Patients with SARS-CoV-2-Induced Viral Sepsis Simultaneously Show Immune Activation, Impaired Immune Function and a Procoagulatory Disease State
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Ethics
2.3. Cellular Analyzes
2.4. Flow Cytometry
2.5. Multiplex ELISA
2.6. Functional Immunoassays–Stimulation of Monocytes and T Cells
2.7. Phagocytosis Assay (E. coli-pHrodo)
2.8. Isolation and Quantification of Mitochondrial DNA
2.9. Data and Statistical Analysis
3. Results
3.1. Study Population
3.2. Severe COVID-19 Patients Show Increased Granulocyte but Decreased Lymphocyte Numbers
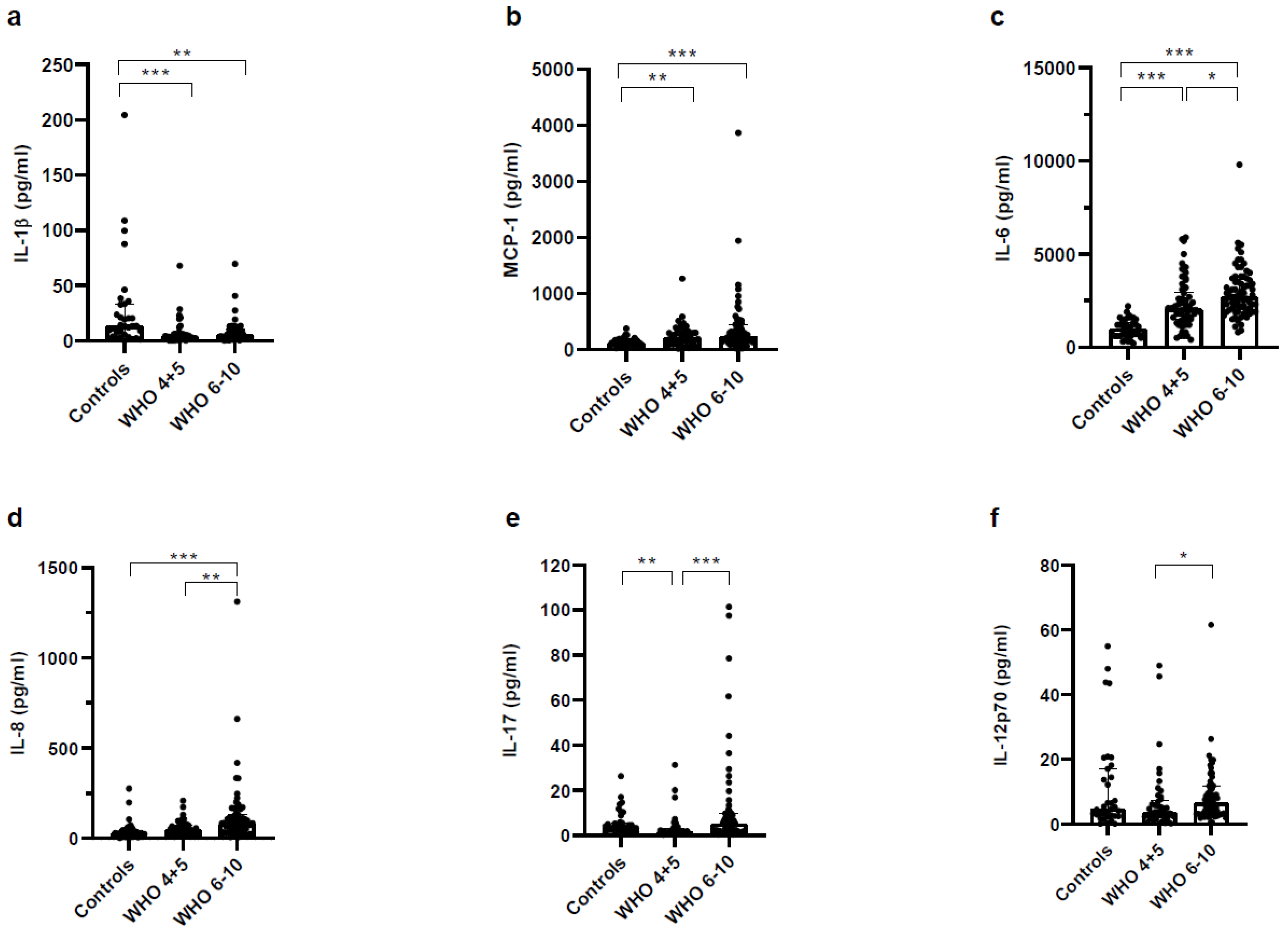
3.3. Higher Concentrations of Pro- and Anti-Inflammatory Mediators in Plasma of Severe COVID-19 Patients
3.4. Higher Concentrations of Pro- and Anti-Inflammatory Soluble Receptors in Plasma of Severe COVID-19 Patients
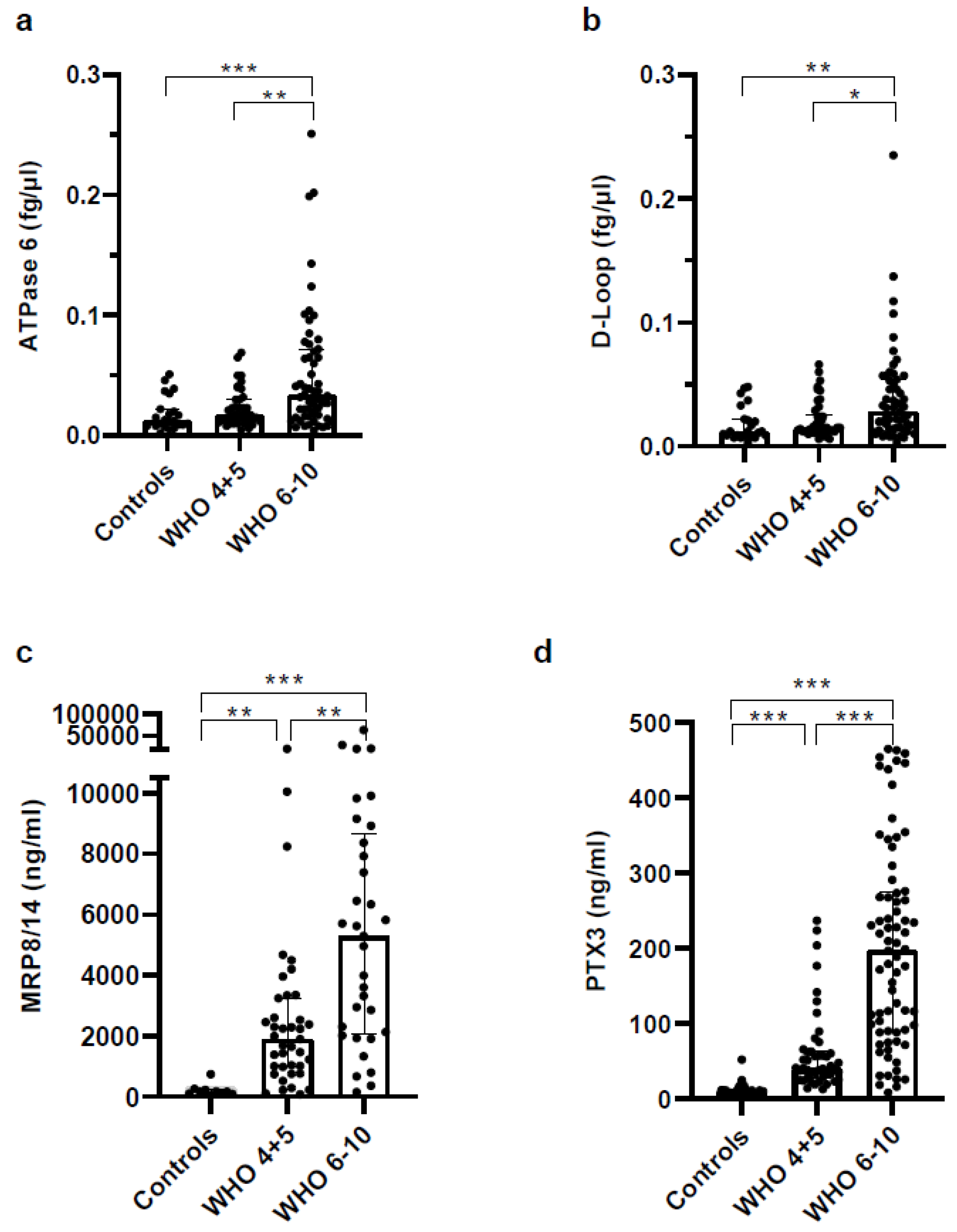
3.5. Biomarkers Reflecting Organ Dysfunction Are Significantly Upregulated in Severe COVID-19 Patients
3.6. Concentrations of Soluble Pattern Recognition Receptors Are Increased in COVID Patients but Strongly Increased in Plasma of Severe COVID-19 Patients
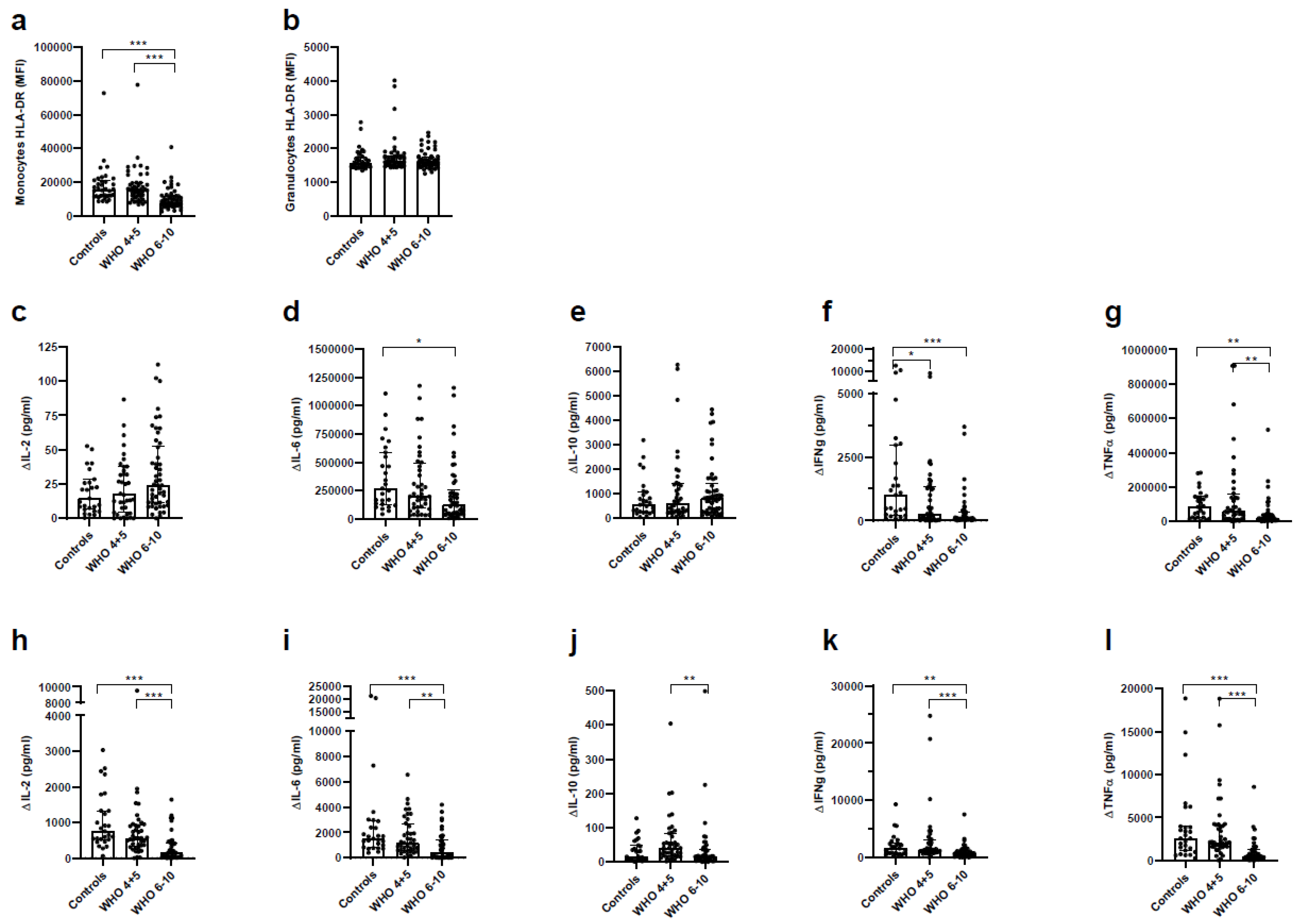
3.7. Impaired Immune Function of T Cells and Monocytes in Severe COVID-19 Patients
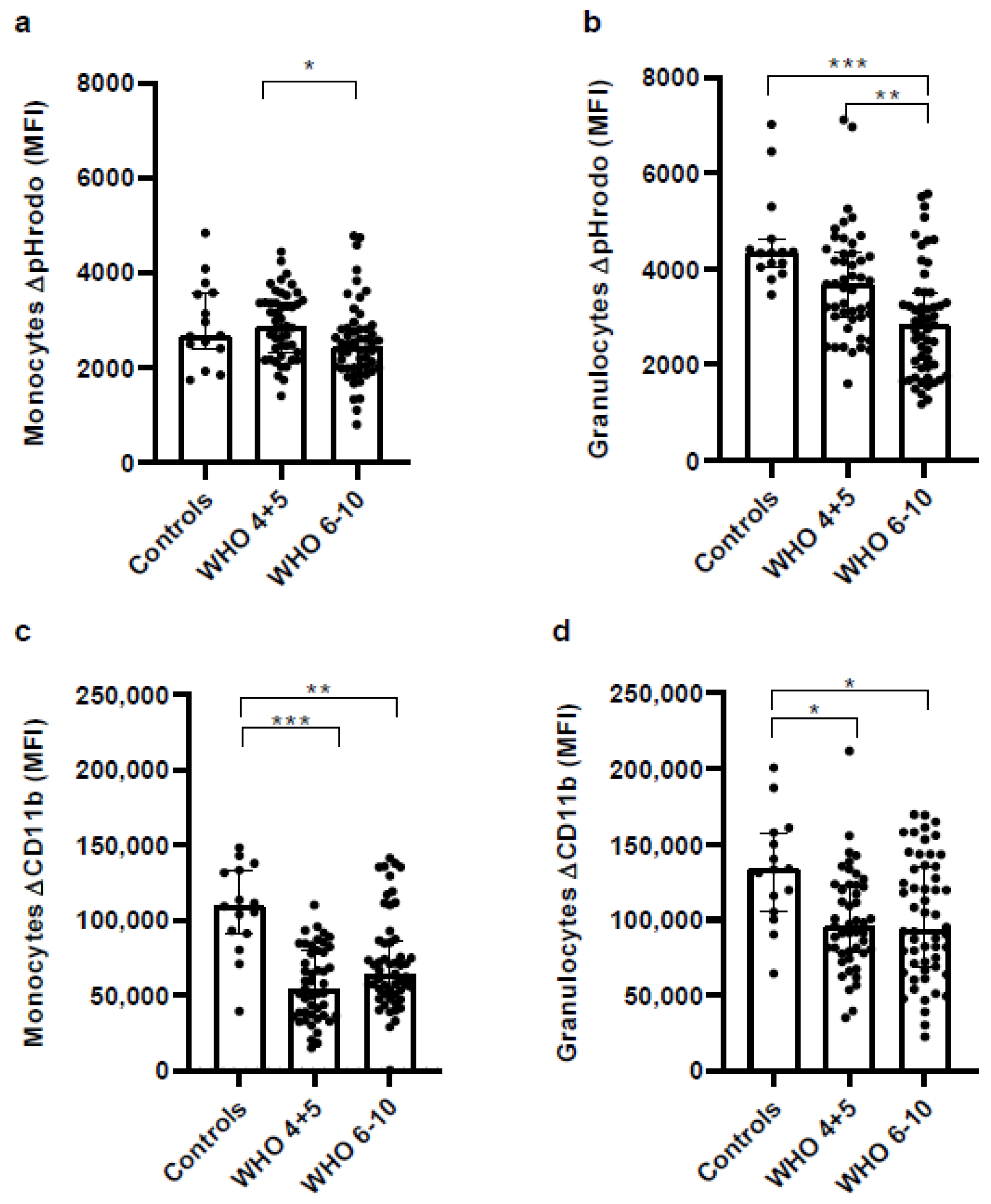
3.8. Impaired Phagocytic Activity of Monocytes and Granulocytes in Severe COVID-19 Patients
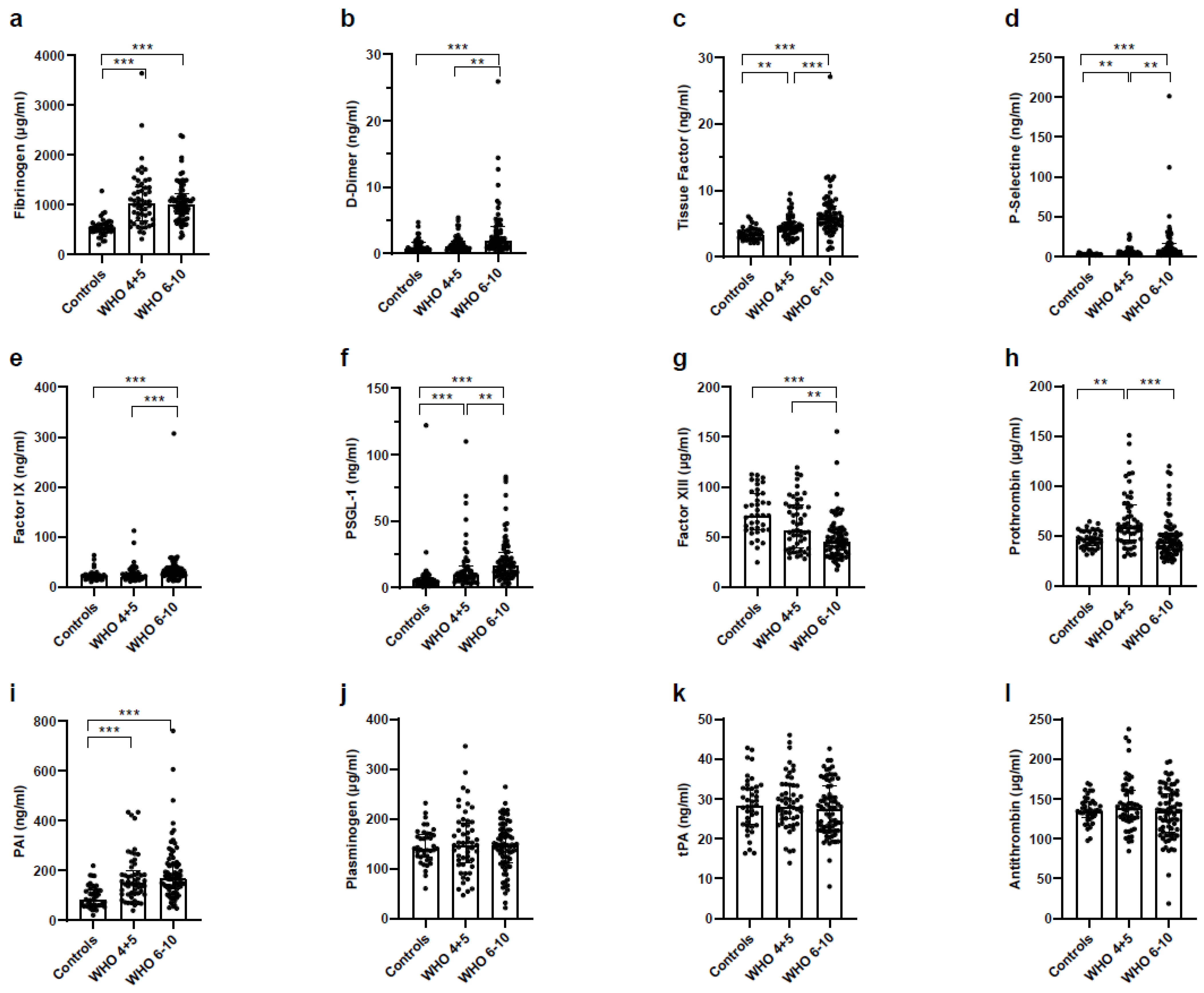
3.9. Increased Coagulation and Decreased Fibrinolysis in Severe COVID-19 Patients
3.10. Only Minor Phenotypic and Functional Changes over Time in COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Godoy, L.C.; Goligher, E.C.; Lawler, P.R.; Slutsky, A.S.; Zarychanski, R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ 2020, 192, E1156–E1161. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Cron, R.Q. One-two punch of cytokine storm syndrome. Blood 2020, 136, 645–646. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, N.; Jin, R.; Feng, Y.; Wang, S.; Gao, S.; Gao, R.; Wu, G.; Tian, D.; Tan, W.; et al. Immune suppression in the early stage of COVID-19 disease. Nat. Commun. 2020, 11, 5859. [Google Scholar] [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Loftus, T.J.; Ungaro, R.; Dirain, M.; Efron, P.A.; Mazer, M.B.; Remy, K.E.; Hotchkiss, R.S.; Zhong, L.; Bacher, R.; Starostik, P.; et al. Overlapping but Disparate Inflammatory and Immunosuppressive Responses to SARS-CoV-2 and Bacterial Sepsis: An Immunological Time Course Analysis. Front Immunol. 2021, 12, 792448. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Remy, K.E.; Brakenridge, S.C.; Francois, B.; Daix, T.; Deutschman, C.S.; Monneret, G.; Jeannet, R.; Laterre, P.-F.; Hotchkiss, R.S.; Moldawer, L.L. Immunotherapies for COVID-19: Lessons learned from sepsis. Lancet Respir. Med. 2020, 8, 946–949. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Cui, X.; Chen, W.; Zhou, H.; Gong, Y.; Zhu, B.; Lv, X.; Guo, H.; Duan, J.; Zhou, J.; Marcon, E.; et al. Pulmonary Edema in COVID-19 Patients: Mechanisms and Treatment Potential. Front. Pharmacol. 2021, 12, 664349. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Nossent, E.J.; Schuurman, A.R.; Reijnders, T.D.; Saris, A.; Jongerius, I.; Blok, S.G.; de Vries, H.; Duitman, J.; Noordegraaf, A.V.; Meijboom, L.J.; et al. Pulmonary Procoagulant and Innate Immune Responses in Critically Ill COVID-19 Patients. Front. Immunol. 2021, 12, 664209. [Google Scholar] [CrossRef]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.T.; Franken, L.; Adamzik, M.; Schumak, B.; Scherag, A.; Engler, A.; Schonborn, N.; Walden, J.; Koch, S.; Baba, H.A.; et al. Mitochondrial DNA: An Endogenous Trigger for Immune Paralysis. Anesthesiology 2016, 124, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Frosch, M.; Strey, A.; Vogl, T.; Wulffraat, N.M.; Kuis, W.; Sunderkötter, C.; Harms, E.; Sorg, C.; Roth, J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000, 43, 628–637. [Google Scholar] [CrossRef]
- Cron, R.Q. COVID-19 cytokine storm: Targeting the appropriate cytokine. Lancet Rheumatol. 2021, 3, e236–e237. [Google Scholar] [CrossRef] [PubMed]
- Cron, R.Q. No perfect therapy for the imperfect COVID-19 cytokine storm. Lancet Rheumatol. 2022, 4, e308–e310. [Google Scholar] [CrossRef]
- Karakike, E.; Giamarellos-Bourboulis, E.J.; Kyprianou, M.; Fleischmann-Struzek, C.; Pletz, M.W.; Netea, M.G.; Reinhart, K.; Kyriazopoulou, E. Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2021, 49, 2042–2057. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe COVID-19 induces a TGF-beta-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef]
- Witkowski, M.; Tizian, C.; Ferreira-Gomes, M.; Niemeyer, D.; Jones, T.C.; Heinrich, F.; Frischbutter, S.; Angermair, S.; Hohnstein, T.; Mattiola, I.; et al. Untimely TGFbeta responses in COVID-19 limit antiviral functions of NK cells. Nature 2021, 600, 295–301. [Google Scholar] [CrossRef]
- Bonnet, B.; Cosme, J.; Dupuis, C.; Coupez, E.; Adda, M.; Calvet, L.; Fabre, L.; Saint-Sardos, P.; Bereiziat, M.; Vidal, M.; et al. Severe COVID-19 is characterized by the co-occurrence of moderate cytokine inflammation and severe monocyte dysregulation. eBioMedicine 2021, 73, 103622. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J. Complex immune deregulation in severe COVID-19: More than a mechanism of pathogenesis. eBioMedicine 2021, 73, 103673. [Google Scholar] [CrossRef]
- Masia, M.; Fernandez-Gonzalez, M.; Garcia, J.A.; Padilla, S.; Garcia-Abellan, J.; Botella, A.; Mascarell, P.; Agullo, V.; Gutierrez, F. Robust long-term immunity to SARS-CoV-2 in patients recovered from severe COVID-19 after interleukin-6 blockade. eBioMedicine 2022, 82, 104153. [Google Scholar] [CrossRef]
- Sinha, P. Is a “cytokine storm” relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensiv. Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Opal, S.M. Activating Immunity to Fight a Foe—A New Path. N. Engl. J. Med. 2020, 382, 1270–1272. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Campbell, R.A.; Hisada, Y.; Denorme, F.; Grover, S.P.; Bouck, E.G.; Middleton, E.A.; Wolberg, A.S.; Rondina, M.T.; Mackman, N. Comparison of the coagulopathies associated with COVID-19 and sepsis. Res. Pract. Thromb. Haemost. 2021, 5, e12525. [Google Scholar] [CrossRef]
- Bouck, E.G.; Denorme, F.; Holle, L.A.; Middelton, E.A.; Blair, A.; De Laat, B.; Schiffman, J.D.; Yost, C.C.; Rondina, M.T.; Wolberg, A.S.; et al. COVID-19 and Sepsis Are Associated With Different Abnormalities in Plasma Procoagulant and Fibrinolytic Activity. Arterioscler. Thromb. Vasc. Biol. 2020, 41, 401–414. [Google Scholar] [CrossRef]
- Pluta, J.; Cieniewicz, A.; Trzebicki, J. COVID-19: Coagulation disorders and anticoagulant treatment in patients hospitalised in ICU. Anaesthesiol. Intensiv. Ther. 2021, 53, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pluta, J.; Pihowicz, A.; Horban, A.; Trzebicki, J. DIC, SIC or CAC—The haemostatic profile in COVID-19 patients hospitalised in the intensive care unit: A single-centre retrospective analysis. Anaesthesiol. Intensiv. Ther. 2021, 53, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Bunch, C.M.; Moore, E.E.; Moore, H.B.; Neal, M.D.; Thomas, A.V.; Zackariya, N.; Zhao, J.; Zackariya, S.; Brenner, T.J.; Berquist, M.; et al. Immuno-Thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation. Front. Surg. 2022, 9, 889999. [Google Scholar] [CrossRef] [PubMed]
- Coveney, A.P.; Wang, W.; Kelly, J.; Liu, J.H.; Blankson, S.; Wu, Q.D.; Redmond, H.P.; Wang, J.H. Myeloid-related protein 8 induces self-tolerance and cross-tolerance to bacterial infection via TLR4- and TLR2-mediated signal pathways. Sci. Rep. 2015, 5, 13694. [Google Scholar] [CrossRef] [PubMed]
- Freise, N.; Burghard, A.; Ortkras, T.; Daber, N.; Chasan, A.I.; Jauch, S.-L.; Fehler, O.; Hillebrand, J.; Schakaki, M.; Rojas, J.; et al. Signaling mechanisms inducing hyporesponsiveness of phagocytes during systemic inflammation. Blood 2019, 134, 134–146. [Google Scholar] [CrossRef]
- Nakahira, K.; Kyung, S.-Y.; Rogers, A.J.; Gazourian, L.; Youn, S.; Massaro, A.F.; Quintana, C.; Osorio, J.C.; Wang, Z.; Zhao, Y.; et al. Circulating Mitochondrial DNA in Patients in the ICU as a Marker of Mortality: Derivation and Validation. PLoS Med. 2013, 10, e1001577. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Yu, B.; Li, L.; Luo, L.; Wu, F.; Wu, B. Association between circulating mononuclear cell mitochondrial DNA copy number and in-hospital mortality in septic patients: A prospective observational study based on the Sepsis-3 definition. PLoS ONE 2019, 14, e0212808. [Google Scholar] [CrossRef]
- Vogl, T.; Eisenblatter, M.; Voller, T.; Zenker, S.; Hermann, S.; van Lent, P.; Faust, A.; Geyer, C.; Petersen, B.; Roebrock, K.; et al. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat. Commun. 2014, 5, 4593. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Schwandt, T.; Schumak, B.; Gielen, G.H.; Jüngerkes, F.; Schmidbauer, P.; Klocke, K.; Staratschek-Jox, A.; Van Rooijen, N.; Kraal, G.; Ludwig-Portugall, I.; et al. Expression of type I interferon by splenic macrophages suppresses adaptive immunity during sepsis. EMBO J. 2011, 31, 201–213. [Google Scholar] [CrossRef]
- Austermann, J.; Friesenhagen, J.; Fassl, S.K.; Ortkras, T.; Burgmann, J.; Barczyk-Kahlert, K.; Faist, E.; Zedler, S.; Pirr, S.; Rohde, C.; et al. Alarmins MRP8 and MRP14 Induce Stress Tolerance in Phagocytes under Sterile Inflammatory Conditions. Cell Rep. 2014, 9, 2112–2123. [Google Scholar] [CrossRef]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef]
- Karimi, A.; Shobeiri, P.; Kulasinghe, A.; Rezaei, N. Novel Systemic Inflammation Markers to Predict COVID-19 Prognosis. Front. Immunol. 2021, 12, 741061. [Google Scholar] [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.-G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e18. [Google Scholar] [CrossRef]
- Chapuis, N.; Ibrahimi, N.; Belmondo, T.; Goulvestre, C.; Berger, A.-E.; Mariaggi, A.-A.; Andrieu, M.; Chenevier-Gobeaux, C.; Bayle, A.; Campos, L.; et al. Dynamics of circulating calprotectin accurately predict the outcome of moderate COVID-19 patients. eBioMedicine 2022, 80, 104077. [Google Scholar] [CrossRef]



| Demographics | Total (n = 127) | WHO 4–5 (n = 52) | WHO 6–10 (n = 75) | p-Value |
|---|---|---|---|---|
| Male/Female | 82/45 | 30/22 | 52/23 | 0.1917 $ |
| Age (years), median (range) | 61 (19–88) | 61 (21–88) | 62 (19–86) | 0.7021 § |
| BMI (kg/m2), median (range) | 27.8 (18.4–52.7) | 25.45 (18.4–39) | 29.2 (20.1–52.7) | 0.0006 § |
| Charlson Comorbidity Index, median (range) | 1 (0–9) | 1 (0–9) | 1 (0–9) | 0.1316 § |
| SOFA on admission, median (range) | 2 (0–11) | 0 (0–3) | 2 (0–11) | <0.0001 § |
| SAPS II on admission, median (range) | 27 (0–54) | 22 (0–28) | 31 (0–54) | <0.0001 § |
| GCS, median (range) | 15 (3–15) | 15 (15) | 15 (3–15) | 0.5126 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limmer, A.; Engler, A.; Kattner, S.; Gregorius, J.; Pattberg, K.T.; Schulz, R.; Schwab, J.; Roth, J.; Vogl, T.; Krawczyk, A.; et al. Patients with SARS-CoV-2-Induced Viral Sepsis Simultaneously Show Immune Activation, Impaired Immune Function and a Procoagulatory Disease State. Vaccines 2023, 11, 435. https://doi.org/10.3390/vaccines11020435
Limmer A, Engler A, Kattner S, Gregorius J, Pattberg KT, Schulz R, Schwab J, Roth J, Vogl T, Krawczyk A, et al. Patients with SARS-CoV-2-Induced Viral Sepsis Simultaneously Show Immune Activation, Impaired Immune Function and a Procoagulatory Disease State. Vaccines. 2023; 11(2):435. https://doi.org/10.3390/vaccines11020435
Chicago/Turabian StyleLimmer, Andreas, Andrea Engler, Simone Kattner, Jonas Gregorius, Kevin Thomas Pattberg, Rebecca Schulz, Jansje Schwab, Johannes Roth, Thomas Vogl, Adalbert Krawczyk, and et al. 2023. "Patients with SARS-CoV-2-Induced Viral Sepsis Simultaneously Show Immune Activation, Impaired Immune Function and a Procoagulatory Disease State" Vaccines 11, no. 2: 435. https://doi.org/10.3390/vaccines11020435
APA StyleLimmer, A., Engler, A., Kattner, S., Gregorius, J., Pattberg, K. T., Schulz, R., Schwab, J., Roth, J., Vogl, T., Krawczyk, A., Witzke, O., Zelinskyy, G., Dittmer, U., Brenner, T., & Berger, M. M. (2023). Patients with SARS-CoV-2-Induced Viral Sepsis Simultaneously Show Immune Activation, Impaired Immune Function and a Procoagulatory Disease State. Vaccines, 11(2), 435. https://doi.org/10.3390/vaccines11020435








