pregnancy
| Name | Pfizer/BioNTech | Moderna | SinoVac, Sinopharm | Bharat Biotech (Covaxin) | Serum Institute of India (Covishield) | Oxford/AstraZeneca | Janssen Biotech, Inc. (Johnson & Johnson) |
|---|---|---|---|---|---|---|---|
| Age | ≥12 | ≥18 | |||||
| Dosage | 2 doses | Single dose | |||||
| Dose interval | 3 weeks | 4 weeks | 3–4 weeks | 4–6 weeks | 12–16 weeks | 8–12 weeks | - |
| Type of vaccine | mRNA | Inactivated virus | Adenoviral vector | ||||
| Side effects | Local pain, redness, swelling, fatigue, headache, muscle pain, nausea and vomiting, and fever | ||||||
| Severe allergic reaction, myocarditis, and pericarditis | Diarrhea, cough, Joint pain, and allergies | Itching, rashes, and allergic reaction | Influenza-like illness, thrombocytopenia, and venous thrombotic events | Guillain-Barre syndrome, thrombosis with thrombocytopenia syndrome, and post-vaccination syndrome | |||
| References | [2,10,58,63,64,65,66] | ||||||
6. Modes of COVID-19 Infection Transmission from the Mother to the Fetus
7. Prevention, Management, and NICU Admission of Preterm Infants of COVID-19-Infected Mothers
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Kelley, C.F.; Horton, J.P.; Jamieson, D. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: What obstetricians need to know. J. Obstet. Gynaecol. 2021, 137, 408. [Google Scholar] [CrossRef] [PubMed]
- Dennison Himmelfarb, C.R.; Baptiste, D. Coronavirus Disease (COVID-19): Implications for Cardiovascular and Socially At-risk Populations. J. Cardiovasc. Nurs. 2020, 35, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Hancean, M.G.; Lerner, J.; Perc, M.; Oana, I.; Bunaciu, D.A.; Stoica, A.A.; Ghita, M.C. Occupations and their impact on the spreading of COVID-19 in urban communities. Sci. Rep. 2022, 12, 14115. [Google Scholar] [CrossRef]
- Hancean, M.G.; Ghita, M.C.; Perc, M.; Lerner, J.; Oana, I.; Mihaila, B.E.; Stoica, A.A.; Bunaciu, D.A. Disaggregated data on age and sex for the first 250 days of the COVID-19 pandemic in Bucharest, Romania. Sci. Data 2022, 9, 253. [Google Scholar] [CrossRef]
- Karasek, D.; Baer, R.J.; McLemore, M.R.; Bell, A.J.; Blebu, B.E.; Casey, J.A.; Coleman-Phox, K.; Costello, J.M.; Felder, J.N.; Flowers, E.; et al. The association of COVID-19 infection in pregnancy with preterm birth: A retrospective cohort study in California. Lancet Reg. Health Am. 2021, 2, 100027. [Google Scholar] [CrossRef]
- Matar, R.; Alrahmani, L.; Monzer, N.; Debiane, L.G.; Berbari, E.; Fares, J.; Fitzpatrick, F.; Murad, M.H. Clinical Presentation and Outcomes of Pregnant Women With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2020, 72, 521–533. [Google Scholar] [CrossRef]
- Wainstock, T.; Yoles, I.; Sergienko, R.; Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021, 39, 6037–6040. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. COVID-19 Vaccine in Pregnant and Lactating Women: A Review of Existing Evidence and Practice Guidelines. Infect. Dis. Rep. 2021, 13, 685–699. [Google Scholar] [CrossRef]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; Perry, A.; Bourne, T.; Lees, C.C. Pregnancy and neonatal outcomes of COVID-19: Coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021, 57, 573–581. [Google Scholar] [CrossRef]
- Zeng, L.; Xia, S.; Yuan, W.; Yan, K.; Xiao, F.; Shao, J.; Zhou, W. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. JAMA Pediatr. 2020, 174, 722–725. [Google Scholar] [CrossRef]
- Papapanou, M.; Papaioannou, M.; Petta, A.; Routsi, E.; Farmaki, M.; Vlahos, N.; Siristatidis, C. Maternal and Neonatal Characteristics and Outcomes of COVID-19 in Pregnancy: An Overview of Systematic Reviews. Int. J. Environ. Res. Public Health 2021, 18, 596. [Google Scholar] [CrossRef]
- Quinn, J.A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29-October 14, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1635–1640. [Google Scholar] [CrossRef]
- Bobei, T.I.; Haj Hamoud, B.; Sima, R.M.; Gorecki, G.P.; Poenaru, M.O.; Olaru, O.G.; Ples, L. The Impact of SARS-CoV-2 Infection on Premature Birth-Our Experience as COVID Center. Medicina 2022, 58, 587. [Google Scholar] [CrossRef]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Heath, P.; Ladhani, S.; et al. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020, 25, 100446. [Google Scholar] [CrossRef]
- Elshafeey, F.; Magdi, R.; Hindi, N.; Elshebiny, M.; Farrag, N.; Mahdy, S.; Sabbour, M.; Gebril, S.; Nasser, M.; Kamel, M.; et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 150, 47–52. [Google Scholar] [CrossRef]
- Smith, V.; Seo, D.; Warty, R.; Payne, O.; Salih, M.; Chin, K.L.; Ofori-Asenso, R.; Krishnan, S.; da Silva Costa, F.; Vollenhoven, B.; et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS ONE 2020, 15, e0234187. [Google Scholar] [CrossRef]
- Singh, V.; Choudhary, A.; Datta, M.R.; Ray, A. Maternal and Neonatal Outcomes of COVID-19 in Pregnancy: A Single-Centre Observational Study. Cureus 2021, 13, e13184. [Google Scholar] [CrossRef]
- Pierce-Williams, R.A.M.; Burd, J.; Felder, L.; Khoury, R.; Bernstein, P.S.; Avila, K.; Penfield, C.A.; Roman, A.S.; DeBolt, C.A.; Stone, J.L.; et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: A United States cohort study. Am. J. Obstet. Gynecol. MFM 2020, 2, 100134. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.P.; Maru, S.; Krishnan, P.; Carroll-Bennett, R.; Sanchez, J.; Noble, L.; Wasserman, R. Newborns of COVID-19 mothers: Short-term outcomes of colocating and breastfeeding from the pandemic’s epicenter. J. Perinatol. 2020, 40, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulos, L.; Myers, T.R.; Gee, J.; Lipkind, H.S.; Kharbanda, E.O.; Ryan, D.S.; Williams, J.T.B.; Naleway, A.L.; Klein, N.P.; Hambidge, S.J.; et al. SARS-CoV-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics—Eight U.S. Health Care Centers, March 1-May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J.; Whitaker, M.; O’Halloran, A.; Chai, S.J.; Kirley, P.D.; Alden, N.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; Anderson, E.J.; et al. Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19—COVID-NET, 13 States, March 1–August 22, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1347–1354. [Google Scholar] [CrossRef]
- Berghella, V.; Boelig, R.; Roman, A.; Burd, J.; Anderson, K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am. J. Obstet. Gynecol. MFM 2020, 2, 100258. [Google Scholar] [CrossRef]
- Meyer, R.; Bart, Y.; Tsur, A.; Yinon, Y.; Friedrich, L.; Maixner, N.; Levin, G. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am. J. Obstet. Gynecol. 2021, 224, 234–237. [Google Scholar] [CrossRef]
- Hedermann, G.; Hedley, P.L.; Baekvad-Hansen, M.; Hjalgrim, H.; Rostgaard, K.; Poorisrisak, P.; Breindahl, M.; Melbye, M.; Hougaard, D.M.; Christiansen, M.; et al. Danish premature birth rates during the COVID-19 lockdown. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 93–95. [Google Scholar] [CrossRef]
- Philip, R.K.; Purtill, H.; Reidy, E.; Daly, M.; Imcha, M.; McGrath, D.; O’Connell, N.H.; Dunne, C.P. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: A ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob. Health 2020, 5, e003075. [Google Scholar] [CrossRef]
- Jasper, B.; Stillerova, T.; Anstey, C.; Weaver, E. Reduction in preterm birth rates during and after the COVID-19 lockdown in Queensland Australia. Aust. New Zealand J. Obstet. Gynaecol. 2022, 62, 851–858. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Jafari, M.; Pormohammad, A.; Sheikh Neshin, S.A.; Ghorbani, S.; Bose, D.; Alimohammadi, S.; Basirjafari, S.; Mohammadi, M.; Rasmussen-Ivey, C.; Razizadeh, M.H.; et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, 1–16. [Google Scholar] [CrossRef]
- Ntounis, T.; Prokopakis, I.; Koutras, A.; Fasoulakis, Z.; Pittokopitou, S.; Valsamaki, A.; Chionis, A.; Kontogeorgi, E.; Lampraki, V.; Peraki, A.; et al. Pregnancy and COVID-19. J. Clin. Med. 2022, 11, 6645. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., 3rd; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22-October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Chinn, J.; Sedighim, S.; Kirby, K.A.; Hohmann, S.; Hameed, A.B.; Jolley, J.; Nguyen, N.T. Characteristics and Outcomes of Women with COVID-19 Giving Birth at US Academic Centers during the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, 6–11. [Google Scholar] [CrossRef]
- Nachega, J.B.; Sam-Agudu, N.A.; Machekano, R.N.; Rosenthal, P.J.; Schell, S.; de Waard, L.; Bekker, A.; Gachuno, O.W.; Kinuthia, J.; Mwongeli, N.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Pregnancy in Sub-Saharan Africa: A 6-Country Retrospective Cohort Analysis. Clin. Infect. Dis. 2022, 75, 1950–1961. [Google Scholar] [CrossRef]
- DeSisto, C.L.; Wallace, B.; Simeone, R.M.; Polen, K.; Ko, J.Y.; Meaney-Delman, D.; Ellington, S.R. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization—United States, March 2020–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1640–1645. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Sayad, R.; Mahmoud, I.A.; El-Monem, A.M.A.; Badry, S.H.; Ibrahim, I.H.; Hafez, M.H.R.; El-Mokhtar, M.A.; Sayed, I.M. “Anosmia” the mysterious collateral damage of COVID-19. J. NeuroVirology 2022, 28, 189–200. [Google Scholar] [CrossRef]
- De Rose, D.U.; Piersigilli, F.; Ronchetti, M.P.; Santisi, A.; Bersani, I.; Dotta, A.; Danhaive, O.; Auriti, C.; Study Group of Neonatal Infectious Diseases of The Italian Society of Neonatology. Novel Coronavirus disease (COVID-19) in newborns and infants: What we know so far. Ital. J. Pediatr. 2020, 46, 56. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Warner, S.; Richter, A.; Stamataki, Z.; Kelly, D. Understanding COVID-19: Are children the key? BMJ Paediatr. Open 2021, 5, e001063. [Google Scholar] [CrossRef]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, C.M.; Chang, K.T.; Brown, M.M.; Chu, V.T.; Yousaf, A.R.; Anyalechi, N.; Aryee, P.A.; Kirking, H.L.; Lumsden, M.; Mayweather, E.; et al. SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp—Georgia, June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, E.; Levin, T.L. COVID-19 in pediatric patients: A case series from the Bronx, NY. Pediatr. Radiol. 2020, 50, 1369–1374. [Google Scholar] [CrossRef]
- Vimercati, A.; De Nola, R.; Trerotoli, P.; Metta, M.E.; Cazzato, G.; Resta, L.; Malvasi, A.; Lepera, A.; Ricci, I.; Capozza, M.; et al. COVID-19 Infection in Pregnancy: Obstetrical Risk Factors and Neonatal Outcomes-A Monocentric, Single-Cohort Study. Vaccines 2022, 10, 166. [Google Scholar] [CrossRef]
- Angelidou, A.; Sullivan, K.; Melvin, P.R.; Shui, J.E.; Goldfarb, I.T.; Bartolome, R.; Chaudhary, N.; Vaidya, R.; Culic, I.; Singh, R.; et al. Association of Maternal Perinatal SARS-CoV-2 Infection with Neonatal Outcomes during the COVID-19 Pandemic in Massachusetts. JAMA Netw. Open 2021, 4, e217523. [Google Scholar] [CrossRef]
- Mackin, D.W.; Walker, S.P. The historical aspects of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 13–22. [Google Scholar] [CrossRef]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Syan, S.K.; Gohari, M.R.; Levitt, E.E.; Belisario, K.; Gillard, J.; DeJesus, J.; MacKillop, J. COVID-19 Vaccine Perceptions and Differences by Sex, Age, and Education in 1367 Community Adults in Ontario. Front. Public Health 2021, 9, 719665. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Bookstein Peretz, S.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Levin, E.G.; Regev Yochay, G.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e154834. [Google Scholar] [CrossRef]
- Shanes, E.D.; Otero, S.; Mithal, L.B.; Mupanomunda, C.A.; Miller, E.S.; Goldstein, J.A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination in Pregnancy: Measures of Immunity and Placental Histopathology. Obstet. Gynecol. 2021, 138, 281–283. [Google Scholar] [CrossRef]
- Prahl, M.; Golan, Y.; Cassidy, A.G.; Matsui, Y.; Li, L.; Alvarenga, B.; Chen, H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy. Nat. Commun. 2022, 13, 4422. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e301–303.e317. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.R.; Racherla, S.; Tirumala, R.; Madathala, R.R.; Gajula, V. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: A cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 2021, 225, 458–460. [Google Scholar] [CrossRef]
- Prasad, S.; Kalafat, E.; Blakeway, H.; Townsend, R.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Ladhani, S.; et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022, 13, 2414. [Google Scholar] [CrossRef]
- Fu, W.; Sivajohan, B.; McClymont, E.; Albert, A.; Elwood, C.; Ogilvie, G.; Money, D. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynecol. Obstet. 2022, 156, 406–417. [Google Scholar] [CrossRef]
- Piekos, S.N.; Hwang, Y.M.; Roper, R.T.; Sorensen, T.; Price, N.D.; Hood, L.; Hadlock, J.J. The effect of COVID-19 vaccination and booster on maternal-fetal outcomes: A retrospective multicenter cohort study. medRxiv 2022. [Google Scholar] [CrossRef]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; DeRiso, E.A.; Davis, C.; Bordt, E.A.; De Guzman, R.M.; Shook, L.L.; Yonker, L.M.; Fasano, A.; Akinwunmi, B.; Lauffenburger, D.A.; et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021, 13, eabi8631. [Google Scholar] [CrossRef] [PubMed]
- Zavala, E.; Krubiner, C.B.; Jaffe, E.F.; Nicklin, A.; Gur-Arie, R.; Wonodi, C.; Faden, R.R.; Karron, R.A. Global disparities in public health guidance for the use of COVID-19 vaccines in pregnancy. BMJ Glob. Health 2022, 7, e007730. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Liu, Y.; Pang, W.; Zhang, D.; Wang, K.; Chen, Y. Associations of COVID-19 vaccination during pregnancy with adverse neonatal and maternal outcomes: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1044031. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, T.; Chen, Z.; Zhang, L.; Gao, Q.; Liu, G.; Zheng, J.; Ding, F. Systematic review and meta-analysis of neonatal outcomes of COVID-19 vaccination in pregnancy. Pediatr. Res. 2023, 1–9. [Google Scholar] [CrossRef]
- Magon, N.; Prasad, S.; Mahato, C.; Sharma, J.B. COVID-19 vaccine and pregnancy: A safety weapon against pandemic. Taiwan J. Obstet. Gynaecol. 2022, 61, 201–209. [Google Scholar] [CrossRef]
- Lu, Q.; Shi, Y. Coronavirus disease (COVID-19) and neonate: What neonatologist need to know. J. Med. Virol. 2020, 92, 564–567. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Auriti, C.; De Rose, D.U.; Tzialla, C.; Caforio, L.; Ciccia, M.; Manzoni, P.; Stronati, M. Vertical Transmission of SARS-CoV-2 (COVID-19): Are Hypotheses More than Evidences? Am. J. Perinatol. 2020, 37, S31–S38. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020, 20, 559–564. [Google Scholar] [CrossRef]
- Schwartz, D.A. An Analysis of 38 Pregnant Women With COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef]
- Patane, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100145. [Google Scholar] [CrossRef]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine Transmission of SARS-COV-2 Infection in a Preterm Infant. Pediatr. Infect. Dis. J. 2020, 39, e265–e267. [Google Scholar] [CrossRef]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020, 192, E647–E650. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Stagno, S. Can SARS-CoV-2 Infection Be Acquired In Utero?: More Definitive Evidence Is Needed. JAMA 2020, 323, 1788–1789. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 224, 35–53.e33. [Google Scholar] [CrossRef]
- Costa, S.; Posteraro, B.; Marchetti, S.; Tamburrini, E.; Carducci, B.; Lanzone, A.; Valentini, P.; Buonsenso, D.; Sanguinetti, M.; Vento, G.; et al. Excretion of SARS-CoV-2 in human breast milk. Clin. Microbiol. Infect. 2020, 26, 1430–1432. [Google Scholar] [CrossRef]
- Gross, R.; Conzelmann, C.; Muller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Munch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Krogstad, P.; Bertrand, K.; Contreras, D.; Tobin, N.H.; Bode, L.; Aldrovandi, G. Evaluation for SARS-CoV-2 in Breast Milk From 18 Infected Women. JAMA 2020, 324, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Atanu, F.O.; El-Zamkan, M.A.; Diab, H.M.; Ahmed, A.S.; Al-Maiahy, T.J.; Obaidullah, A.J.; Alshehri, S.; Ghoniem, M.M.; et al. Maternal Transmission of SARS-CoV-2: Safety of Breastfeeding in Infants Born to Infected Mothers. Front. Pediatr. 2021, 9, 738263. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, W.; Botha, E.; Niela-Vilen, H.; Reimers, P. Breastfeeding during the COVID-19 pandemic—A literature review for clinical practice. Int. Breastfeed. J. 2020, 15, 82. [Google Scholar] [CrossRef]
- Darcy Mahoney, A.; White, R.D.; Velasquez, A.; Barrett, T.S.; Clark, R.H.; Ahmad, K.A. Impact of restrictions on parental presence in neonatal intensive care units related to coronavirus disease 2019. J. Perinatol. 2020, 40, 36–46. [Google Scholar] [CrossRef]
- WHO. Breastfeeding and COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 (accessed on 1 February 2023).
- Hu, X.; Gao, J.; Wei, Y.; Chen, H.; Sun, X.; Chen, J.; Luo, X.; Chen, L. Managing Preterm Infants Born to COVID-19 Mothers: Evidence from a Retrospective Cohort Study in Wuhan, China. Neonatology 2020, 117, 592–598. [Google Scholar] [CrossRef]
- Society of Pediatrics, C.M.A.; Editorial Board, C.J.o.P. [Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children (first interim edition)]. Zhonghua Er Ke Za Zhi Chin. J. Pediatr. 2020, 58, 169–174. [Google Scholar] [CrossRef]
- Yeo, K.T.; Biswas, A.; Ying Ho, S.K.; Kong, J.Y.; Bharadwaj, S.; Chinnadurai, A.; Yip, W.Y.; Ab Latiff, N.F.; Quek, B.H.; Yeo, C.L.; et al. Guidance for the clinical management of infants born to mothers with suspected/confirmed COVID-19 in Singapore. Singap. Med. J. 2022, 63, 489–496. [Google Scholar] [CrossRef]
- Tegethoff, M.; Pryce, C.; Meinlschmidt, G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: A systematic review. Endocr. Rev. 2009, 30, 753–789. [Google Scholar] [CrossRef]
- Magala Ssekandi, A.; Sserwanja, Q.; Olal, E.; Kawuki, J.; Bashir Adam, M. Corticosteroids Use in Pregnant Women with COVID-19: Recommendations from Available Evidence. J. Multidiscip. Healthc. 2021, 14, 659–663. [Google Scholar] [CrossRef]
- Vardhelli, V.; Pandita, A.; Pillai, A.; Badatya, S.K. Perinatal COVID-19: Review of current evidence and practical approach towards prevention and management. Eur. J. Pediatr. 2021, 180, 1009–1031. [Google Scholar] [CrossRef]
- Travers, C.P.; Clark, R.H.; Spitzer, A.R.; Das, A.; Garite, T.J.; Carlo, W.A. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: Prospective cohort study. BMJ 2017, 356, j1039. [Google Scholar] [CrossRef]
- De Luca, D. Managing neonates with respiratory failure due to SARS-CoV-2. Lancet Child Adolesc. Health 2020, 4, e8. [Google Scholar] [CrossRef]
- Vavouraki, E. The impact of COVID-19 pandemic on the healthcare of premature babies. Eur. J. Midwifery 2020, 4, 21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.K.; Sijercic, V.C.; Sayad, R.; Ruthig, G.R.; Abdelwahab, S.F.; El-Mokhtar, M.A.; Sayed, I.M. Risks and Preventions for Pregnant Women and Their Preterm Infants in a World with COVID-19: A Narrative Review. Vaccines 2023, 11, 640. https://doi.org/10.3390/vaccines11030640
Ahmed AK, Sijercic VC, Sayad R, Ruthig GR, Abdelwahab SF, El-Mokhtar MA, Sayed IM. Risks and Preventions for Pregnant Women and Their Preterm Infants in a World with COVID-19: A Narrative Review. Vaccines. 2023; 11(3):640. https://doi.org/10.3390/vaccines11030640
Chicago/Turabian StyleAhmed, Abdulrahman K., Victor Coll Sijercic, Reem Sayad, Gregory R. Ruthig, Sayed F. Abdelwahab, Mohamed A. El-Mokhtar, and Ibrahim M. Sayed. 2023. "Risks and Preventions for Pregnant Women and Their Preterm Infants in a World with COVID-19: A Narrative Review" Vaccines 11, no. 3: 640. https://doi.org/10.3390/vaccines11030640
APA StyleAhmed, A. K., Sijercic, V. C., Sayad, R., Ruthig, G. R., Abdelwahab, S. F., El-Mokhtar, M. A., & Sayed, I. M. (2023). Risks and Preventions for Pregnant Women and Their Preterm Infants in a World with COVID-19: A Narrative Review. Vaccines, 11(3), 640. https://doi.org/10.3390/vaccines11030640






