Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru
Abstract
:1. Introduction
2. Materials and Methods
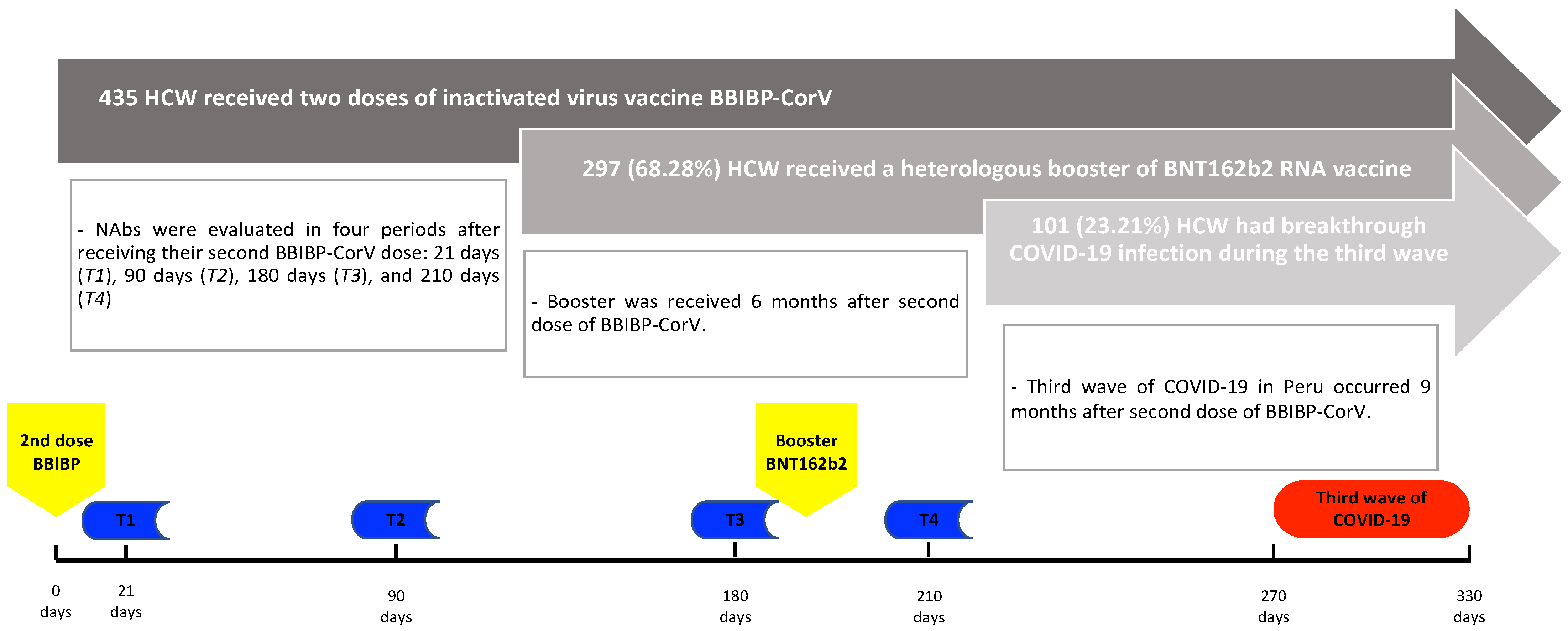
2.1. Population and Study Design
2.2. Outcome
2.3. Statistical Analysis
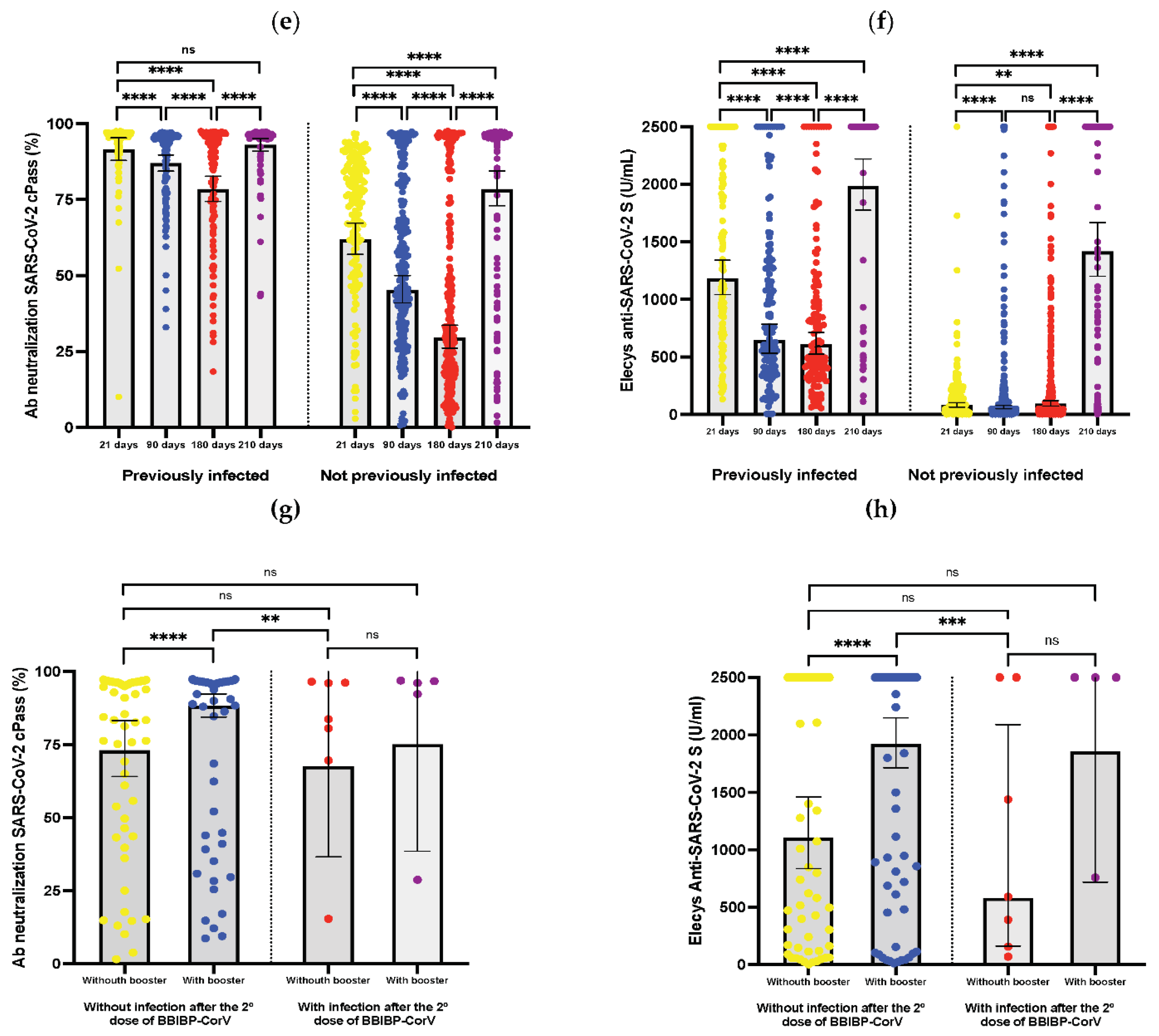
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: A randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 22, 196–208. [Google Scholar] [CrossRef] [PubMed]
- AlHosani, F.I.; Stanciole, A.E.; Aden, B.; Timoshkin, A.; Najim, O.; Zaher, W.A.; AlDhaheri, F.A.; Al Mazrouie, S.; Rizvi, T.A.; Mustafa, F. Impact of the Sinopharm’s BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: Results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE). Vaccine 2022, 40, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Hueda-Zavaleta, M.; de la Torre, J.C.G.; Aguila, J.A.C.-D.; Muro-Rojo, C.; De La Cruz-Escurra, N.; Siles, D.A.; Minchón-Vizconde, D.; Copaja-Corzo, C.; Bardales-Silva, F.; Benites-Zapata, V.A.; et al. Evaluation of the Humoral Immune Response of a Heterologous Vaccination between BBIBP-CorV and BNT162b2 with a Temporal Separation of 7 Months, in Peruvian Healthcare Workers with and without a History of SARS-CoV-2 Infection. Vaccines 2022, 10, 502. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Graichen, H. What is the difference between the first and the second/third wave of COVID-19?—German perspective. J. Orthop. 2021, 24, A1–A3. [Google Scholar] [CrossRef]
- Rampal, L.; Liew, B.S. Malaysia’s third COVID-19 wave—A paradigm shift required. Med. J. Malays. 2021, 76, 1–4. [Google Scholar]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Zhangfan, F.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-COVID-19-vaccine-certain-populations (accessed on 1 June 2022).
- Dosis de Refuerzo: Minsa Inició Vacunación Contra la COVID-19 a Colegios Profesionales de la Salud. Available online: https://www.gob.pe/institucion/minsa/noticias/553216-dosis-de-refuerzo-minsa-inicio-vacunacion-contra-la-COVID-19-a-colegios-profesionales-de-la-salud (accessed on 1 June 2022).
- De la Torre, J.C.G.; Cáceres-DelAguila, J.A.; Muro-Rojo, C.; De La Cruz-Escurra, N.; Copaja-Corzo, C.; Hueda-Zavaleta, M.; Siles, D.A.; Benites-Zapata, V.A. Humoral Immune Response Induced by the BBIBP-CorV Vaccine (Sinopharm) in Healthcare Workers: A Cohort Study. Trop. Med. Infect. Dis. 2022, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.S.; Ogun, O.A.; Simmons, S.R.; Zamparo, J.M.; et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: A retrospective cohort study. Lancet Reg. Health-Am. 2022, 9, 100198. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2021, 22, 483–495. [Google Scholar] [CrossRef] [PubMed]
- COVID-19: Minsa Anuncia El Fin de la Tercera Ola de la Pandemia en El Perú. Available online: https://elperuano.pe/noticia/142675-COVID-19-minsa-anuncia-el-fin-de-la-tercera-ola-de-la-pandemia-en-el-peru (accessed on 7 June 2022).
- Instituto Nacional de SALUD. Distribución de Casos Por Las VOC DELTA—ÓMICRON. Available online: https://web.ins.gob.pe/es/COVID19/georreferenciacion-casos-variante-preocupacion-variable-delta (accessed on 7 June 2022).
- Perú Inicia Plan de Vacunación Contra COVID-19. Available online: https://elperuano.pe/noticia/114960-peru-inicia-plan-de-vacunacion-contra-COVID-19 (accessed on 7 June 2022).
- Chin, E.T.; Leidner, D.; Zhang, Y.; Long, E.; Prince, L.; Schrag, S.J.; Verani, J.R.; Wiegand, R.E.; Alarid-Escudero, F.; Goldhaber-Fiebert, J.D.; et al. Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccines among Incarcerated People in California State Prisons: Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e838–e845. [Google Scholar] [CrossRef]
- Ministerio de Salud Inició la Aplicación de Dosis de Refuerzo de la Vacuna Contra la COVID-19. Available online: https://www.gob.pe/institucion/minsa/noticias/545255-ministerio-de-salud-inicio-la-aplicacion-de-dosis-de-refuerzo-de-la-vacuna-contra-la-COVID-19 (accessed on 7 June 2022).
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-authorizes-first-test-detects-neutralizing-antibodies-recent-or (accessed on 7 June 2022).
- Roche—Doing Now What Patients Need Next. Available online: https://www.roche.com/media/releases/med-cor-2020-12-02 (accessed on 7 June 2022).
- Jung, J.; Rajapakshe, D.; Julien, C.; Devaraj, S. Analytical and clinical performance of cPass neutralizing antibodies assay. Clin. Biochem. 2021, 98, 70–73. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.-J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and Clinical Evaluation of a SARS-CoV-2 Surrogate Virus Neutralisation Test (SVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Riester, E.; Findeisen, P.; Hegel, J.K.; Kabesch, M.; Ambrosch, A.; Rank, C.M.; Pessl, F.; Laengin, T.; Niederhauser, C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J. Virol. Methods 2021, 297, 114271. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud. Secuenciación Genómica Del Virus SARS-CoV-2 en El Perú. Available online: https://web.ins.gob.pe/es/COVID19/secuenciamiento-sars-cov2 (accessed on 7 June 2022).
- Ledda, C.; Costantino, C.; Motta, G.; Cunsolo, R.; Stracquadanio, P.; Liberti, G.; Maltezou, H.C.; Rapisarda, V. SARS-CoV-2 mRNA Vaccine Breakthrough Infections in Fully Vaccinated Healthcare Personnel: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 9. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Holtgrave, D.R.; Dorabawila, V.; Conroy, M.; Greene, D.; Lutterloh, E.; Backenson, B.; Hoefer, D.; Morne, J.; Bauer, U.; et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status—New York, May 3–July 25, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1150–1155. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, Y.; Zhang, H.; Zhang, Q.; Fu, Z.; Lin, K.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: Interim results from a prospective open-label study. Emerg. Microbes Infect. 2022, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cui, T.; Huang, M.; Liu, S.; Su, X.; Li, G.; Song, T.; Li, W.; Zhong, N.; Xu, M.; et al. Heterologous boosting with third dose of coronavirus disease recombinant subunit vaccine increases neutralizing antibodies and T cell immunity against different severe acute respiratory syndrome coronavirus 2 variants. Emerg. Microbes Infect. 2022, 11, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes Infect. 2021, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Herrera, N.; Araujo-Castillo, R.V.; Mestanza, O.; Galarza, M.; Rojas-Serrano, N.; Solari-Zerpa, L. SARS-CoV-2 Lambda and Gamma variants competition in Peru, a country with high seroprevalence. Lancet Reg. Health Am. 2022, 6, 100112. [Google Scholar] [CrossRef]
- Mileto, D.; Micheli, V.; Fenizia, C.; Cutrera, M.; Gagliardi, G.; Mancon, A.; Bracchitta, F.; De Silvestri, A.; Rizzardini, G.; Lombardi, A.; et al. Reduced neutralization of SARS-CoV-2 Omicron variant by BNT162b2 vaccinees’ sera: A preliminary evaluation. Emerg. Microbes Infect. 2022, 11, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults—United States, March–July 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1156–1162. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune Correlates Analysis of the mRNA-1273 COVID-19 Vaccine Efficacy Trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Coppeta, L.; Somma, G.; Ferrari, C.; Mazza, A.; Rizza, S.; Aurilio, M.T.; Perrone, S.; Magrini, A.; Pietroiusti, A. Persistence of Anti-S Titre among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19. Vaccines 2021, 9, 947. [Google Scholar] [CrossRef]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Maeda, K.; Matsuda, K.; Tanaka, A.; Horii, K.; Okudera, K.; Takeuchi, J.S.; Mizoue, T.; Konishi, M.; Ozeki, M.; et al. Coronavirus Disease 2019 (COVID-19) Breakthrough Infection and Post-Vaccination Neutralizing Antibodies among Healthcare Workers in a Referral Hospital in Tokyo: A Case-Control Matching Study. Clin. Infect. Dis. 2021, 75, e683–e691. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Pradenas, E.; Trinité, B.; Urrea, V.; Marfil, S.; Ávila-Nieto, C.; de la Concepción, M.L.R.; Tarrés-Freixas, F.; Pérez-Yanes, S.; Rovirosa, C.; Ainsua-Enrich, E.; et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med 2021, 2, 313–320.e4. [Google Scholar] [CrossRef]
- Modenese, A.; Casolari, L.; Rossi, G.; Della Vecchia, E.; Glieca, F.; D’Elia, C.; Garavini, D.; Righi, E.; Mariani, S.; Venturelli, L.; et al. Factors Associated with SARS-CoV-2 Infection Risk among Healthcare Workers of an Italian University Hospital. Healthcare 2021, 9, 1495. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 435) | VBI (n= 101) | Not VBI (n = 334) | p-Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years * | 34.0 (28–42.5) | 33 (27–38) | 34 (29–43) | 0.084 a |
| Sex (%) | 0.027 b | |||
| Female | 340 (78.16) | 87 (86.14) | 253 (75.75) | |
| Male | 95 (21.84) | 14 (13.86) | 81 (24.25) | |
| Laboral area (%) | 0.044 | |||
| Phlebotomy | 57 (13.10) | 14 (13.86) | 43 (12.87) | |
| Customer service | 144 (33.10) | 27 (26.73) | 117 (35.03) | |
| Maintenance service | 65 (14.95) | 12 (11.89) | 53 (15.87) | |
| Analytic process | 122 (28.05) | 40 (39.60) | 82 (24.55) | |
| Administrative | 47 (10.80) | 8 (7.92) | 39 (11.68) | |
| Infection before the third wave (%) | 138 (31.72) | 27 (26.73) | 111 (33.23) | 0.219 b |
| Before the first dose of BBIBP-CorV | 122 (28.04) | 26 (25.74) | 96 (28.74) | 0.556 b |
| After the first dose of BBIBP-CorV | 11 (2.52) | 0 (0.00) | 11 (3.30) | 0.075 c |
| After the second dose of BBIBP-CorV | 14 (3.21) | 6 (5.94) | 8 (2.40) | 0.077 c |
| Number of doses | 0.633 b | |||
| Two doses of BBIBP-CorV | 138 (31.72) | 34 (33.66) | 104 (31.14) | |
| Two doses of BBIBP-CorV plus booster BNT162b2 | 297 (68.28) | 67 (66.34) | 230 (68.86) | |
| Time from the first infection until the third wave | 507.5 (317–558) | 541 (344–599) | 492 (317–557) | 0.084 a |
| Days since first infection until VBI | - | 539.5 (310–579) | - | |
| Days since infection before the first dose of BBIBP-CorV until VBI | - | 555 (366–610) | - | |
| Days since infection after the second dose of BBIBP-CorV until VBI | - | 175.5 (135–208) | - | |
| Humoral response rates21 days after the second dose (%) | ||||
| Elecys Anti-SARS-CoV-2 S (n = 303; VBI = 74, not VBI = 229) (+) | ||||
| ≥0.8 | 296 (97.69) | 73 (98.64) | 223 (97.38) | 0.999 c |
| ≥500 | 120 (39.60) | 27 (36.48) | 93 (40.61) | 0.528 b |
| ≥1000 | 84 (27.72) | 17 (22.97) | 67 (29.25) | 0.379 c |
| Ab neutralization cPass (n = 280; VBI = 69, not VBI = 211) (+) | ||||
| ≥30% | 265 (94.64) | 66 (95.65) | 199 (94.31) | 0.999 c |
| ≥60% | 230 (82.14) | 51 (73.91) | 179 (84.83) | 0.047 c |
| ≥90% | 125 (44.64) | 25 (36.23) | 100 (47.39) | 0.125 c |
| 90 days after the second dose (%) | ||||
| Elecys Anti-SARS-CoV-2 S (n = 384; VBI = 91, not VBI = 293) (+) | ||||
| ≥0.8 | 372 (96.88) | 86 (94.50) | 286 (97.61) | 0.165 c |
| ≥500 | 128 (33.33) | 26 (28.57) | 102 (34.81) | 0.270 b |
| ≥1000 | 76 (19.79) | 15 (16.48) | 61 (20.81) | 0.452 c |
| Ab neutralization cPass (n = 356; VBI = 83, not VBI = 273) (+) | ||||
| ≥30% | 311 (87.36) | 71 (85.54) | 240 (87.91) | 0.574 c |
| ≥60% | 217 (60.96) | 48 (57.83) | 169 (61.90) | 0.523 c |
| ≥90% | 122 (34.27) | 22 (24.17) | 100 (36.63) | 0.089 b |
| 180 days after the second dose (%) | ||||
| Elecys Anti-SARS-CoV-2 S (n = 395; VBI = 92, not VBI = 303) (+) | ||||
| ≥0.8 | 384 (97.22) | 88 (95.65) | 296 (97.68) | 0.290 c |
| ≥500 | 132 (33.42) | 20 (21.73) | 112 (36.96) | 0.008 c |
| ≥1000 | 75 (18.99) | 11 (11.95) | 64 (21.12) | 0.050 c |
| Ab neutralization cPass (n = 383; VBI = 88, not VBI = 295) (+) | ||||
| ≥30% | 261 (68.15) | 54 (61.36) | 207 (70.16) | 0.120 b |
| ≥60% | 185 (48.30) | 30 (34.09) | 155 (52.54) | 0.002 b |
| ≥90% | 112 (29.24) | 17 (19.31) | 95 (32.20) | 0.020 b |
| 210 days after second dose (%) | ||||
| Elecys Anti-SARS-CoV-2 S (n = 366; VBI = 91; not VBI = 275) (+) | ||||
| ≥0.8 | 365 (99.73) | 90 (98.90) | 275 (100.0) | 0.249 c |
| ≥500 | 323 (88.25) | 78 (85.71) | 245 (89.09) | 0.452 c |
| ≥1000 | 307 (83.88) | 75 (82.41) | 232 (84.36) | 0.742 c |
| Ab neutralization cPass (n = 364; VBI = 90, not VBI = 274) (+) | ||||
| ≥30% | 345 (94.78) | 84 (93.33) | 261 (95.25) | 0.584 c |
| ≥60% | 329 (90.38) | 80 (88.88) | 249 (90.87) | 0.544 c |
| ≥90% | 307 (84.34) | 76 (84.44) | 231 (84.30) | 0.999 c |
| Variable | cRR (95% CI) | p-Value | aRR (95% CI) | p-Value |
|---|---|---|---|---|
| Male sex | 0.575 (0.343–0.965) | 0.037 | 0.430 (0.226–0.816) | 0.010 |
| Previously infected before third wave | 0.785 (0.530–1.162) | 0.227 | 1.688 (0.800–3.559) | 0.169 |
| Infection before the first dose of BBIBP-CorV | 0.889 (0.599–1.319) | 0.560 | ||
| Infection after the first dose of BBIBP-CorV | 1.899 (1.010–3.569) | 0.046 | ||
| Time since the first infection until the third wave | 1.001 (0.998–1.004) | 0.210 | ||
| Nº doses | ||||
| Two doses BBIBP-CorV | Ref. | - | ||
| Two doses BBIBP-CorV plus booster BNT162b2 | 0.874 (0.612–1.247) | 0.459 | ||
| Ab neutralization cPass at 21 days ≥ 60% | 0.615 (0.395–0.958) | 0.032 | 0.621 (0.397–0.971) | 0.037 |
| Ab neutralization cPass at 180 days ≥ 60% | 0.553 (0.373–0. 820) | 0.003 | 0.588 (0.396–0.874) | 0.009 |
| Ab neutralization cPass at 180 days ≥ 90% | 0.579 (0.357–0.938) | 0.026 | 0.598 (0.371–0.964) | 0.035 |
| Elecys Anti-SARS-CoV-2 S at 180 days ≥ 500 | 0.553 (0.353–0.867) | 0.010 | 0.585 (0.373–0.916) | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hueda-Zavaleta, M.; Gómez de la Torre, J.C.; Cáceres-DelAguila, J.A.; Muro-Rojo, C.; De La Cruz-Escurra, N.; Copaja-Corzo, C.; Aragón-Ayala, C.J.; Benítes-Zapata, V.A. Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru. Vaccines 2023, 11, 447. https://doi.org/10.3390/vaccines11020447
Hueda-Zavaleta M, Gómez de la Torre JC, Cáceres-DelAguila JA, Muro-Rojo C, De La Cruz-Escurra N, Copaja-Corzo C, Aragón-Ayala CJ, Benítes-Zapata VA. Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru. Vaccines. 2023; 11(2):447. https://doi.org/10.3390/vaccines11020447
Chicago/Turabian StyleHueda-Zavaleta, Miguel, Juan C. Gómez de la Torre, José Alonso Cáceres-DelAguila, Cecilia Muro-Rojo, Nathalia De La Cruz-Escurra, Cesar Copaja-Corzo, Carlos J. Aragón-Ayala, and Vicente A. Benítes-Zapata. 2023. "Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru" Vaccines 11, no. 2: 447. https://doi.org/10.3390/vaccines11020447
APA StyleHueda-Zavaleta, M., Gómez de la Torre, J. C., Cáceres-DelAguila, J. A., Muro-Rojo, C., De La Cruz-Escurra, N., Copaja-Corzo, C., Aragón-Ayala, C. J., & Benítes-Zapata, V. A. (2023). Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru. Vaccines, 11(2), 447. https://doi.org/10.3390/vaccines11020447









