Knowledge on Parental Hesitancy toward COVID-19 Vaccination of Children 5–11 Years Old
Abstract
1. Background
2. Methods
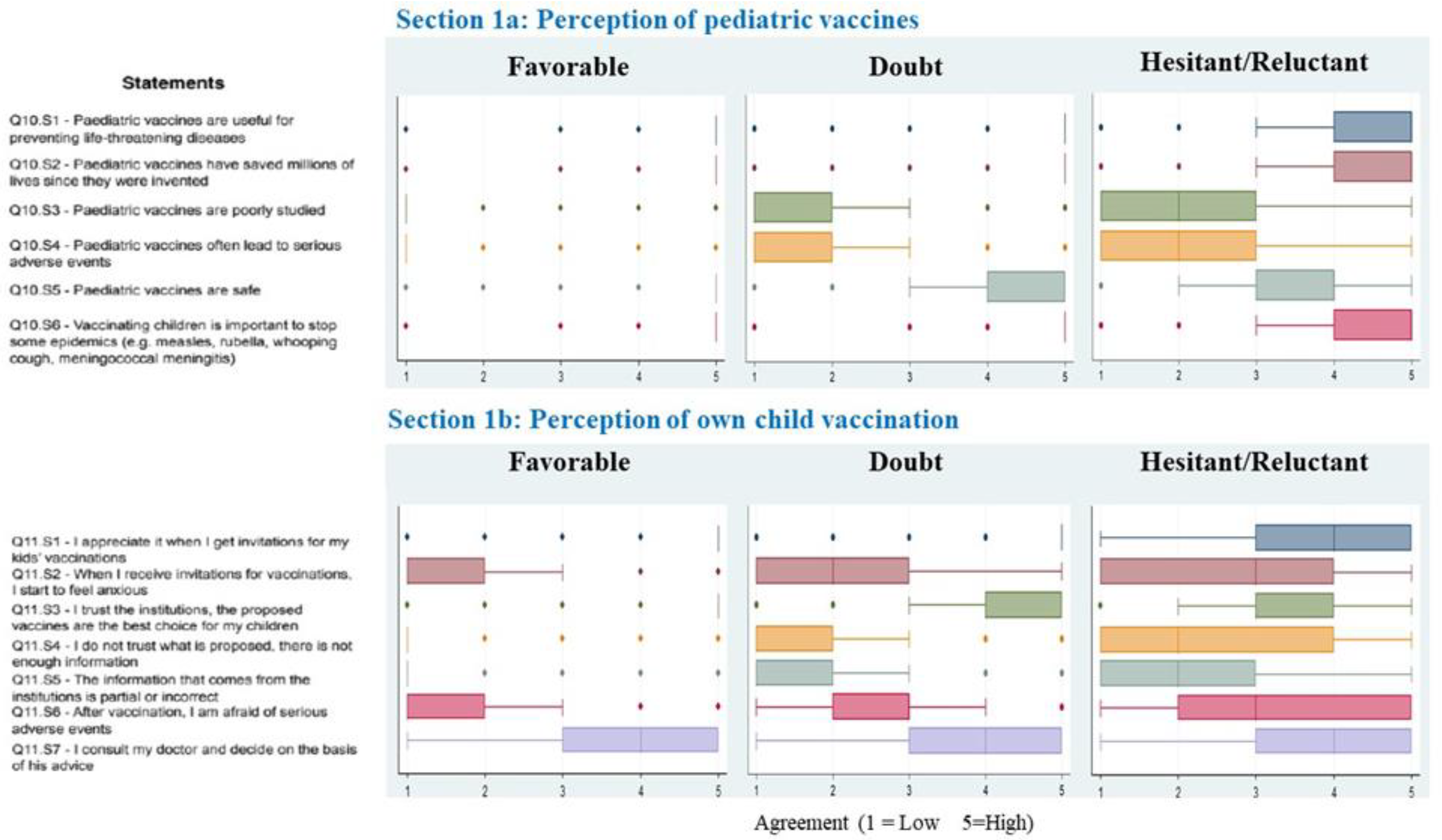
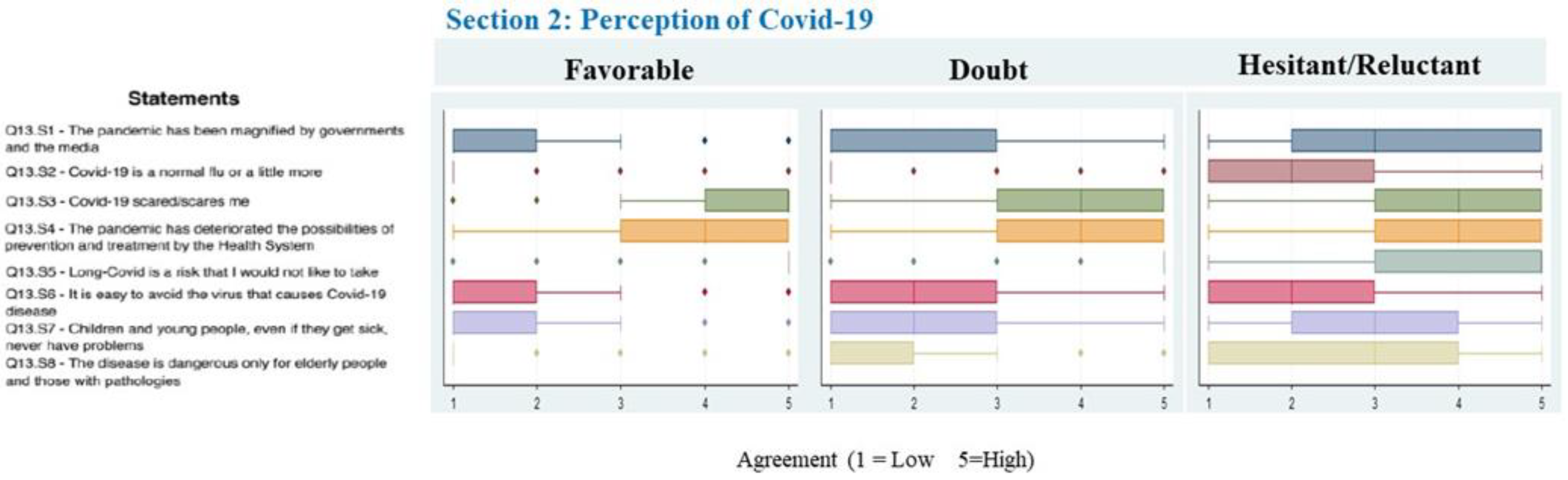
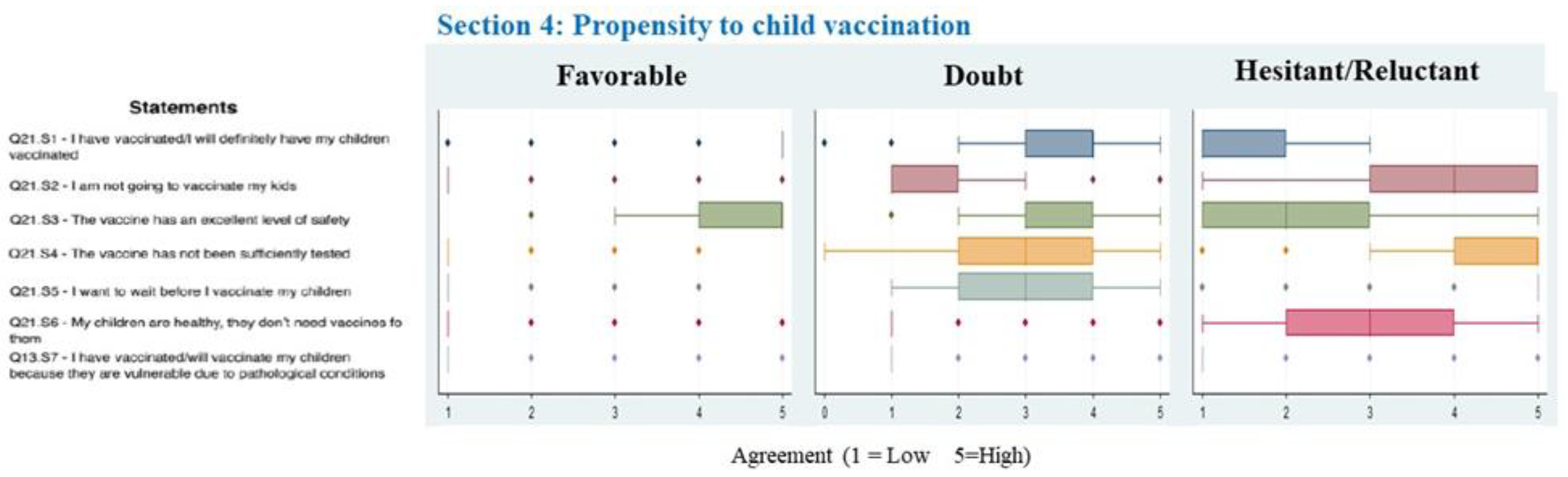
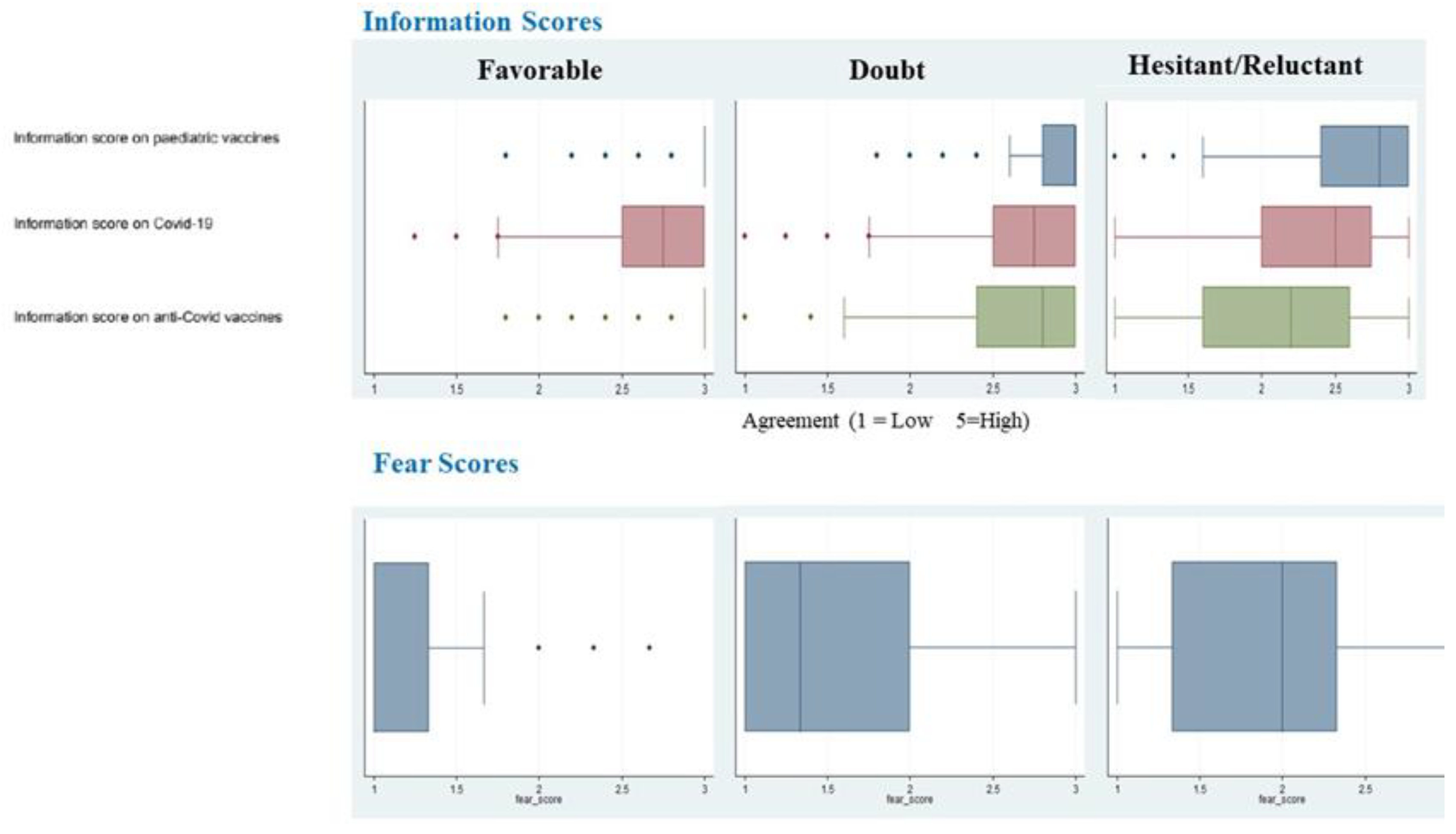
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orenstein, W.A.; Ahmed, R. Simply put: Vaccination saves lives. Proc. Natl. Acad. Sci. USA 2017, 114, 4031–4033. [Google Scholar] [CrossRef]
- Butler, R. Vaccine Hesitancy: What it means and what we need to know in order to tackle it. Vaccine 2016, 34, 1643–1649. [Google Scholar]
- Esposito, S.; Principi, N.; Cornaglia, G.; ESCMID Vaccine Study Group (EVASG). Barriers to the vaccination of children and adolescents and possible solutions. Clin. Microbiol. Infect. 2014, 20 (Suppl. S5), 25–31. [Google Scholar] [CrossRef]
- Kempe, A.; Saville, A.W.; Albertin, C.; Zimet, G.; Breck, A.; Helmkamp, L.; Vangala, S.; Dickinson, L.M.; Rand, C.; Humiston, S.; et al. Parental Hesitancy About Routine Childhood and Influenza Vaccinations: A National Survey. Pediatrics 2020, 146, e20193852. [Google Scholar] [CrossRef]
- Dube, E.; Gagnon, D.; Zhou, Z.; Deceuninck, G. Parental Vaccine Hesitancy in Quebec (Canada). PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- My, C.; Danchin, M.; Willaby, H.W.; Pemberton, S.; Leask, J. Parental attitudes, beliefs, behaviours and concerns towards childhood vaccinations in Australia: A national online survey. Aust. Fam. Phys. 2017, 46, 145–151. [Google Scholar]
- Hadjipanayis, A.; van Esso, D.; Del Torso, S.; Dornbusch, H.J.; Michailidou, K.; Minicuci, N.; Pancheva, R.; Mujkic, A.; Geitmann, K.; Syridou, G.; et al. Vaccine confidence among parents: Large scale study in eighteen European countries. Vaccine 2020, 38, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Tremolati, E.; Bellasio, M.; Chiarelli, G.; Marchisio, P.; Tiso, B.; Mosca, F.; Pardi, G.; Principi, N.; V.I.P. Study Group. Attitudes and knowledge regarding influenza vaccination among hospital health workers caring for women and children. Vaccine 2007, 25, 5283–5289. [Google Scholar] [CrossRef] [PubMed]
- Gellin, B.G.; Maibach, E.W.; Marcuse, E.K. Do parents understand immunizations? A national telephone survey. Pediatrics 2000, 106, 1097–1102. [Google Scholar] [CrossRef]
- Freed, G.L.; Clark, S.J.; Butchart, A.T.; Singer, D.C.; Davis, M.M. Parental vaccine safety concerns in 2009. Pediatrics 2010, 125, 654–659. [Google Scholar] [CrossRef]
- Napolitano, F.; Miraglia Del Giudice, G.; Angelillo, S.; Fattore, I.; Licata, F.; Pelullo, C.P.; Di Giuseppe, G. Hesitancy towards Childhood Vaccinations among Parents of Children with Underlying Chronic Medical Conditions in Italy. Vaccines 2022, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Srivastav, A.; Lindley, M.C.; Fisher, A.; Kim, D.; Greby, S.M.; Lee, J.; Singleton, J.A. Parental Vaccine Hesitancy and Association With Childhood Diphtheria, Tetanus Toxoid, and Acellular Pertussis; Measles, Mumps, and Rubella; Rotavirus; and Combined 7-Series Vaccination. Am. J. Prev. Med. 2022, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Srivastav, A.; Vaish, A.; Singleton, J.A. Population Attributable Fraction of Nonvaccination of Child and Adolescent Vaccines Attributed to Parental Vaccine Hesitancy, 2018–2019. Am. J. Epidemiol. 2022, 191, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Measles Outbreaks. Strategic Response Plan 2021–2023. Available online: https://apps.who.int/iris/bitstream/handle/10665/340657/9789240018600-eng.pdf (accessed on 30 December 2022).
- Dempsey, A.F.; Schaffer, S.; Singer, D.; Butchart, A.; Davis, M.; Freed, G.L. Alternative vaccination schedule preferences among parents of young children. Pediatrics 2011, 128, 848–856. [Google Scholar] [CrossRef]
- Gust, D.A.; Darling, N.; Kennedy, A.; Schwartz, B. Parents with doubts about vaccines: Which vaccines and reasons why. Pediatrics 2008, 122, 718–725. [Google Scholar] [CrossRef]
- Fauci, A.S. The story behind COVID-19 vaccines. Science 2021, 372, 109. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Why It Is Important to Develop an Effective and Safe Pediatric COVID-19 Vaccine. Vaccines 2021, 9, 127. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 30 December 2022).
- European Medicines Agency. Treatments and Vaccines for COVID-19. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19 (accessed on 30 December 2022).
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where do we Stand? Arch. Med. Res. 2022, 53, 1–8. [Google Scholar] [CrossRef]
- Esposito, S.; Autore, G.; Argentiero, A.; Ramundo, G.; Perrone, S.; Principi, N. Update on COVID-19 Therapy in Pediatric Age. Pharmaceuticals 2022, 15, 1512. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N. Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Paediatr. Drugs 2021, 23, 119–129. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N.; Azzari, C.; Cardinale, F.; Di Mauro, G.; Galli, L.; Gattinara, G.C.; Fainardi, V.; Guarino, A.; Lancella, L.; et al. Italian intersociety consensus on management of long covid in children. Ital. J. Pediatr. 2022, 48, 42. [Google Scholar] [CrossRef]
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, S.L.; Zhang, B.Y.; Li, S.N.J.; Burgert, C.; Shulha, H.P.; Kitchin, V.; Sauvé, L.; Sadarangani, M. Child transmission of SARS-CoV-2: A systematic review and meta-analysis. BMC Pediatr. 2022, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Giordano, R.; Paini, G.; Puntoni, M.; Principi, N.; Caminiti, C. Can we get out of the COVID pandemic without adequate vaccination coverage in the pediatric population? Ital. J. Pediatr. 2022, 48, 150. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, G.; Napoli, A.; Corea, F.; Folcarelli, L.; Angelillo, I.F. Evaluating COVID-19 Vaccine Willingness and Hesitancy among Parents of Children Aged 5–11 Years with Chronic Conditions in Italy. Vaccines 2022, 10, 396. [Google Scholar] [CrossRef]
- Esposito, S.; Pediatricians of Emilia-Romagna Region, Italy. Manifesto of the pediatricians of Emilia-Romagna region, Italy, in favor of vaccination against COVID in children 5–11 years old. Ital. J. Pediatr. 2022, 48, 40. [Google Scholar] [CrossRef] [PubMed]
- Zona, S.; Partesotti, S.; Bergomi, A.; Rosafio, C.; Antodaro, F.; Esposito, S. Anti-COVID Vaccination for Adolescents: A Survey on Determinants of Vaccine Parental Hesitancy. Vaccines 2021, 9, 1309. [Google Scholar] [CrossRef]
- Montalti, M.; Rallo, F.; Guaraldi, F.; Bartoli, L.; Po, G.; Stillo, M.; Perrone, P.; Squillace, L.; Dallolio, L.; Pandolfi, P.; et al. Would Parents Get Their Children Vaccinated Against SARS-CoV-2? Rate and Predictors of Vaccine Hesitancy According to a Survey over 5000 Families from Bologna, Italy. Vaccines 2021, 9, 366. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Shah, M.D.; Delgado, J.R.; Thomas, K.; Vizueta, N.; Cui, Y.; Vangala, S.; Shetgiri, R.; Kapteyn, A. Parents’ Intentions and Perceptions About COVID-19 Vaccination for Their Children: Results from a National Survey. Pediatrics 2021, 148, e2021052335. [Google Scholar] [CrossRef]
- Teasdale, C.A.; Borrell, L.N.; Kimball, S.; Rinke, M.L.; Rane, M.; Fleary, S.A.; Nash, D. Plans to Vaccinate Children for Coronavirus Disease 2019: A Survey of United States Parents. J. Pediatr. 2021, 237, 292–297. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Bagateli, L.E.; Saeki, E.Y.; Fadda, M.; Agostoni, C.; Marchisio, P.; Milani, G.P. COVID-19 Vaccine Hesitancy among Parents of Children and Adolescents Living in Brazil. Vaccines 2021, 9, 1115. [Google Scholar] [CrossRef]
- AIFA. Italian Medicines Agency. AIFA Approves Comirnaty Vaccine for Ages 5 to 11. Available online: https://www.aifa.gov.it/en/-/aifa-approva-il-vaccino-comirnaty-per-la-fascia-di-et%C3%A0-5-11-anni (accessed on 12 January 2023).
- Società Italiana di Pediatria. Vaccinazione COVID-19 nei Bambini, le Raccomandazioni dei Pediatri alle Famiglie. Available online: https://sip.it/2021/11/26/vaccinazione-covid-19-nei-bambini-le-raccomandazioni-dei-pediatri-alle-famiglie/ (accessed on 30 December 2022).
- Ministero della Salute. Conversione in Legge, Con Modificazioni, del Decreto-Legge 7 Giugno 2017, n. 73, Recante Disposizioni Urgenti in Materia di Prevenzione Vaccinale. (17G00132). Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=60201 (accessed on 12 January 2023).
- Napoli, A.; Miraglia Del Giudice, G.; Corea, F.; Folcarelli, L.; Angelillo, I.F. Parents’ reasons to vaccinate their children aged 5–11 years against COVID-19 in Italy. Front. Med. 2022, 9, 949693. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Gagnon, D.; Pelletier, C. COVID-19 vaccination in 5–11 years old children: Drivers of vaccine hesitancy among parents in Quebec. Hum. Vaccines Immunother. 2022, 18, 2028516. [Google Scholar] [CrossRef] [PubMed]
- Goulding, M.; Ryan, G.W.; Minkah, P.; Borg, A.; Gonzalez, M.; Medina, N.; Suprenant, P.; Rosal, M.C.; Lemon, S.C. Parental perceptions of the COVID-19 vaccine for 5- to 11-year-old children: Focus group findings from Worcester Massachusetts. Hum. Vaccines Immunother. 2022, 18, 2120721. [Google Scholar] [CrossRef] [PubMed]
- McGregor, S.; Goldman, R.D. Determinants of parental vaccine hesitancy. Can. Fam. Physician 2021, 67, 339–341. [Google Scholar] [CrossRef]
- Lafnitzegger, A.; Gaviria-Agudelo, C. Vaccine Hesitancy in Pediatrics. Adv. Pediatr. 2022, 69, 163–176. [Google Scholar] [CrossRef]
- Presidenza del Consiglio dei Ministri-Unità Completamento Campagna Vaccinale-Ministero della Salute. Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 12 January 2023).
- Tan, S.H.X.; Cook, A.R.; Heng, D.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Cohen-Stavi, C.J.; Magen, O.; Barda, N.; Yaron, S.; Peretz, A.; Netzer, D.; Giaquinto, C.; Judd, A.; Leibovici, L.; Hernán, M.A.; et al. BNT162b2 Vaccine Effectiveness against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 227–236. [Google Scholar] [CrossRef]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef]
- Khan, F.L.; Nguyen, J.L.; Singh, T.G.; Puzniak, L.A.; Wiemken, T.L.; Schrecker, J.P.; Taitel, M.S.; Zamparo, J.M.; Jodar, L.; McLaughlin, J.M. Estimated BNT162b2 Vaccine Effectiveness against Infection with Delta and Omicron Variants among US Children 5 to 11 Years of Age. JAMA Netw. Open 2022, 5, e2246915. [Google Scholar] [CrossRef]
- Sacco, C.; Del Manso, M.; Mateo-Urdiales, A.; Rota, M.C.; Petrone, D.; Riccardo, F.; Bella, A.; Siddu, A.; Battilomo, S.; Proietti, V.; et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: A retrospective analysis of January–April, 2022. Lancet 2022, 400, 97–103. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention COVID-19 Vaccination for Children. Available online: https://www.cdc.gov/vaccines/covid-19/planning/children.html#covid19-vax-recommendations (accessed on 30 December 2022).
- Ladhani, S.N. COVID-19 vaccination for children aged 5–11 years. Lancet 2022, 400, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Dargham, S.R.; Loka, S.; Shaik, R.M.; Chemaitelly, H.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Yassine, H.M.; Al-Khatib, H.A.; et al. Coronavirus Disease 2019 Disease Severity in Children Infected With the Omicron Variant. Clin. Infect. Dis. 2022, 75, e361–e367. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Carter, M.J.; Ronny Cheung, C.; Ladhani, S.; Evelina PIMS-TS Study Group. Lower Risk of Multisystem Inflammatory Syndrome in Children (MIS-C) with the Delta and Omicron variants of SARS-CoV-2. Clin. Infect. Dis. 2023, 76, e518–e521. [Google Scholar] [CrossRef] [PubMed]
- Madewell, Z.J.; Yang, Y.; Longini, I.M., Jr.; Halloran, M.E.; Dean, N.E. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status:an updated systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e229317. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Wong, B.L.H.; Ramsay, M.E.; Ladhani, S.N. Should children be vaccinated against COVID-19 now? Arch. Dis. Child. 2021, 106, 1147–1148. [Google Scholar] [CrossRef]
| Data | Total | Male N = 548 | Female N = 2869 | p |
|---|---|---|---|---|
| Age, years | 41.3 ± 5.3 | 43.6 ± 6.0 | 40.8 ± 5.0 | <0.001 |
| Group of age, years | <0.001 | |||
| <40 | 1573 (45.8%) | 181 (33.0%) | 1381 (48.1%) | |
| 41–50 | 1656 (48.24%) | 291 (53.1%) | 1361 (47.4%) | |
| >50 | 204 (5.9%) | 76 (13.9%) | 121 (4.4%) | |
| Education | <0.01 | |||
| Lower secondary school | 136 (4.0%) | 28 (5.1%) | 108 (3.8%) | |
| High school | 1172 (34%) | 203 (37.0%) | 966 (33.7%) | |
| BA | 508 (14.8%) | 64 (11.7%) | 442 (15.4%) | |
| MA | 1108 (32.35) | 154 (28.1%) | 947 (33.0%) | |
| PhD | 419 (12.2%) | 87 (15.9%) | 330 (11.5%) | |
| No answer | 90 (2.62%) | 12 (2.2%) | 76 (2.65%) | |
| Work condition | <0.001 | |||
| Permanent employment | 2138 (62.3%) | 379 (69.2%) | 1704 (61.1%) | |
| Temporary employment | 253 (7.4%) | 21 (3.8%) | 232 (8.1%) | |
| Self-employed | 626 (18.2%) | 135 (24.6%) | 487(17.0%) | |
| Unemployed/unpaid work | 293 (8.5%) | 4 (0.7%) | 289 (10.1%) | |
| Retired | 7 (0.2) | 3 (0.5%) | 4 (0.1%) | |
| No answer | 116 (3.38%) | 6 (1.1%) | 103 (3.6%) | |
| Number of children | 0.30 | |||
| 1 | 1115 (32.5%) | 188 (34.3%) | 921 (32.1%) | |
| 2 | 1887 (55.0%) | 285 (52.0%) | 1594 (55.6%) | |
| >2 | 431 (12.6%) | 75 (13.7%) | 354 (12.3%) | |
| Number of children 5–11 years old | 0.56 | |||
| 1 | 2485 (72.4%) | 2802 (72.6%) | 391 (71.3%) | |
| >1 | 948 (27.6%) | 787 (27.4%) | 157 (28.7%) | |
| Nationality | 0.67 | |||
| Italian | 3381 (98.5%) | 538 (98.1%) | 2829 (98.6%) | |
| Foreign | 43 (1.3%) | 98 (1.6%) | 34 (1.2%) | |
| No answer | 9 (0.3%) | 1 (0.2%) | 6 (0.2%) | |
| Group | <0.001 | |||
| Favorable | 1223 (35.6%) | 300 (57.8%) | 1159 (40.4%) | |
| Doubtful | 1459 (42.5%) | 149 (27.2%) | 1072 (37.4%) | |
| Hesitant/Reluctant | 751 (21.9%) | 99 (18.1%) | 638 (22.2%) |
| Independent Variable | Favorable | Doubtful | Hesitant/Reluctant | ||
|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||
| Age, years | 1 (Ref.) | - | |||
| <40 | 1 (Ref.) | 1 (Ref.) | |||
| 41–50 | 0.73 | 0.62–0.85 | 0.55 | 0.45–0.66 | |
| >50 | 0.62 | 0.45–0.86 | 0.39 | 0.25–0.60 | |
| Gender | 1 (Ref.) | - | |||
| Female | 1 (Ref.) | 1 (Ref.) | |||
| Male | 0.54 | 0.43–0.66 | 0.60 | 0.46–0.76 | |
| No answer/non-binary | 0.81 | - | 0.95 | - | |
| Education | 1 (Ref.) | 0.83–2.01 | |||
| Lower secondary school | 1.01 | 0.65–1.54 | 1.30 | ||
| High school | 1 (Ref.) | 1 (Ref.) | |||
| BA | 0.95 | 0.75–1.20 | 0.67 | 0.51–0.89 | |
| MA | 0.74 | 0.62–0.90 | 0.59 | 0.47–0.73 | |
| PhD | 0.55 | 0.42–0.71 | 0.39 | 0.28–0.53 | |
| No answer | 2.07 | 1.14–3.75 | 3.24 | 1.80–5.85 | |
| Work condition | 1 (Ref.) | ||||
| Employed | 1 (Ref.) | 1 (Ref.) | |||
| Self-employed | 0.92 | 0.76–1.13 | 1.12 | 0.89–1.41 | |
| Unemployed/unpaid | 0.92 | 0.70–1.21 | 0.96 | 0.69–1.32 | |
| Retired/no answer | 1.63 | 1.00–2.65 | 3.89 | 2.44–6.19 | |
| Annual income | 1 (Ref.) | 1.34–3.00 | |||
| <15,000 | 1.36 | 0.95–1.97 | 2.01 | ||
| 15,001–28,000 | 1.25 | 1.02–1.53 | 1.47 | 1.15–1.88 | |
| 28,001–55,000 | 1 (Ref.) | 1 (Ref.) | |||
| 55,001–75,000 | 0.80 | 0.62–1.02 | 0.69 | 0.49–0.95 | |
| >75,000 | 0.67 | 0.50–0.88 | 0.92 | 0.67–1.27 | |
| No answer | 1.43 | 1.12–1.82 | 2.60 | 1.99–3.38 | |
| Number of children | 1 (Ref.) | - | |||
| 1 | 1 (Ref.) | 1 (Ref.) | |||
| 2 | 0.86 | 0.73–1.01 | 1.10 | 0.90–1.33 | |
| >2 | 0.72 | 0.55–0.92 | 1.12 | 0.84–1.50 | |
| Number of children 5–11 yrs | 1 (Ref.) | - | |||
| 1 | 1 (Ref.) | 1 (Ref.) | |||
| >1 | 0.95 | 0.80–1.13 | 1.29 | 1.06–1.56 | |
| Nationality | 1 (Ref.) | - | |||
| Italian | 1 (Ref.) | 1 (Ref.) | |||
| Foreign | 0.84 | 0.77–0.90 | 1.62 | 0.78–3.31 | |
| No answer | - | - | |||
| Area of residence | 1 (Ref.) | ||||
| North | 1 (Ref.) | 1 (Ref.) | |||
| Center | 1.01 | 0.81–1.25 | 0.82 | 0.63–1.08 | |
| South and Islands | 0.86 | 0.62–1.19 | 0.65 | 0.43–1.00 | |
| No answer | 2.63 | 1.61–4.30 | 4.76 | 2.93–7.74 | |
| Independent Variable | Favorable | Doubtful | Hesitant/Reluctant | ||
|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||
| Age | 1 (Ref.) | - | |||
| <40 years | 1 (Ref.) | 1 (Ref.) | |||
| 41–50 | 0.70 | 0.59–0.83 | 0.57 | 0.45–0.72 | |
| >50 | 0.63 | 0.44–0.90 | 0.32 | 0.18–0.57 | |
| Gender | 1 (Ref.) | - | |||
| Female | 1 (Ref.) | 1 (Ref.) | |||
| Male | 0.66 | 0.52–0.83 | 0.68 | 0.48–0.95 | |
| No answer/non-binary | 0.15 | - | - | ||
| Work condition | 1 (Ref.) | ||||
| Employed | 1 (Ref.) | 1 (Ref.) | |||
| Self-employed | 1.01 | 0.81–1.26 | 1.15 | 0.85–1.54 | |
| Unemployed/unpaid | 0.70 | 0.52–0.95 | 0.61 | 0.40–0.93 | |
| Retired/no answer | 1.35 | 0.80–2.33 | 2.10 | 1.11–3.96 | |
| Inf. score on vaccines (pre-pandemic) | 1 (Ref.) | 0.25 | 0.15–0.42 | 0.12 | 0.07–0.22 |
| Inf. score on COVID-19 | 1 (Ref.) | 0.40 | 0.30–0.54 | 0.05 | 0.04–0.08 |
| Fear Score | 1 (Ref.) | 5.25 | 13.96–432 | 12.67 | 9.90–16.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S.; Rosafio, C.; Partesotti, S.; Fiore, M.; Antodaro, F.; Bergomi, A.; Neglia, C.; Argentiero, A.; Principi, N.; Zona, S. Knowledge on Parental Hesitancy toward COVID-19 Vaccination of Children 5–11 Years Old. Vaccines 2023, 11, 587. https://doi.org/10.3390/vaccines11030587
Esposito S, Rosafio C, Partesotti S, Fiore M, Antodaro F, Bergomi A, Neglia C, Argentiero A, Principi N, Zona S. Knowledge on Parental Hesitancy toward COVID-19 Vaccination of Children 5–11 Years Old. Vaccines. 2023; 11(3):587. https://doi.org/10.3390/vaccines11030587
Chicago/Turabian StyleEsposito, Susanna, Cristiano Rosafio, Simonetta Partesotti, Michele Fiore, Francesco Antodaro, Andrea Bergomi, Cosimo Neglia, Alberto Argentiero, Nicola Principi, and Stefano Zona. 2023. "Knowledge on Parental Hesitancy toward COVID-19 Vaccination of Children 5–11 Years Old" Vaccines 11, no. 3: 587. https://doi.org/10.3390/vaccines11030587
APA StyleEsposito, S., Rosafio, C., Partesotti, S., Fiore, M., Antodaro, F., Bergomi, A., Neglia, C., Argentiero, A., Principi, N., & Zona, S. (2023). Knowledge on Parental Hesitancy toward COVID-19 Vaccination of Children 5–11 Years Old. Vaccines, 11(3), 587. https://doi.org/10.3390/vaccines11030587







