Deepening Our Understanding of COVID-19 Vaccine Decision-Making amongst Healthcare Workers in Southwest Virginia, USA Using Exploratory and Confirmatory Factor Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Study Design
2.2. Statistical Methods
3. Results
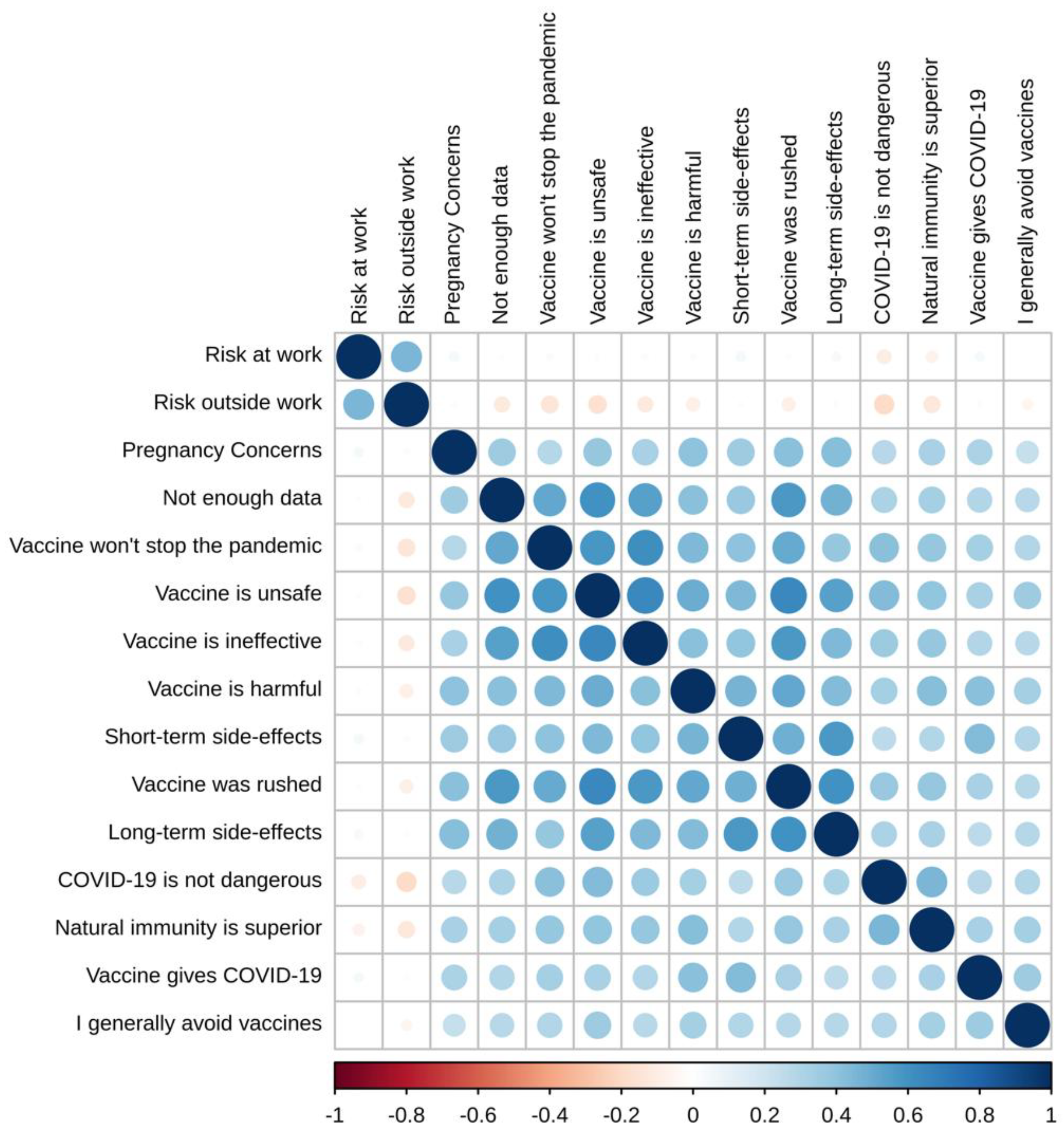
3.1. Descriptive Statistics
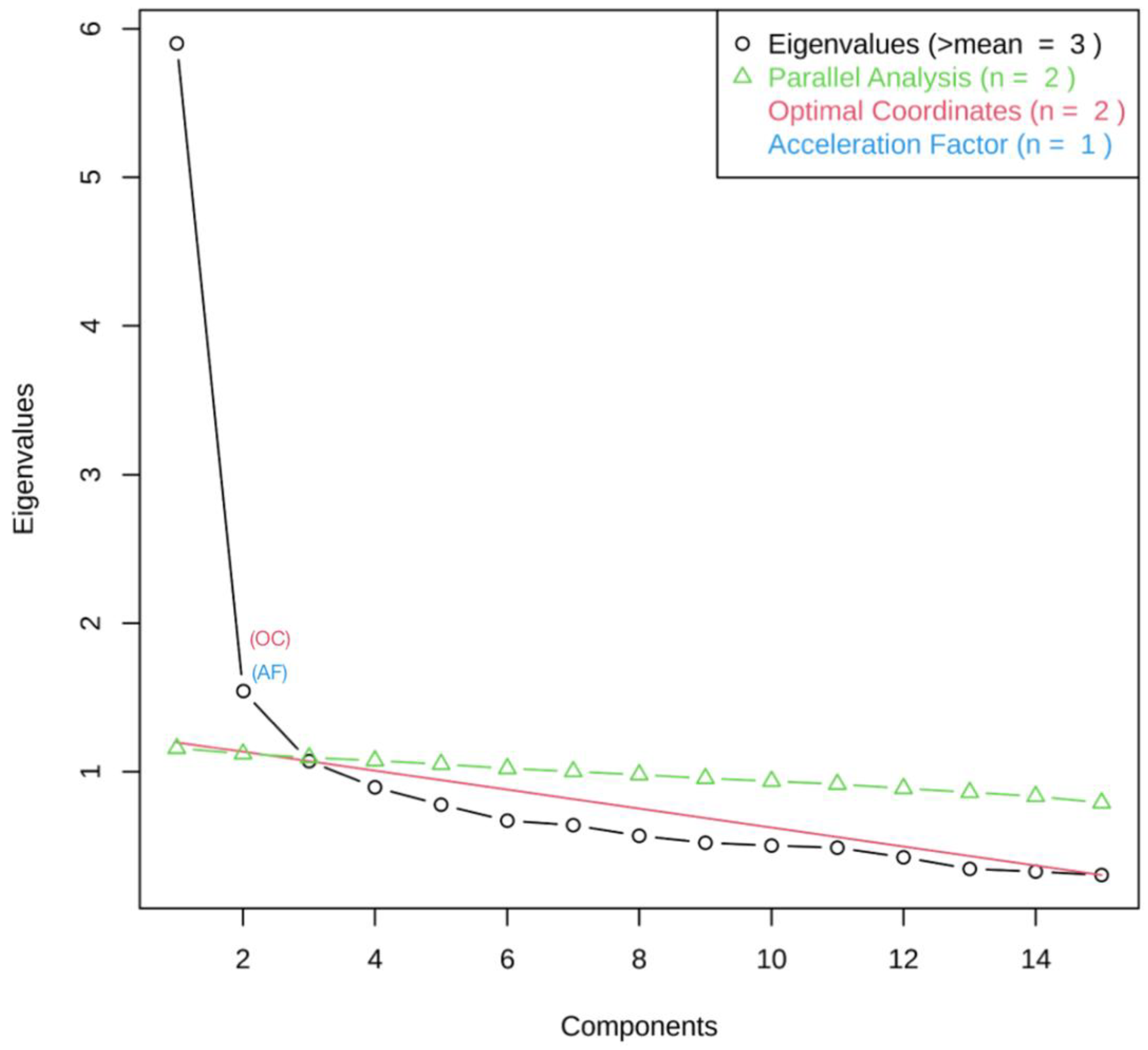
3.2. Exploratory Factor Analysis (EFA)
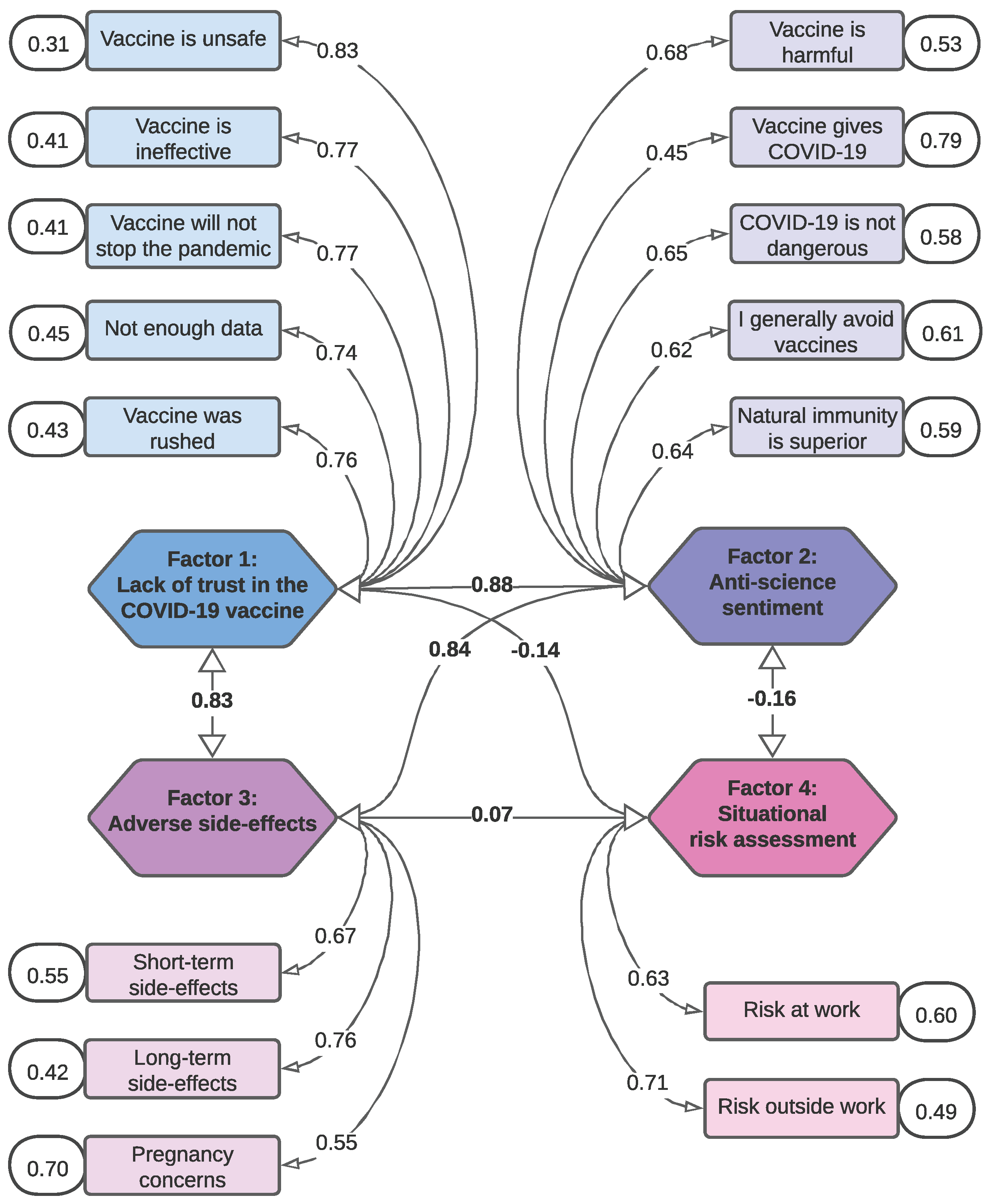
- Factor 1: Lack of trust in the COVID-19 vaccine
- Factor 2: Anti-science sentiment
- Factor 3: Adverse side-effects
- Factor 4: Situational risk assessment
3.3. Confirmatory Factor Analysis (CFA)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCW | Healthcare Worker |
| EFA | Exploratory Factor Analysis |
| CFA | Confirmatory Factor Analysis |
| TLI | Tucker Lewis Index |
| CFI | Comparative Fit Index |
| RMSEA | Root Mean Square Error of Approximation |
Appendix A
| Exploratory Factor Analysis | Confirmatory Factor Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| Factor 1: Lack of trust in the COVID-19 vaccine | 1.000 | 0.767 *** | 0.724 *** | −0.211 *** | 1.000 | 0.882 *** | 0.835 *** | −0.142 ** |
| Factor 2: Anti-science sentiment | 0.767 *** | 1.000 | 0.660 *** | −0.143 *** | 0.882 *** | 1.000 | 0.843 *** | −0.163 ** |
| Factor 3: Adverse side-effects | 0.724 *** | 0.660 *** | 1.000 | 0.041 | 0.835 *** | 0.843 *** | 1.000 | 0.066 |
| Factor 4: Situational risk assessment | −0.211 *** | −0.143 *** | 0.041 | 1.000 | −0.142 ** | −0.163 ** | 0.066 | 1.000 |
References
- CDC. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 21 January 2023).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Dubé, È.; Ward, J.K.; Verger, P.; MacDonald, N.E. Vaccine Hesitancy, Acceptance, and Anti-Vaccination: Trends and Future Prospects for Public Health. Annu. Rev. Public Health 2021, 42, 175–191. [Google Scholar] [CrossRef]
- Sallam, M.; Al-Sanafi, M.; Sallam, M. A Global Map of COVID-19 Vaccine Acceptance Rates per Country: An Updated Concise Narrative Review. J. Multidiscip. Healthc. 2022, 15, 21–45. [Google Scholar] [CrossRef]
- Albrecht, D. Vaccination, politics and COVID-19 impacts. BMC Public Health 2022, 22, 96. [Google Scholar] [CrossRef]
- Leong, C.; Jin, L.; Kim, D.; Kim, J.; Teo, Y.Y.; Ho, T.H. Assessing the impact of novelty and conformity on hesitancy towards COVID-19 vaccines using mRNA technology. Commun. Med. 2022, 2, 61. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Danaee, M.; Ahmed, J.; Lachyan, A.; Cai, C.Z.; Lin, Y.; Hu, Z.; Tan, S.Y.; Lu, Y.; et al. COVID-19 vaccination intention and vaccine characteristics influencing vaccination acceptance: A global survey of 17 countries. Infect. Dis. Poverty 2021, 10, 122. [Google Scholar] [CrossRef]
- de Albuquerque Veloso Machado, M.; Roberts, B.; Wong, B.L.H.; van Kessel, R.; Mossialos, E. The Relationship Between the COVID-19 Pandemic and Vaccine Hesitancy: A Scoping Review of Literature Until August 2021. Front. Public Health 2021, 9, 747787. [Google Scholar] [CrossRef]
- Syan, S.K.; Gohari, M.R.; Levitt, E.E.; Belisario, K.; Gillard, J.; DeJesus, J.; MacKillop, J. COVID-19 Vaccine Perceptions and Differences by Sex, Age, and Education in 1,367 Community Adults in Ontario. Front. Public Health 2021, 9, 719665. [Google Scholar] [CrossRef]
- Gilboa, M.; Tal, I.; Levin, E.G.; Segal, S.; Belkin, A.; Zilberman-Daniels, T.; Biber, A.; Rubin, C.; Rahav, G.; Regev-Yochay, G. Coronavirus disease 2019 (COVID-19) vaccination uptake among healthcare workers. Infect. Control. Hosp. Epidemiol. 2022, 43, 1433–1438. [Google Scholar] [CrossRef]
- King, W.C.; Rubinstein, M.; Reinhart, A.; Mejia, R. Time trends, factors associated with, and reasons for COVID-19 vaccine hesitancy: A massive online survey of US adults from January–May 2021. PLoS ONE 2021, 16, e0260731. [Google Scholar] [CrossRef]
- Toth-Manikowski, S.M.; Swirsky, E.S.; Gandhi, R.; Piscitello, G. COVID-19 vaccination hesitancy among health care workers, communication, and policy-making. Am. J. Infect. Control. 2022, 50, 20–25. [Google Scholar] [CrossRef]
- Swann, M.C.; Bendetson, J.; Johnson, A.; Jatta, M.; Schleupner, C.J.; Baffoe-Bonnie, A. Examining drivers of coronavirus disease 2019 (COVID-19) vaccine hesitancy among healthcare workers. Infect. Control. Hosp. Epidemiol. 2022, 43, 1813–1821. [Google Scholar] [CrossRef]
- Caiazzo, V.; Witkoski Stimpfel, A. Vaccine hesitancy in American healthcare workers during the COVID-19 vaccine roll out: An integrative review. Public Health 2022, 207, 94–104. [Google Scholar] [CrossRef]
- Dzieciolowska, S.; Hamel, D.; Gadio, S.; Dionne, M.; Gagnon, D.; Robitaille, L.; Cook, E.; Caron, I.; Talib, A.; Parkes, L.; et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: A multicenter survey. Am. J. Infect. Control. 2021, 49, 1152–1157. [Google Scholar] [CrossRef]
- Elliott, T.R.; Perrin, P.B.; Powers, M.B.; Jacobi, K.S.; Warren, A.M. Predictors of Vaccine Hesitancy among Health Care Workers during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 7123. [Google Scholar] [CrossRef]
- Kwok, K.O.; Li, K.K.; Wei, W.I.; Tang, A.; Wong, S.Y.S.; Lee, S.S. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey. Int. J. Nurs. Stud. 2021, 114, 103854. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Intention of health care workers to accept COVID-19 vaccination and related factors: A systematic review and meta-analysis. Asian Pac. J. Trop. Med. 2021, 14, 543–554. [Google Scholar] [CrossRef]
- Huang, D.; Ganti, L.; Graham, E.W.; Shah, D.; Aleksandrovskiy, I.; Al-Bassam, M.; Fraunfelter, F.; Falgiani, M.; Leon, L.; Lopez-Ortiz, C. COVID-19 Vaccine Hesitancy Among Healthcare Providers. Health Psychol. Res. 2022, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, S.; Iheaku, O.; Farrque, M.; Hanna, J.; Johnson, K.B.; Wiley, Z.; Franks, N.M.; Carroll, K.; Shin, S.R.; Sims, K.M.; et al. COVID-19 Vaccine Hesitancy Among Health Care Workers in Four Health Care Systems in Atlanta. Open Forum Infect. Dis. 2022, 9, ofac224. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Hanley, S.; Stewart, T.; Salmon, D.A.; Ortiz, C.; Trief, P.M.; Asiago Reddy, E.; Morley, C.P.; Thomas, S.J.; Anderson, K.B. Healthcare Personnel (HCP) Attitudes About Coronavirus Disease 2019 (COVID-19) Vaccination after Emergency Use Authorization. Clin. Infect. Dis. 2022, 75, e814–e821. [Google Scholar] [CrossRef]
- Browne, S.K.; Feemster, K.A.; Shen, A.K.; Green-McKenzie, J.; Momplaisir, F.M.; Faig, W.; Offit, P.A.; Kuter, B.J. Coronavirus disease 2019 (COVID-19) vaccine hesitancy among physicians, physician assistants, nurse practitioners, and nurses in two academic hospitals in Philadelphia. Infect. Control. Hosp. Epidemiol. 2022, 43, 1424–1432. [Google Scholar] [CrossRef]
- Peterson, C.J.; Lee, B.; Nugent, K. COVID-19 Vaccination Hesitancy among Healthcare Workers—A Review. Vaccines 2022, 10, 948. [Google Scholar] [CrossRef]
- Gadoth, A.; Halbrook, M.; Martin-Blais, R.; Gray, A.; Tobin, N.H.; Ferbas, K.G.; Aldrovandi, G.M.; Rimoin, A.W. Cross-sectional Assessment of COVID-19 Vaccine Acceptance Among Health Care Workers in Los Angeles. Ann. Intern. Med. 2021, 174, 882–885. [Google Scholar] [CrossRef]
- Díaz Luévano, C.; Sicsic, J.; Pellissier, G.; Chyderiotis, S.; Arwidson, P.; Olivier, C.; Gagneux-Brunon, A.; Botelho-Nevers, E.; Bouvet, E.; Mueller, J. Quantifying healthcare and welfare sector workers’ preferences around COVID-19 vaccination: A cross-sectional, single-profile discrete-choice experiment in France. BMJ Open 2021, 11, e055148. [Google Scholar] [CrossRef]
- Koh, S.W.C.; Liow, Y.; Loh, V.W.K.; Liew, S.J.; Chan, Y.H.; Young, D. COVID-19 vaccine acceptance and hesitancy among primary healthcare workers in Singapore. BMC Prim. Care 2022, 23, 81. [Google Scholar] [CrossRef]
- Paris, C.; Bénézit, F.; Geslin, M.; Polard, E.; Baldeyrou, M.; Turmel, V.; Tadié, É.; Garlantezec, R.; Tattevin, P. COVID-19 vaccine hesitancy among healthcare workers. Infect. Dis. Now 2021, 51, 484–487. [Google Scholar] [CrossRef]
- Thompson, B. Exploratory and Confirmatory Factor Analysis: Understanding Concepts and Applications; American Psychological Association: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Xiang, B.; Wong, H.M.; Cao, W.; Perfecto, A.P.; McGrath, C.P.J. Development and validation of the Oral health behavior questionnaire for adolescents based on the health belief model (OHBQAHBM). BMC Public Health 2020, 20, 701. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, X.; Naqvi, A.A.; Zhang, Q.; Zang, X. Translation and validation of the Chinese version of the general medication adherence scale (GMAS) in patients with chronic illness. Curr. Med. Res. Opin. 2021, 37, 829–837. [Google Scholar] [CrossRef]
- Rhea, K.C.; Cater, M.W.; McCarter, K.; Tuuri, G. Psychometric Analyses of the Eating and Food Literacy Behaviors Questionnaire with University Students. J. Nutr. Educ. Behav. 2020, 52, 1008–1017. [Google Scholar] [CrossRef]
- Yu, X.; Xu, C.; Ni, Y.; Chang, R.; Wang, H.; Gong, R.; Wang, Y.; Wang, S.; Cai, Y. Pre-Exposure Prophylaxis (PrEP) Adherence Questionnaire: Psychometric Validation among Sexually Transmitted Infection Patients in China. Int. J. Environ. Res. Public Health 2021, 18, 10980. [Google Scholar] [CrossRef]
- Brown, C.L.; Perrin, E.M. Defining picky eating and its relationship to feeding behaviors and weight status. J. Behav. Med. 2020, 43, 587–595. [Google Scholar] [CrossRef]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. 2022. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 21 January 2023).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Schumacker, R.E.; Lomax, R.G. A Beginner’s Guide to Structural Equation Modeling, 3rd ed.; Routledge: New York, NY, USA, 2010. [Google Scholar]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 9 March 2022).
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 2. [Google Scholar] [CrossRef]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef]
- Al-Sanafi, M.; Sallam, M. Psychological Determinants of COVID-19 Vaccine Acceptance among Healthcare Workers in Kuwait: A Cross-Sectional Study Using the 5C and Vaccine Conspiracy Beliefs Scales. Vaccines 2021, 9, 701. [Google Scholar] [CrossRef]
- Geiger, M.; Rees, F.; Lilleholt, L.; Santana, A.P.; Zettler, I.; Wilhelm, O.; Betsch, C.; Böhm, R. Measuring the 7Cs of Vaccination Readiness. Eur. J. Psychol. Assess. 2022, 38, 261–269. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Levy, M.E.; Gaglani, M.; Irving, S.A.; Stockwell, M.; Dascomb, K.; DeSilva, M.B.; Reese, S.E.; Liao, I.C.; Ong, T.C.; et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA Vaccine Doses Among Immunocompetent Adults During Periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 Sublineages Predominated—VISION Network, 10 States, December 2021–June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Srivastav, A.; de Perio, M.A.; Laney, A.S.; Black, C.L. Influenza and COVID-19 Vaccination Coverage Among Health Care Personnel—United States, 2021–22. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Centers for Medicare & Medicaid Services Data. Available online: https://data.cms.gov/covid-19/covid-19-nursing-home-data (accessed on 21 January 2023).
- Chou, W.Y.S.; Budenz, A. Considering Emotion in COVID-19 Vaccine Communication: Addressing Vaccine Hesitancy and Fostering Vaccine Confidence. Health Commun. 2020, 35, 1718–1722. [Google Scholar] [CrossRef]
- Kaim, A.; Siman-Tov, M.; Jaffe, E.; Adini, B. Effect of a Concise Educational Program on COVID-19 Vaccination Attitudes. Front. Public Health 2021, 9, 767447. [Google Scholar] [CrossRef]
- Li, P.C.; Theis, S.R.; Kelly, D.; Ocampo, T.; Berglund, A.; Morgan, D.; Markert, R.; Fisher, E.; Burtson, K. Impact of an Education Intervention on COVID-19 Vaccine Hesitancy in a Military Base Population. Mil. Med. 2022, 187, e1516–e1522. [Google Scholar] [CrossRef]
- Evans, C.T.; DeYoung, B.J.; Gray, E.L.; Wallia, A.; Ho, J.; Carnethon, M.; Zembower, T.R.; Hirschhorn, L.R.; Wilkins, J.T. Coronavirus disease 2019 (COVID-19) vaccine intentions and uptake in a tertiary-care healthcare system: A longitudinal study. Infect. Control. Hosp. Epidemiol. 2021, 43, 1806–1812. [Google Scholar] [CrossRef]
- Hornsey, M.J.; Fielding, K.S. Attitude roots and Jiu Jitsu persuasion: Understanding and overcoming the motivated rejection of science. Am. Psychol. 2017, 72, 459–473. [Google Scholar] [CrossRef]
| Characteristic | n (%) |
| Sex | |
| Female | 2019 (82.1%) |
| Male | 423 (17.2%) |
| Non-Binary | 5 (0.2%) |
| Prefer Not to Say | 12 (0.5%) |
| Age Group | |
| 18–24 | 114 (4.6%) |
| 25–34 | 560 (22.8%) |
| 35–44 | 545 (22.2%) |
| 45–54 | 613 (24.9%) |
| 55–64 | 545 (22.2%) |
| 65+ | 82 (3.3%) |
| Race/Ethnicity | |
| Asian | 41 (1.7%) |
| Black/ African American | 127 (5.2%) |
| Hispanic/Latino(a) | 39 (1.6%) |
| Native American or Alaska Native | 16 (0.7%) |
| Native Hawaiian or Other Pacific Islander | 1 (0.04%) |
| White/Caucasian | 2181 (88.7%) |
| Two or More Races | 32 (1.3%) |
| Unknown | 22 (0.9%) |
| Role at Carilion * | |
| Nursing | 827 (33.6%) |
| Provider | 194 (7.9%) |
| Management | 280 (11.4%) |
| Other Responsibilities (Patient Care) | 605 (24.6%) |
| Other Responsibilities (Non-Patient Care) | 553 (22.5%) |
| Work Setting | |
| Outpatient | 767 (31.2%) |
| Inpatient | 696 (28.3%) |
| Both | 464 (18.9%) |
| Other | 490 (19.9%) |
| Unsure | 42 (1.7%) |
| Hesitancy Status | |
| Vaccine Hesitant | 446 (18.1%) |
| Vaccine Acceptant | 2013 (81.9%) |
| Survey Item | |
| Vaccine is ineffective | |
| Mean (SD) | 2.20 (±1.02) |
| Median [Min, Max] | 2 [1, 5] |
| Vaccine is unsafe | |
| Mean (SD) | 2.19 (±1.15) |
| Median [Min, Max] | 2 [1, 5] |
| Vaccine won’t stop the pandemic | |
| Mean (SD) | 2.07 (±1.07) |
| Median [Min, Max] | 2 [1, 5] |
| Vaccine was rushed | |
| Mean (SD) | 2.48 (±1.25) |
| Median [Min, Max] | 2 [1, 5] |
| Vaccine gives COVID-19 | |
| Mean (SD) | 1.32 (±0.74) |
| Median [Min, Max] | 1 [1, 5] |
| Natural immunity is superior | |
| Mean (SD) | 2.30 (±1.11) |
| Median [Min, Max] | 2 [1, 5] |
| I generally avoid vaccines | |
| Mean (SD) | 1.62 (±1.06) |
| Median [Min, Max] | 1 [1, 5] |
| Vaccine is harmful | |
| Mean (SD) | 2.00 (±1.10) |
| Median [Min, Max] | 2 [1, 5] |
| COVID-19 is not dangerous | |
| Mean (SD) | 1.45 (±0.86) |
| Median [Min, Max] | 1 [1, 5] |
| Long-term side-effects | |
| Mean (SD) | 3.09 (±1.31) |
| Median [Min, Max] | 3 [1, 5] |
| Short-term side-effects | |
| Mean (SD) | 2.23 (±1.21) |
| Median [Min, Max] | 2 [1, 5] |
| Pregnancy concerns | |
| Mean (SD) | 1.98 (±1.18) |
| Median [Min, Max] | 1 [1, 5] |
| Risk at work | |
| Mean (SD) | 3.00 (±1.29) |
| Median [Min, Max] | 3 [1, 5] |
| Risk outside work | |
| Mean (SD) | 2.96 (±1.13) |
| Median [Min, Max] | 3 [1, 5] |
| Survey Item | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Vaccine is ineffective | 0.827 | – | – | – |
| Vaccine is unsafe | 0.717 | – | – | – |
| Vaccine won’t stop the pandemic | 0.709 | – | – | – |
| Not enough data | 0.615 | – | – | – |
| Vaccine was rushed | 0.492 | – | 0.387 | – |
| Vaccine gives COVID-19 | – | 0.633 | – | – |
| Natural immunity is superior | – | 0.503 | – | – |
| I generally avoid vaccines | – | 0.499 | – | – |
| Vaccine is harmful | – | 0.457 | – | – |
| COVID-19 is not dangerous | – | 0.373 | – | – |
| Long-term side-effects | – | – | 0.694 | – |
| Short-term side-effects | – | 0.339 | 0.491 | – |
| Pregnancy concerns | – | – | – | – |
| Risk at work | – | – | – | 0.686 |
| Risk outside work | – | – | – | 0.677 |
| Proportion of Variance Explained | 0.407 | 0.259 | 0.202 | 0.133 |
| Number of Survey Items | 5 | 5 | 3 | 2 |
| Cronbach’s Alpha | 0.880 | 0.733 | 0.701 | 0.637 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendetson, J.; Swann, M.C.; Lozano, A.; West, J.; Hanlon, A.L.; Crandell, I.; Jatta, M.; Schleupner, C.J.; Baffoe-Bonnie, A. Deepening Our Understanding of COVID-19 Vaccine Decision-Making amongst Healthcare Workers in Southwest Virginia, USA Using Exploratory and Confirmatory Factor Analysis. Vaccines 2023, 11, 556. https://doi.org/10.3390/vaccines11030556
Bendetson J, Swann MC, Lozano A, West J, Hanlon AL, Crandell I, Jatta M, Schleupner CJ, Baffoe-Bonnie A. Deepening Our Understanding of COVID-19 Vaccine Decision-Making amongst Healthcare Workers in Southwest Virginia, USA Using Exploratory and Confirmatory Factor Analysis. Vaccines. 2023; 11(3):556. https://doi.org/10.3390/vaccines11030556
Chicago/Turabian StyleBendetson, Jesse, Mandy C. Swann, Alicia Lozano, Jennifer West, Alexandra L. Hanlon, Ian Crandell, Maimuna Jatta, Charles J. Schleupner, and Anthony Baffoe-Bonnie. 2023. "Deepening Our Understanding of COVID-19 Vaccine Decision-Making amongst Healthcare Workers in Southwest Virginia, USA Using Exploratory and Confirmatory Factor Analysis" Vaccines 11, no. 3: 556. https://doi.org/10.3390/vaccines11030556
APA StyleBendetson, J., Swann, M. C., Lozano, A., West, J., Hanlon, A. L., Crandell, I., Jatta, M., Schleupner, C. J., & Baffoe-Bonnie, A. (2023). Deepening Our Understanding of COVID-19 Vaccine Decision-Making amongst Healthcare Workers in Southwest Virginia, USA Using Exploratory and Confirmatory Factor Analysis. Vaccines, 11(3), 556. https://doi.org/10.3390/vaccines11030556






