Evaluating the Intestinal Immunity of Asian Seabass (Lates calcarifer, Bloch 1790) following Field Vaccination Using a Feed-Based Oral Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed-Based Vaccine Preparation
2.2. Fish
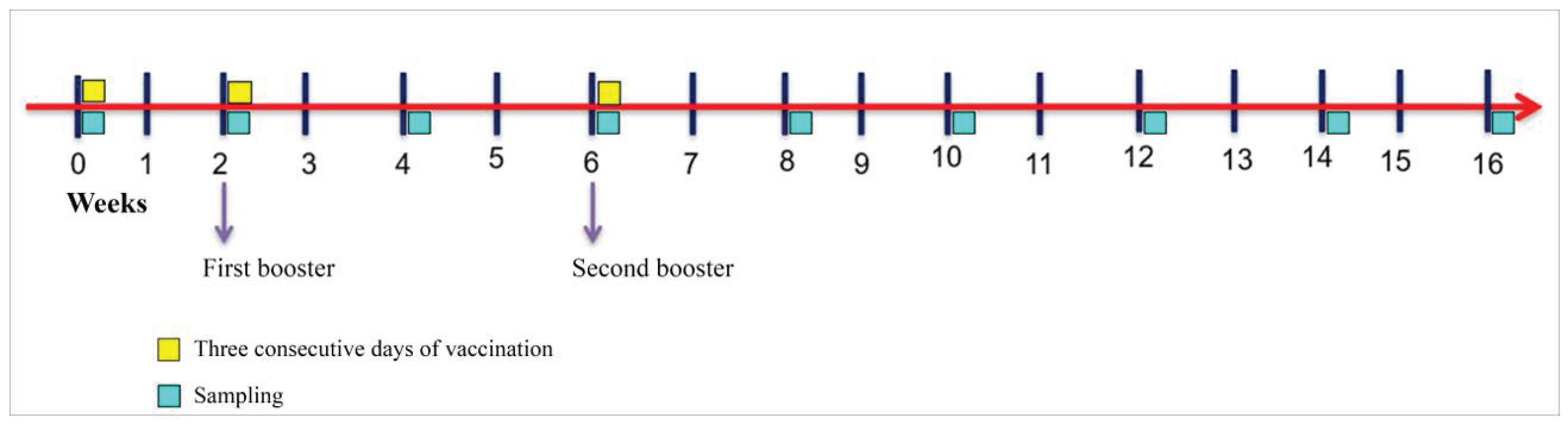
2.3. Experimental Design and Vaccine Regimen
2.4. Sampling
2.5. Incidence Rate
2.6. Measurement of Gut Specific IgM against V. harveyi
2.7. Measurement of Gut Lysozyme Activity
2.8. Gut Histopathology
2.9. GALT Histomorphometric Analysis
2.10. Statistical Analysis
3. Results
3.1. Clinical Findings and Gross Lesions
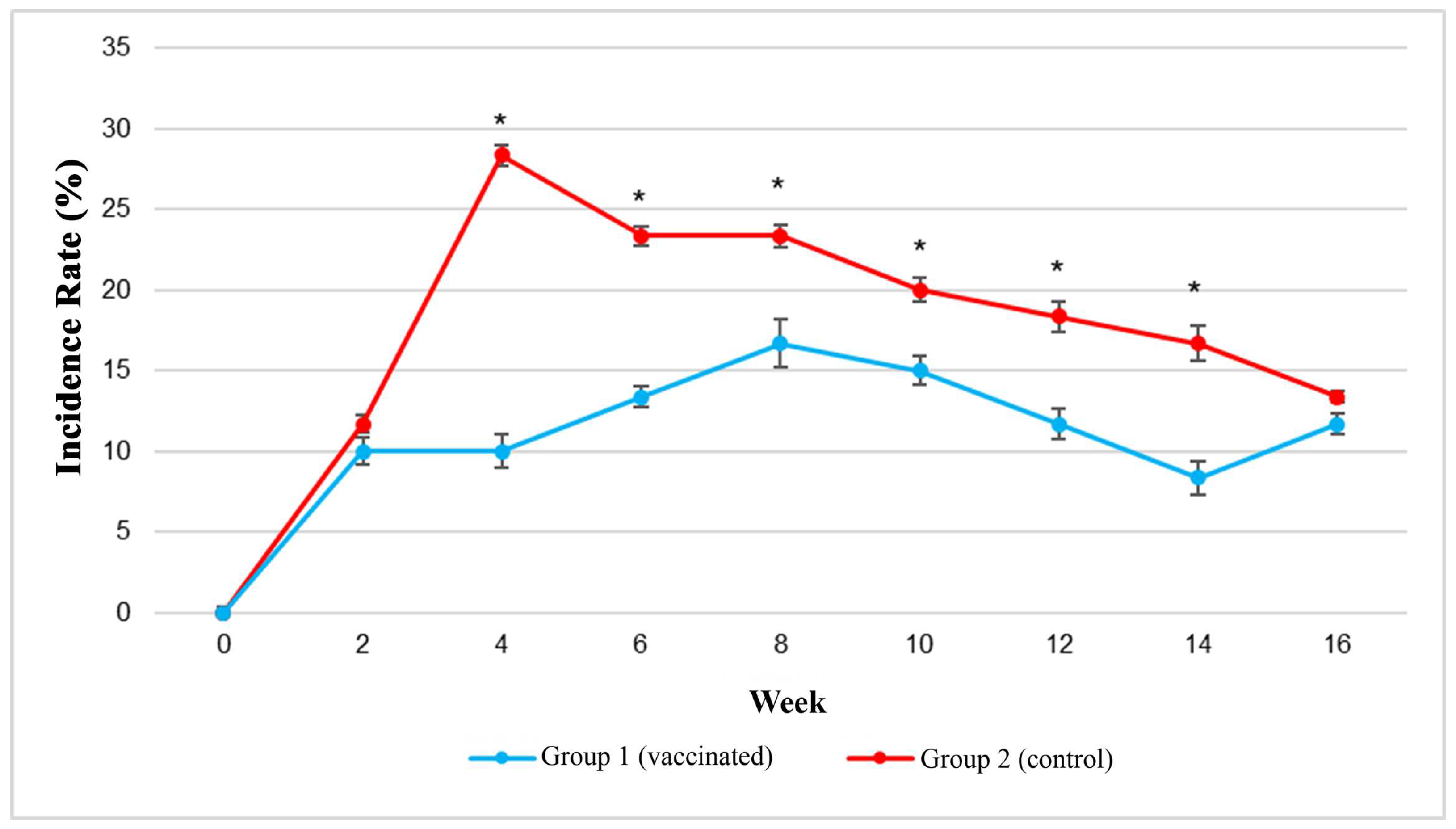
3.2. Incidence Rates of Vibriosis
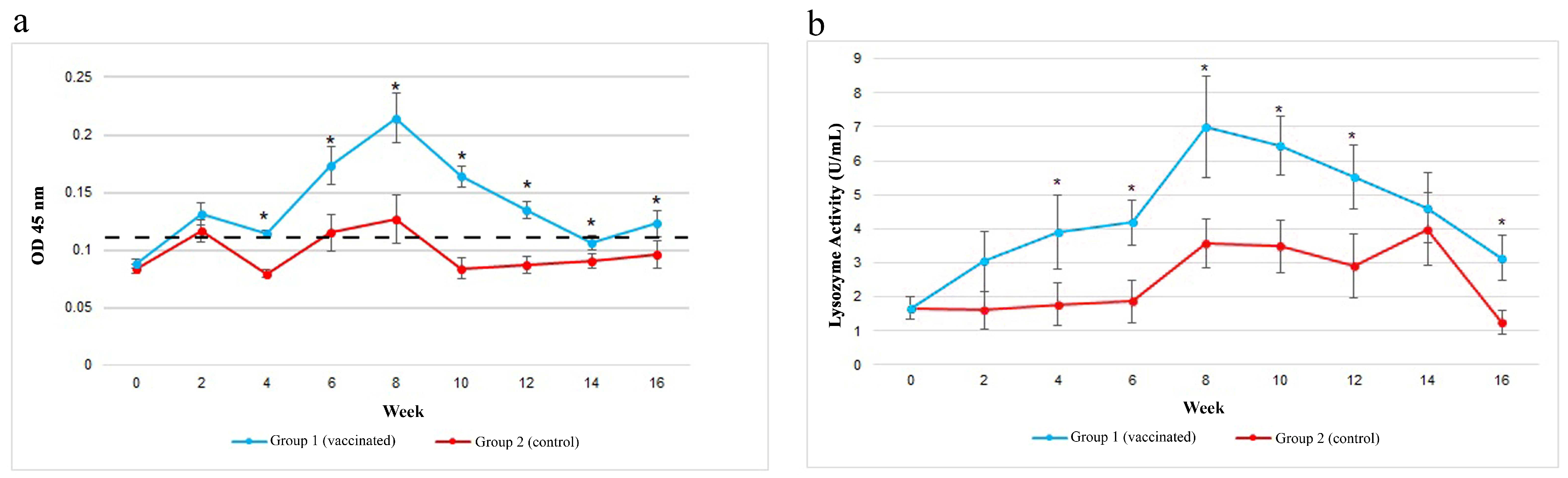
3.3. Gut Specific IgM against V. harveyi
3.4. Gut Lysozyme Activities
3.5. Gut Histopathology
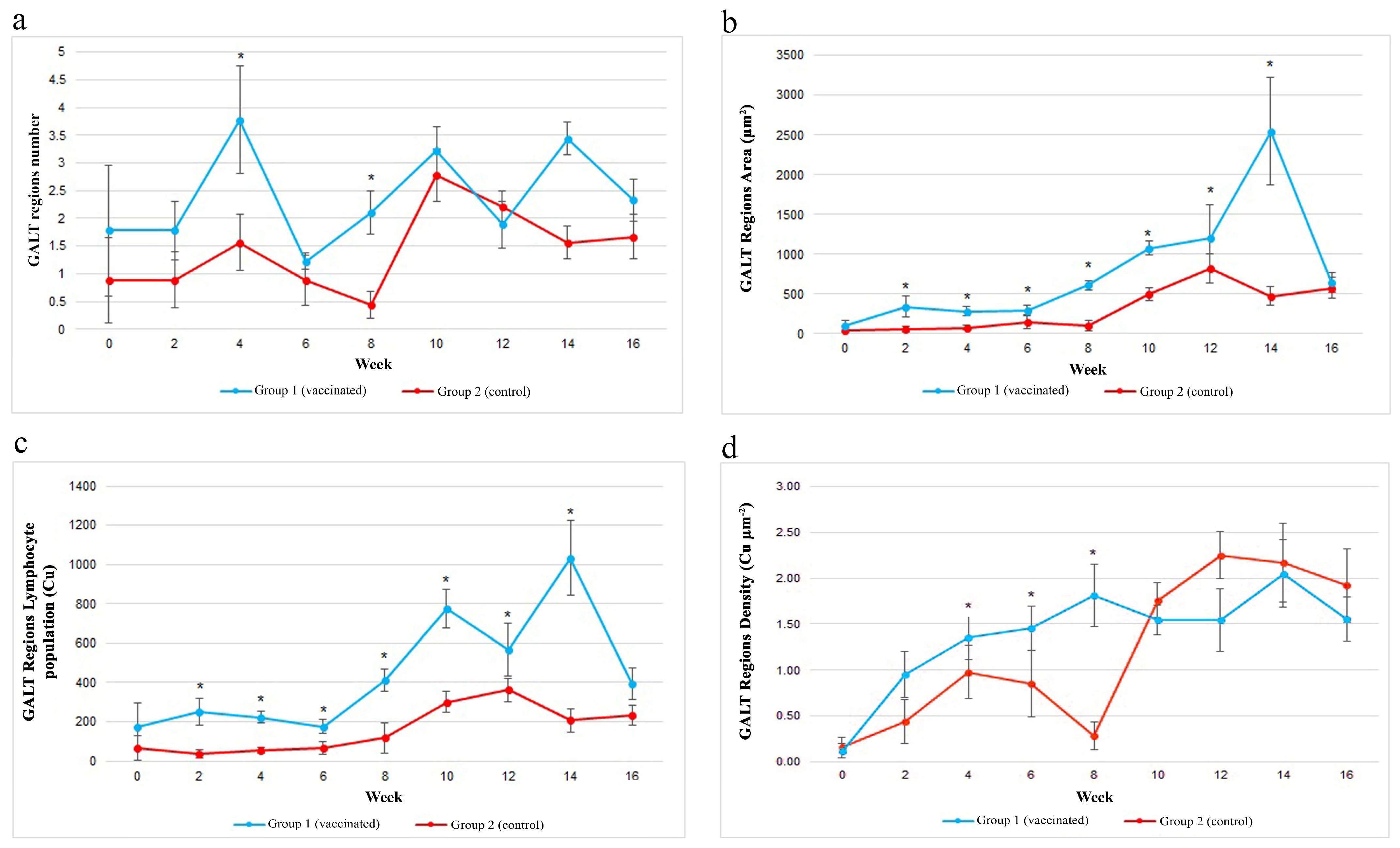
3.6. Assessment of GALT Regions
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Zamri Saad, M.; Nasruddin, N.S.; Al-saari, N.; Mino, S.; Sawabe, T. Vibriosis in cultured marine fishes: A review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.-A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2018, 31, 3–22. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Chen, H.; Liu, S.; Guo, Z.; Cheng, C.; Ma, H.; Feng, J. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci. Rep. 2020, 10, 14329. [Google Scholar] [CrossRef]
- Manchanayake, T.; Salleh, A.; Amal, M.N.A.; Yasin, I.S.M.; Zamri-Saad, M. Pathology and pathogenesis of Vibrio infection in fish: A review. Aquac. Rep. 2023, 28, 101459. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Krupesha Sharma, S.R.; Rathore, G.; Verma, D.K.; Sadhu, N.; Philipose, K.K. Vibrio alginolyticus infection in Asian seabass (Lates calcarifer, Bloch) reared in open sea floating cages in India. Aquac. Res. 2011, 44, 86–92. [Google Scholar] [CrossRef]
- Mohd Yazid, S.H.; Mohd Daud, H.; Azmai, M.N.A.; Mohamad, N.; Mohd Nor, N. Estimating the Economic Loss Due to Vibriosis in Net-Cage Cultured Asian Seabass (Lates calcarifer): Evidence From the East Coast of Peninsular Malaysia. Front. Vet. Sci. 2021, 8, 644009. [Google Scholar] [CrossRef]
- Mohamad, A.; Zamri-Saad, M.; Amal, M.N.A.; Al-saari, N.; Monir, M.S.; Chin, Y.K.; Md Yasin, I.S. Vaccine Efficacy of a Newly Developed Feed-Based Whole-Cell Polyvalent Vaccine against Vibriosis, Streptococcosis and Motile Aeromonad Septicemia in Asian Seabass, Lates calcarifer. Vaccines 2021, 9, 368. [Google Scholar] [CrossRef]
- Adamek, M.; Matras, M.; Rebl, A.; Stachnik, M.; Falco, A.; Bauer, J.; Miebach, A.-C.; Teitge, F.; Jung-Schroers, V.; Abdullah, M.; et al. Don’t Let It Get Under Your Skin!—Vaccination Protects the Skin Barrier of Common Carp From Disruption Caused by Cyprinid Herpesvirus 3. Front. Immunol. 2022, 13, 787021. [Google Scholar] [CrossRef]
- Annas, S.; Zamri-Saad, M. Intranasal Vaccination Strategy to Control the COVID-19 Pandemic from a Veterinary Medicine Perspective. Animals 2021, 11, 1876. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.G.; Qin, D.C.; Mu, Q.J.; Dong, Z.R.; Luo, Y.Z.; Ai, T.S.; Xu, Z. Mucosal immune responses and protective efficacy in yellow catfish after immersion vaccination with bivalent inactivated Aeromonas veronii and Edwardsiella ictaluri vaccine. Water Biol. Secur. 2022, 1, 100032. [Google Scholar] [CrossRef]
- Abu Nor, N.; Zamri-Saad, M.; Md Yasin, I.-S.; Salleh, A.; Mustaffa-Kamal, F.; Matori, M.F.; Azmai, M.N. Efficacy of whole cell inactivated vibrio harveyi vaccine against vibriosis in a marine red hybrid tilapia (Oreochromis niloticus × O. mossambicus) model. Vaccines 2020, 8, 734. [Google Scholar] [CrossRef]
- Wali, A.; Balkhi, H. Fish vaccination and therapeutics. Int. J. Multidiscip. Res. Dev. 2016, 3, 2349–4182. [Google Scholar]
- Salinas, I.; Parra, D. Fish mucosal immunity: Intestine. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2015; pp. 135–170. [Google Scholar] [CrossRef]
- Amir-Danial, Z.; Zamri-Saad, M.; Amal, M.N.A.; Annas, S.; Mohamad, A.; Jumria, S.; Manchanayake, T.; Arbania, A.; Ina-Salwany, M.Y. Field Efficacy of a Feed-Based Inactivated Vaccine against Vibriosis in Cage-Cultured Asian Seabass, Lates calcarifer, in Malaysia. Vaccines 2023, 11, 9. [Google Scholar] [CrossRef]
- Mohamad, A.; Mursidi, F.-A.; Zamri-Saad, M.; Amal, M.N.A.; Annas, S.; Monir, M.S.; Loqman, M.; Hairudin, F.; Al-saari, N.; Ina-Salwany, M.Y. Laboratory and Field Assessments of Oral Vibrio Vaccine Indicate the Potential for Protection against Vibriosis in Cultured Marine Fishes. Animals 2022, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusof, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2013, 45, 87–96. [Google Scholar] [CrossRef]
- Amalina, N.Z.; Dzarifah, Z.; Amal, M.N.A.; Yusof, M.T.; Zamri-Saad, M.; Al-saari, N.; Tanaka, M.; Mino, S.; Sawabe, T.; Ina-Salwany, M.Y. Recent update on the prevalence of Vibrio species among cultured grouper in Peninsular Malaysia. Aquac. Res. 2019, 50, 3202–3210. [Google Scholar] [CrossRef]
- Ismail, M.S.; Syafiq, M.R.; Siti-Zahrah, A.; Fahmi, S.; Shahidan, H.; Hanan, Y.; Amal, M.N.A.; Zamri Saad, M. The effect of feed-based vaccination on tilapia farm endemic for streptococcosis. Fish Shellfish Immunol. 2017, 60, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Byadgi, O.; Uyen, N.H.N.; Chou, R.L.; Guo, J.J.; Lee, Y.H.; Lee, J.W.; Cheng, T.C. Immunogenicity of inactivated formalin-killed Photobacterium damselae subsp. piscicida combined with Toll-like receptor 9 agonist in Cobia Rachycentron canadum. Aquaculture 2018, 492, 369–378. [Google Scholar] [CrossRef]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Yusof, M.T.; Monir, M.S.; Mohamad, A.; Muhamad-Sofie, M.H.N.; Lee, J.Y.; Chin, Y.K.; et al. Comparative Pathogenicity of Aeromonas spp. in cultured red hybrid tilapia (Oreochromis niloticus × O. mossambicus). Biology 2021, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Nawi, M.; Omar, N.; Sabri, M.Y.; Siti-Zahrah, A.; Saad, M.; Latifah, H. The effects of oral vaccination of Streptococcus agalactiae on stimulating gut-associated lymphoid tissues (GALTs) in Tilapia (Oreochromis spp.). Pertanika J. Trop. Agric. Sci. 2011, 34, 137–143. [Google Scholar]
- Hayat, M.; Mohd Yusoff, M.S.; Samad, M.J.; Abdul Razak, I.S.; Md Yasin, I.S.; Thompson, K.D.; Hasni, K. Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia. Vaccines 2021, 9, 51. [Google Scholar] [CrossRef]
- Kwan, G.T.; Finnerty, S.H.; Wegner, N.C.; Tresguerres, M. Quantification of Cutaneous Ionocytes in Small Aquatic Organisms. Bio-Protocol 2019, 9, e3227. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Polycarpo, G.D.V.; Moromizato, B.S.; Sidekerskis, A.P.D.; Silva, T.D.D.; Reis, I.C.D.; Fierro-Castro, C. Lysozyme activity as an indicator of innate immunity of tilapia (Oreochromis niloticus) when challenged with LPS and Streptococcus agalactiae. Rev. Bras. Zootec. 2021, 50, e20210053. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Ding, L.G.; Huang, Z.Y.; Xu, H.Y.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Hayat, M.; Sabri, M.Y.; Intan-Shameha, A.R.; Ina-Salwany, M.Y.; Thompson, K.D. Localisation of antigens in the gut post-challenge with Streptococcus iniae in vaccinated and non-vaccinated red hybrid tilapia (Oreochromis sp.). Aquac. Int. 2020, 28, 1739–1752. [Google Scholar] [CrossRef]
- Attaya, A.; Secombes, C.J.; Wang, T. Effective isolation of GALT cells: Insights into the intestine immune response of rainbow trout (Oncorhynchus mykiss) to different bacterin vaccine preparations. Fish Shellfish Immunol. 2020, 105, 378–392. [Google Scholar] [CrossRef]
- Parra, D.; Korytář, T.; Takizawa, F.; Sunyer, J.O. B cells and their role in the teleost gut. Dev. Comp. Immunol. 2016, 64, 150–166. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Nawi, M.; Zamri-Saad, M.; Nik-Haiha, N.Y.; Zuki, M.A.B.; Effendy, A.W.M. Histological assessments of intestinal immuno-morphology of tiger grouper juvenile, Epinephelus fuscoguttatus. SpringerPlus 2013, 2, 611. [Google Scholar] [CrossRef] [PubMed]




| Lesions | Group 1 (Vaccinated) | Group 2 (Control) |
|---|---|---|
| Inflammation | 0.46 ± 0.08 | 0.56 ± 0.10 |
| Congestion | 0.36 ± 0.07 | 0.30 ± 0.08 |
| Haemorrhage | 0.18 ± 0.05 | 0.28 ± 0.08 |
| Overall | 0.33 ± 0.04 | 0.38 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, T.; Manchanayake, T.; Danial, A.; Zamri-Saad, M.; Azmai, M.N.A.; Md Yasin, I.S.; Mohd Nor, N.; Salleh, A. Evaluating the Intestinal Immunity of Asian Seabass (Lates calcarifer, Bloch 1790) following Field Vaccination Using a Feed-Based Oral Vaccine. Vaccines 2023, 11, 602. https://doi.org/10.3390/vaccines11030602
Raju T, Manchanayake T, Danial A, Zamri-Saad M, Azmai MNA, Md Yasin IS, Mohd Nor N, Salleh A. Evaluating the Intestinal Immunity of Asian Seabass (Lates calcarifer, Bloch 1790) following Field Vaccination Using a Feed-Based Oral Vaccine. Vaccines. 2023; 11(3):602. https://doi.org/10.3390/vaccines11030602
Chicago/Turabian StyleRaju, Thanusha, Tilusha Manchanayake, Amir Danial, Mohd Zamri-Saad, Mohammad Noor Amal Azmai, Ina Salwany Md Yasin, Norhariani Mohd Nor, and Annas Salleh. 2023. "Evaluating the Intestinal Immunity of Asian Seabass (Lates calcarifer, Bloch 1790) following Field Vaccination Using a Feed-Based Oral Vaccine" Vaccines 11, no. 3: 602. https://doi.org/10.3390/vaccines11030602
APA StyleRaju, T., Manchanayake, T., Danial, A., Zamri-Saad, M., Azmai, M. N. A., Md Yasin, I. S., Mohd Nor, N., & Salleh, A. (2023). Evaluating the Intestinal Immunity of Asian Seabass (Lates calcarifer, Bloch 1790) following Field Vaccination Using a Feed-Based Oral Vaccine. Vaccines, 11(3), 602. https://doi.org/10.3390/vaccines11030602








