Abstract
Background: Understanding immune responses after HBV vaccination is important to prevent HBV infection in PLWH and to achieve successful treatment. Methods: Thirty-two PLWHs with CD4+ cell count > 350 cells/µL and HIV RNA < 200 copies/mL were vaccinated with 20 µg of HBV vaccine at weeks 0, 4, and 24 in this prospective study. We measured total HIV DNA levels, HBsAb titers and HBsAg-specific T-cell responses during follow-up time. Results: All patients achieved protective HBsAb titer after immunization. The magnitude of the IFN-r and TNF-a response to HBsAg was 22.0 (IQR: 6.5–65.0) and 106.50 (IQR: 58.5–203.0) spot-forming cells (SFC)/105 PBMC, respectively at week 0. The level of IFN-r secreted at weeks 12 and weeks 36 to 48 was comparable with that at week 0. However, IFN-r response was higher at weeks 12 than that at weeks 36 to 48 (p = 0.02). The level of TNF-a secreted at weeks 12 was higher than that at week 0 (p < 0.001). Total HIV DNA levels were 2.76 (IQR: 2.47–3.07), 2.77 (IQR: 2.50–3.09), 2.77(IQR: 2.41–2.89) log10 copies/106 PBMCs at weeks 0, 12, 36 to 48, respectively. No correlation was observed between IFN-r and TNF-a levels and HBsAb titer as well as total HIV DNA levels after immunization. Conclusion: Humoral immunity was satisfactory, but cellular immunity and decline in HIV reservoir were not optimal after HBV vaccine immunization in these patients.
1. Introduction
Approximately 7.5% of people living with human immunodeficiency virus (PLWHs) worldwide are coinfected with hepatitis B virus (HBV) due to the same transmission routes [1]. The coinfection prevalence varies in different regions. For example, it is higher than 10% in sub-Saharan Africa, west Africa, east Asia, and India [1,2]. HIV/HBV-coinfected patients are more likely to develop liver-associated diseases [3,4].
Some patients treated with a TDF or TAF-based regimens are protected from HBV infection [5]. However, the new long-acting drugs such as cabotegravir and rilpivirine cannot protect from HBV, and people with HBV are not eligible for this treatment [6]. Therefore, vaccination is strongly suggested for a better treatment choice and, consequently, a better quality of life in PLWHs with negative hepatitis B serology [7]. The standard recombinant HBV vaccine could achieve 95% seroprotection in HIV-negative adults [8,9]. Unfortunately, the immunogenicity of the HBV vaccine achieved in PLWH ranges from 35% to 70% [10]. Furthermore, a study demonstrated that PLWHs who reached optimal vaccine responses failed to maintain HBV immunity for a long time [11]. The mean time to lose an effective antibody to hepatitis B surface antigen (HBsAb) was 2, 3.7, and 4.4 years for patients with an HBsAb titer of 10–100 IU/I, >100–1000 IU/I, and >1000 IU/I at primary vaccination, respectively [12]. Age, dose of the HBV vaccine, CD4+ cell counts, low HIV RNA levels and combination antiretroviral therapy (cART) were associated with HBV vaccine responses in PLWHs [10,13,14].
Cellular immunity plays a crucial role in HBV vaccination. Previous studies have evaluated cellular immune responses to HBV vaccination by different variables in HIV-negative people [15,16]. Simons et al. demonstrated that interferon-gamma (IFN-r) and tumor necrosis factor-alpha (TNF-a)-based T-cell response to hepatitis B surface antigen (HBsAg) could last at least 32 years regardless of HBsAb level in HIV-negative subjects vaccinated with HBV vaccine during childhood [16]. However, Chawansuntati et al. found no statistical differences in the frequencies of IFN-r and TNF-a production of total or memory CD4+ or CD8+ cells during the 12 months of study in PLWHs vaccinated with standard or double doses of HBV vaccine at 0, 1, 6 months [17].
CD4+ cells play an important role in the process of HBV antibody production following vaccination. Then, effective vaccination could produce long-lived memory T cells that recognize and respond to HBV infection. However, central memory CD4+ cells contribute to maintain the viral reservoir in PLWHs [18]. Bekele et al. demonstrated the influence of HBV vaccination in affecting the size of HIV reservoirs in 22 HIV-infected children. They showed that total HIV DNA levels after both hepatitis A virus (HAV) and HBV vaccination were reduced compared to baseline, although the decline was not statistically significant [19]. The influence of HBV vaccination in the size of the HIV reservoir in PLWHs has not been well evaluated.
CD4+ cell count > 350 cells/µL and HIV RNA < 200 copies/mL after cART are considered as a successful treatment in PLWHs [20]. The humoral and cellular immune responses to HBV vaccination in these satisfactory patients when they are vaccinated with 20 µg of recombinant HBV DNA vaccine at weeks 0, 4, and 24 are unknown at present. Could HBV vaccination reduce the size of HIV reservoirs in HIV-infected adults? Based on these facts, we aim to observe the long-term humoral immunity to HBV vaccination and to analyze the changes in cellular immunity as well as HIV reservoirs during the follow-up period in a prospective study.
2. Materials and Methods
2.1. Study Design and Population
We performed a prospective study from December 2018 to January 2020 at the Department of Infectious Disease at Peking Union Medical College Hospital (PUMCH) clinics. We included 32 PLWHs over 18 years old. They were seronegative for HBsAg, HBsAb, antibody to hepatitis B core antigen (anti-HBc), and antibody to hepatitis C virus (anti-HCV), and without a history of HBV vaccination in previous five years. Patients were eligible to participate if they received cART and had CD4+ cell count >350 cells/µL and HIV viral load <200 copies/mL for the past 6 months. Exclusion criteria included being pregnant or breastfeeding; acute elevations of liver enzymes within the past three months (alanine aminotransferase (ALT) or aspartate aminotransferase (AST) two or more times the normal upper); having a history of hypersensitivity to any component of the vaccine or other immunocompromised conditions Furthermore HIV.
The enrolled individuals were given 20 ug recombinant HBV DNA vaccine at weeks 0, 4, and 24. The diagram of the participants is shown in Figure S1. Ten participants did not vaccinate for personal reasons. The main reason was that they needed to take three weeks of leave to vaccinate, which they thought would affect their work. Furthermore, they still had concerns about the safety and effectiveness of vaccines. The patients were followed-up every 12 weeks in our clinics; therefore, HBsAb was tested, and full blood was collected to separate PBMC at weeks 0, 4, and 36 in the original plan. However, some patients could not come for follow-up at week 36 due to the outbreak of the COVID-19 epidemic. Thus, they were followed-up between weeks 36 and 48 (Figure S2). Furthermore, HBsAb was tested annually in every patient, whether vaccinated or not, in order to evaluate HBV infection in our clinics. Therefore, we could observe the dynamics of HBsAb titer in longer follow-up duration for enrolled participants. Enzyme-linked immunosorbent assay (Elisa) was used to measure HBsAb, and the cut-off value of this test was 10 IU/mL. The serological response was defined as HBsAb > 10 IU/mL.
2.2. Total HIV DNA Determination
Total HIV DNA was extracted from peripheral blood containing 0.25–1 million PBMCs using the QIAsymphony DNA Mini Kit (QIAGEN, Hilden, Germany). The SUPBIO total HIV DNA Quantitative PCR Kit (SUPBIO, Guangzhou, China) was used for simultaneously quantitating total HIV DNA and cell number, following the manufacturer’s instructions as previously described [21]. The linear quantification range of the SUPBIO total HIV DNA quantitative kit was 20 copies/106 PBMCs to 100,000 copies/106 PBMCs.
2.3. Lymphocyte Subsets Phenotyping
Immunophenotyping of peripheral blood lymphocytes was analyzed by three-color flow cytometry (Epics XL flow cytometry; Bechman Coulter, USA) using commercially available monoclonal antibodies as previously described [22]. The percentages and counts of the following lymphocyte subsets were measured, including CD3−CD16+ CD56+ NK cells, CD3−CD4+ cells, CD3−CD8+ cells, memory CD4+ cells, CD4+ CD45RA+CD62+ naïve cells, CD8+ HLA-DR+ cells, CD8+ CD38+ cells, and the CD4/CD8 ratio. The memory CD4+ cells were counted by difference between CD4+ cells and naïve CD4+ cells. Cell counts of lymphocyte subsets were calculated using a dual-platform method with white blood cell counts and lymphocyte differentials obtained from routine blood tests of the same specimen.
2.4. Human IFN-r and TNF-a ELISpot Assay
Isolated PBMCs were incubated at 37 degrees centigrade in 5% CO2 overnight. PBMCs (1.0 × 105) were pulsed with 1 ug/mL recombinant hepatitis B surface protein for 24 h at 37 degrees centigrade in 5% CO2, and then, we tested the cellular immunity responses using a standard Human IFN-r and TNF-a enzyme-linked immunosorbent spot (ELISpot) assay in triplicate wells. Wells containing PBMCs with and without anti-CD3 were used as positive and negative controls, respectively. To quantify antigen-specific responses, mean spots of the control wells were subtracted from the positive wells, and the results were expressed as spot-forming cells (SFC) per 105 PBMCs. Responses were considered positive if results were at least twice the mean of the triplicate negative control wells and >30 SFCs/105 PBMCs.
2.5. Ethics Statement
The Institutional Review Board of Peking Union Medical College Hospital (PUMCH) approved the parent studies, and each participant provided written informed consent.
2.6. Statistical Analysis
Analyses were performed using SPSS 23.0 (IBM Corp, Armonk, NY, USA), with p values < 0.05 signifying statistical significance. Descriptive statistics were presented as median (M) with interquartile ranges (IQRS). The Mann–Whitney U test was conducted for comparison of noncategorical variables. Categorical variables were analyzed by Chi-squared test or Fisher exact test. Association between continuous variables was tested using a nonparametric Spearman rank correlation test.
3. Results
3.1. Characteristics of the Patients
From a total of 1032 follow-up patients seeking HIV care in PUMCH, 61 were eligible for this study. Reasons for exclusion are detailed in Figure S1. Out of the 32 patients who initiated vaccination, the median age was 36 (IQR: 30–50) years, and 29 (90.6%) were male. In total, 75.0% of individuals were infected with HIV by homosexual transmission route. The median nadir CD4+ cell count was 234 (IQR: 91–350) cells/µL, CD4/CD8 ratio was 0.32 (IQR: 0.19–0.38) and HIV RNA load was 4.85 (IQR:4.33–5.30) log10 copies/mL before cART. The median CD4+ cell count was 554 (IQR: 436–621) cells/µL, and HIV RNA level was suppressed in all except for two, in whom it was 68, 79 copies/mL at the first dose of their immunization (week 0). There were no relevant alterations in laboratory values, including liver function tests, hematological counts, and serum creatinine (Table 1).

Table 1.
Pre-vaccine characteristics of enrolled patients.
3.2. The Dynamics of Lymphocyte Subsets and HIV RNA Levels after Receiving HBV Vaccination
At weeks 12 and 36 to 48, the CD4+ cell counts, including naïve CD4+ and memory CD4+ cell counts in all patients, were comparable with that at week 0. Additionally, the percentages of CD8+CD38+ (37.1 (IQR: 29.5–43.2) and 32.8 (IQR: 26.3–41.95), respectively), CD8+HLA-DR+ (37.3 (IQR: 27.0–49.8) and 41.2 (IQR: 28.7–57.0), respectively) (Figure S3), and B cell counts (171 (IQR: 89–259) cells/µL and 178 (IQR: 141–244) cells/µL, respectively) (Figure S4), were similar to that at week 0. Twenty-nine patients achieved HIV RNA load that was effectively suppressed. The remaining three patients had detectable HIV RNA levels, 55, 57, 61 copies/mL, respectively, at weeks 36 to 48. Two of the patients had detectable HIV RNA at 79 copies/mL, 68 copies/mL at week 0.
3.3. HBsAb Levels at Weeks 12
In response to HBV vaccination, the HBsAb levels observed in 31 patients at weeks 12 and 25 (80.6%) were positive responders with a medium HBsAb titer of 157.87 (IQR: 18.13–802.73) IU/mL. Of patients who achieved a protective HBsAb titer, six (24.0%) had anti-HBs titer above 1000 IU/mL, with 10 (40.0%) between 100 and 1000 IU/mL. Six individuals were non-responders with HBsAb < 10 IU/mL, and these subjects were older than the responders (34 (IQR: 29–41) vs. 54 (IQR: 40–57) years, p = 0.012).
HBsAb titer was tested between 36 and 48 weeks for 32 patients. All achieved a protective HBV titer. The medium HBsAb titer was 663.07 (334.39–1000) IU/mL, and 12 patients (37.5%) had HBsAb levels above 1000 IU/mL, with 16 (50.0%) between 100 and 1000 IU/mL. The remaining four patients (12.5%) had HBsAb levels between 10 and 100 IU/mL (Figure 1).
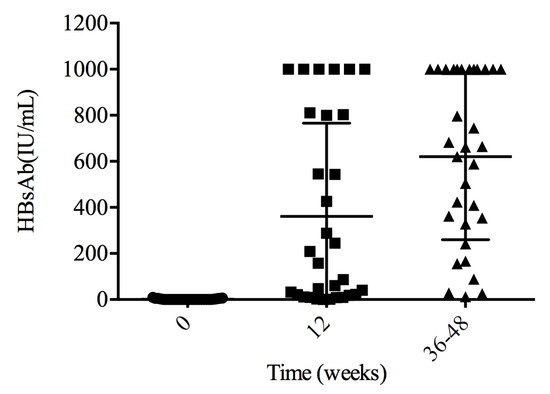
Figure 1.
HBsAb levels after primary vaccination. All patients achieved a protective HBsAb titer between weeks 36 and 48.
3.4. Persistence of Seroprotection following HBV Vaccination
Two or more quantitative HBsAb levels were evaluated in 32 individuals at 102 (IQR: 93–135) weeks of follow-up duration. The medium HBsAb titer was 177.8 (66.28–345.1) IU/mL. HBsAb level was lower than 10 IU/mL in three patients at 1.11, 5.64, 6.43 IU/mL. It was 25.45, 166.76, 28.78 IU/mL after HBV vaccination. Ten patients achieved HBsAb levels between 10 and 100 IU/mL, 16 patients between 100 and 1000 IU/mL, and 3 subjects with more than 1000 IU/mL. Characteristics of participants who did not sustain a protective HBV serological immunity and those who did at 102 (IQR: 93–135) weeks follow-up duration are shown in Table 2 and Table 3, respectively.

Table 2.
Characteristics of participants who did not sustain protective HBV serological immunity at 102 weeks.

Table 3.
Characteristics of participants who sustained protective HBV serological immunity at 102 weeks.
3.5. The Changes in HBsAg-Specific T-Cell Responses during Follow-Up
HBsAg-specific IFN-r or TNF-a-producing T cells were measured by ELISpot analysis in 30 patients. The median magnitude of the IFN-r response to HBsAg was 22 (IQR: 6.5–65.0), 39.3 (IQR: 13.8–117.0), 15.0 (IQR: 5.5–74.0) SFC/105 PBMC, respectively, at weeks 0, 12, 36 to 48. The median magnitude of the TNF-a response to HBsAg was 106.5 (IQR: 58.5–203.0), 198.0 (IQR: 106.5–330.0), 170.8 (IQR: 48.6–247.1) SFC/105 PBMC, respectively, at weeks 0, 12, 36 to 48. The level of IFN-r secreted at weeks 12 and weeks 36 to 48 was comparable with that at week 0. However, IFN-r response was higher at week 12 than that at weeks 36 to 48 (p = 0.02). The level of TNF-a secreted at week 12 was higher than that at week 0 (p < 0.001) (Figure 2).
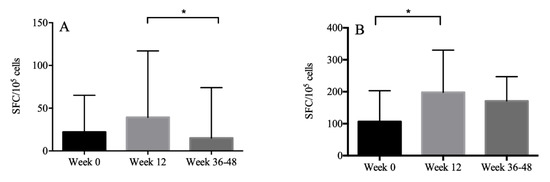
Figure 2.
The magnitude of the IFN-r (A) and TNF-a (B) response to HBsAg presented with median and interquartile ranges. The level of IFN-r secreted at week 12 was higher than that at week 36, and the level of TNF-a secreted at week 12 was higher than that at week 0. *: p < 0.05.
We further explored whether HBsAg-specific T cell responses corresponded with HBsAb titer. We found that there was no correlation between the magnitude of the IFN-r and TNF-a responses to HBsAg and the HBsAb titer after the immunization procedure (p > 0.05) (Figure 3).
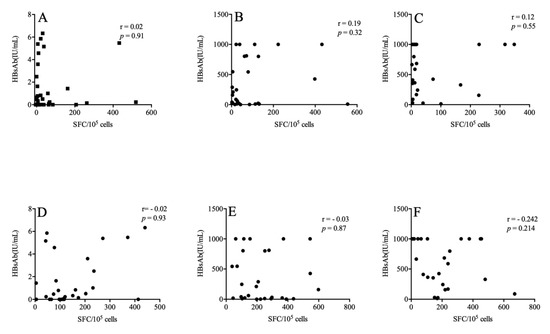
Figure 3.
The association between HBsAg-specific T cell responses and the HBsAb titer. The above figures describe the relationships between HBsAb and IFN-r at weeks 0 (A), 12 (B) and 36–48 (C) respectively. The following are the relationship between HBsAb and TNF-a at weeks 0 (D), 12 (E) and 36–48 (F).
3.6. Total HIV DNA Levels after Receiving HBV Vaccination
HIV DNA levels were measured in 32, 30, 31 patients at weeks 0, 12, 36 to 48, respectively. Total HIV DNA levels were 2.76 (IQR: 2.47–3.07) log10 copies/106 PBMCs at week 0 in the included patients. No significant alteration was observed in the total HIV DNA levels at weeks 12 and 36 to 48 weeks (2.77 (IQR: 2.50–3.09) log10 copies/106 PBMCs, 2.77(IQR: 2.41–2.89) log10 copies/106 PBMCs, respectively) (p > 0.05). No correlation was found between the total HIV DNA levels and HBsAb levels at weeks 12 and 36 to 48 weeks (r = −0.102, p = 0.598; r = −0.167, p = 0.396, respectively). Furthermore, the total HIV DNA levels were not associated with IFN-r and TNF-a levels at weeks 0, 12, and 36 to 48 (p > 0.05).
4. Discussion
In this prospective study, we enrolled 32 PLWHs who achieved successful cART, and they all completed the three-dose vaccination schedule. All patients achieved seroprotection with HBsAb > 10 IU/mL at weeks 36 to 48, indicating that successful cART could promote PLWHs to achieve similar HBV vaccination responses with HIV-negative subjects. Most of them had protective immunity at the median 102-week (IQR: 93–135) follow-up period, suggesting that humoral immunity could persist for a relatively long time. Unfortunately, cellular immunity did not significantly increase after completion of the vaccination schedule. The magnitude of the IFN-r- and TNF-a-based T-cell responses to HBsAg were highest at 12 weeks. Furthermore, we demonstrated that HBV vaccination did not affect the total HIV DNA levels, which were rarely evaluated in previous studies.
The immunogenicity of HBV vaccination in PLWHs varies in different studies, which could be related to the heterogeneous study designs, patient characteristics, and vaccination doses [10]. Landrum et al. demonstrated that higher CD4 cell count at the time of vaccination and use of cART were associated with a higher likelihood of HBV vaccine response [23]. In our study, all patients achieved protective HBsAb between 36 and 48 weeks. Furthermore, 87.5% of patients had ideal seroprotective HBsAb levels. CD4+ cells play an essential role in antibody production by B cells. The optimal serological response may be attributed to the high CD4+ cell count and ideal viral suppression. Of course, there are other cells and processes involved in producing antibodies, such as B cells and regulatory T cells [24]. Future studies are necessary to explore the recovery of these cells and the role they play in modulating immune responses in patients with CD4+ cell count > 350 cells/µL.
HBV vaccination provides long-term immunity in 90% of immunocompetent individuals after three 20 ug doses of anti-HBV vaccine administered over six months. Fauci et al. demonstrated that protective antibody levels persisted in 65% of healthcare workers who had received three vaccine doses [25]. Unfortunately, PLWHs have difficulty in maintaining HBV immunity. In a small sample, Laura et al. reported 69.3% and 26.9% seroprotection after 36 and 84 months among patients with successful vaccination using a 20 mcg three-dose schedule [26]. Cruciani et al. described the persistence of protective HBsAb titers of 70.6% and 32.7% at 12 and 24 months of follow-up [27]. In our study, 90.6% of patients had protective HBsAb titers at 102 (IQR: 93–135) weeks follow-up duration even though HBsAb titers waned over time. Retrospective studies have identified multiple factors affecting long-term immune response, including maximal HBsAb level following primary vaccination, current and nadir CD4+ cell count, HIV viral load, and CD19 cell percentage [12,28,29]. Our data indicated that cART-experienced patients with high CD4+ cell counts had more potency to maintain the persistence of protective HBsAb titers. Moreover, the patients who failed to sustain protective HBsAb had low anti-HBs levels after the HBV vaccination schedule was completed, agreeing with prior studies emphasizing the importance of improving responsiveness after primary immunization.
Previous studies have identified cellular immune responses to HBV vaccination in HIV-negative adults [15,30], which were limited in PLWHs. We showed that the levels of IFN-r secreted at weeks 12 and 36–48 were not significantly higher than that at week 0, which was similar to the results in a previous study [17]. This indicated that the recovery of cellular immune responses in PLWHs was pretty tricky even though ideal humoral immunity was achieved. Future studies could try to vaccinate participants with a double-dose recombinant HBV vaccine at monthly intervals and observe the dynamics of cellular immune responses.
The existence of HIV reservoirs makes curing HIV infection pretty difficult. A previous study evaluated the effect of influenza vaccination on the HIV reservoir, indicating that vaccination may reduce HIV-associated immune activation and lead to reduced HIV replication in PLWHs receiving effective treatment [31]. Our data demonstrated that HBV vaccination did not affect the size of the HIV reservoir in HIV-infected adults. This may be attributed to the fact that total HIV DNA levels decrease significantly from 0 to 1 year of cART [18]. However, patients in our study mainly received treatment for more than 1 year, when the total HIV DNA levels were relatively stable. In general, our results provide some information for the effect of HBV vaccination on the HIV reservoir. Future studies could enlarge the sample size to verify our conclusion.
Our study has several limitations. First, we did not include healthy individuals to immunize HBV vaccine as controls; thus, we can only compare the changes in immune levels before and after vaccination. Second, this study only observed the changes in the number of cells secreting IFN-r and TNF-a before and after vaccination by the ELISPOT method. Multicolor flow cytometry has not observed changes in the proportion of CD4+ and CD8+ cell subsets and the level of cytokines before and after vaccination. Third, the enrolled PLWHs were not vaccinated in the last 5 years, but the proportion of those vaccinated before this period was unknown. Finally, the sample size was small, and we have not followed up with these patients for a long time. The existence of the limitations mentioned above made us more cautious when interpreting the research results. Therefore, we chose to simultaneously evaluate the humoral and cellular immune responses after HBV vaccination among PLWHs with successful treatment. Furthermore, we demonstrated the dynamics of the total HIV DNA levels in these patients, which were rarely shown in previous studies. We hope these measures make our results more scientific and comprehensive.
5. Conclusions
In conclusion, PLWH with successful treatment could achieve ideal humoral immune responses and persistent anti-HBs titers after the completion of a standard HBV vaccination schedule. Unfortunately, the cellular immune response was not optimal, and HBV vaccination did not have an effect on the total HIV DNA levels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11020400/s1, Figure S1: Flow chart for recruitment of the patients attended during the study period. Figure S2: Timeline of vaccination and blood collection Figure S3: The changes in T cell subsets counts. The dynamics of CD4+ cell count (A), naïve CD4+ cell count (B), memory CD4+ cell count (C), CD8+ cell count (D), CD38+ CD8+ percentage (E), HLA-DR+CD8+ percentage (F) before HBV vaccine immunization 12 and 36 weeks after receiving the first HBV vaccine. Figure S4: The changes in B cell counts during the follow-up period. There were no relevant alterations in B cell count.
Author Contributions
Writing—original draft preparation, L.X.; data curation, L.Z., S.K., X.L. (Xiaodi Li), L.L., X.L. (Xiaosheng Liu), X.S., Y.L., X.L. (Xiaoxia Li), W.L., W.C. and Z.L.; project administration, T.L.; funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Technologies R&D Program for the 13th Five-year Plan (2017ZX10202101-001), the CAMS Initiative for Innovative Medicine (2021-I2M-1-037) and the Beijing key clinical specialty project. The funding bodies played no role in the design of the study, data collection, data analysis, interpretation of data or writing of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Peking Union Medical College Hospital.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Datasets used in this analysis are available from the corresponding author on reasonable request.
Acknowledgments
We thank all participants for their contributions to this study and the PUMCH team who established the original cohort.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bollinger, R.C.; Thio, C.L.; Sulkowski, M.S.; McKenzie-White, J.; Thomas, D.L.; Flexner, C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. Lancet. HIV 2020, 7, e443–e448. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Crane, M.; Audsley, J.; Avihingsanon, A.; Sasadeusz, J.; Lewin, S.R. HIV-hepatitis B virus coinfection: Epidemiology, pathogenesis, and treatment. AIDS 2017, 31, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Maini, M.K.; Peppa, D. Shared immunotherapeutic approaches in HIV and hepatitis B virus: Combine and conquer. Curr. Opin. HIV AIDS 2020, 15, 157–164. [Google Scholar] [CrossRef]
- Audsley, J.; Avihingsanon, A.; Littlejohn, M.; Bowden, S.; Matthews, G.V.; Fairley, C.K.; Lewin, S.R.; Sasadeusz, J. Long-Term TDF-Inclusive ART and Progressive Rates of HBsAg Loss in HIV-HBV Coinfection-Lessons for Functional HBV Cure? JAIDS J. Acquir. Immune Defic. Syndr. 2020, 84, 527–533. [Google Scholar] [CrossRef]
- Heuft, M.M.; Houba, S.M.; van den Berk, G.E.; Smissaert van de Haere, T.; van Dam, A.P.; Dijksman, L.M.; Regez, R.M.; Brinkman, K. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014, 28, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Botta, A.; Berruti, M.; Castelli, V.; Lai, V.; Cassol, C.; Lanari, A.; Stella, G.; Shallvari, A.; Bezenchek, A.; et al. Could Long-Acting Cabotegravir-Rilpivirine Be the Future for All People Living with HIV? Response Based on Genotype Resistance Test from a Multicenter Italian Cohort. J. Pers. Med. 2022, 12, 188. [Google Scholar] [CrossRef]
- Schillie, S.; Vellozzi, C.; Reingold, A.; Harris, A.; Haber, P.; Ward, J.W.; Nelson, N.P. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. Morb. Mortal. Wkly. Report. Recomm. Rep. 2018, 67, 1–31. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Abraham, B.; Fourneau, M.; de Clercq, N.; Safary, A. A comparison of two commercial recombinant vaccines for hepatitis B in adolescents. Vaccine 2000, 19, 937–942. [Google Scholar] [CrossRef]
- Van den Ende, C.; Marano, C.; van Ahee, A.; Bunge, E.M.; de Moerlooze, L. The immunogenicity and safety of GSK’s recombinant hepatitis B vaccine in adults: A systematic review of 30 years of experience. Expert Rev. Vaccines 2017, 16, 811–832. [Google Scholar] [CrossRef]
- Farooq, P.D.; Sherman, K.E. Hepatitis B Vaccination and Waning Hepatitis B Immunity in Persons Living with HIV. Curr. HIV AIDS Rep. 2019, 16, 395–403. [Google Scholar] [CrossRef]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.Y. Long-term immune responses to vaccination in HIV-infected patients: A systematic review and meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.B.; Hassing, R.J.; de Vries-Sluijs, T.E.; El Barzouhi, A.; Hansen, B.E.; Schutten, M.; de Man, R.A.; van der Ende, M.E. Long-term response rates of successful hepatitis B vaccination in HIV-infected patients. Vaccine 2013, 31, 1040–1044. [Google Scholar] [CrossRef]
- Tian, Y.; Hua, W.; Wu, Y.; Zhang, T.; Wang, W.; Wu, H.; Guo, C.; Huang, X. Immune Response to Hepatitis B Virus Vaccine Among People Living With HIV: A Meta-Analysis. Front. Immunol. 2021, 12, 745541. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hong, S.; Im, J.H.; Lee, J.S.; Baek, J.H.; Kwon, H.Y. Systematic review and meta-analysis of immune response of double dose of hepatitis B vaccination in HIV-infected patients. Vaccine 2020, 38, 3995–4000. [Google Scholar] [CrossRef]
- Zhu, C.L.; Liu, P.; Chen, T.; Ni, Z.; Lu, L.L.; Huang, F.; Lu, J.; Sun, Z.; Qu, C. Presence of immune memory and immunity to hepatitis B virus in adults after neonatal hepatitis B vaccination. Vaccine 2011, 29, 7835–7841. [Google Scholar] [CrossRef] [PubMed]
- Simons, B.C.; Spradling, P.R.; Bruden, D.J.; Zanis, C.; Case, S.; Choromanski, T.L.; Apodaca, M.; Brogdon, H.D.; Dwyer, G.; Snowball, M.; et al. A Longitudinal Hepatitis B Vaccine Cohort Demonstrates Long-lasting Hepatitis B Virus (HBV) Cellular Immunity Despite Loss of Antibody Against HBV Surface Antigen. J. Infect. Dis. 2016, 214, 273–280. [Google Scholar] [CrossRef]
- Chawansuntati, K.; Chaiklang, K.; Chaiwarith, R.; Praparattanapan, J.; Supparatpinyo, K.; Wipasa, J. Hepatitis B Vaccination Induced TNF-α- and IL-2-Producing T Cell Responses in HIV- Healthy Individuals Higher than in HIV+ Individuals Who Received the Same Vaccination Regimen. J. Immunol. Res. 2018, 2018, 8350862. [Google Scholar] [CrossRef]
- Avettand-Fènoël, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.P.; Rouzioux, C. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef]
- Bekele, Y.; Graham, R.L.; Soeria-Atmadja, S.; Nasi, A.; Zazzi, M.; Vicenti, I.; Naver, L.; Nilsson, A.; Chiodi, F. Hepatitis B Virus Vaccination in HIV-1-Infected Young Adults: A Tool to Reduce the Size of HIV-1 Reservoirs? Front. Immunol. 2017, 8, 1966. [Google Scholar] [CrossRef]
- Kong, Y.; Tian, Y.; Hao, Y.; Chong, X.; Xiao, J.; Yang, D.; Song, C.; Han, J.; Dai, G.; Zhang, F.; et al. Two types of poor immunological responder showing distinct responses to long-term HAART. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2019, 86, 178–187. [Google Scholar] [CrossRef]
- Yue, Y.; Li, Y.; Cui, Y.; Wang, N.; Huang, Y.; Cao, W.; Han, Y.; Zhu, T.; Lyu, W.; Xie, J.; et al. Therapeutic prediction of HIV-1 DNA decay: A multicenter longitudinal cohort study. BMC Infect. Dis. 2021, 21, 592. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Song, X.; Li, Y.; Han, Y.; Zhu, T.; Cao, W.; Li, T. Naïve CD4(+) cell counts significantly decay and high HIV RNA levels contribute to immunological progression in long-term non-progressors infected with HIV by blood products: A cohort study. BMC Immunol. 2021, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.L.; Huppler Hullsiek, K.; Ganesan, A.; Weintrob, A.C.; Crum-Cianflone, N.F.; Barthel, R.V.; Peel, S.; Agan, B.K. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: Another benefit of HAART in those with preserved CD4 count. Vaccine 2009, 27, 4731–4738. [Google Scholar] [CrossRef]
- Hirano, T.; Kadowaki, Y.; Matsunaga, T.; Yoshinaga, K.; Kawano, T.; Moriyama, M.; Suzuki, M. Interaction Between Regulatory T Cells and Antibody-Producing B Cells for Immune Responses at the Upper Respiratory Mucosa Against Nontypeable Haemophilus influenzae: In Vitro Assay Model. Ann. Otol. Rhinol. Laryngol. 2019, 128, 45s–51s. [Google Scholar] [CrossRef]
- La Fauci, V.; Riso, R.; Facciolà, A.; Ceccio, C.; Lo Giudice, D.; Calimeri, S.; Squeri, R. Response to anti-HBV vaccine and 10-year follow-up of antibody levels in healthcare workers. Public Health 2016, 139, 198–202. [Google Scholar] [CrossRef]
- Nicolini, L.A.; Magne, F.; Signori, A.; di Biagio, A.; Sticchi, L.; Paganino, C.; Durando, P.; Viscoli, C. Hepatitis B Virus Vaccination in HIV: Immunogenicity and Persistence of Seroprotection up to 7 Years Following a Primary Immunization Course. AIDS Res. Hum. Retrovir. 2018, 34, 922–928. [Google Scholar] [CrossRef]
- Cruciani, M.; Mengoli, C.; Serpelloni, G.; Lanza, A.; Gomma, M.; Nardi, S.; Rimondo, C.; Bricolo, F.; Consolaro, S.; Trevisan, M.; et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine 2009, 27, 17–22. [Google Scholar] [CrossRef]
- Rey, D.; Piroth, L.; Wendling, M.J.; Miailhes, P.; Michel, M.L.; Dufour, C.; Haour, G.; Sogni, P.; Rohel, A.; Ajana, F.; et al. Safety and immunogenicity of double-dose versus standard-dose hepatitis B revaccination in non-responding adults with HIV-1 (ANRS HB04 B-BOOST): A multicentre, open-label, randomised controlled trial. Lancet. Infect. Dis. 2015, 15, 1283–1291. [Google Scholar] [CrossRef]
- Abzug, M.J.; Warshaw, M.; Rosenblatt, H.M.; Levin, M.J.; Nachman, S.A.; Pelton, S.I.; Borkowsky, W.; Fenton, T. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J. Infect. Dis. 2009, 200, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Jilg, W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 2006, 24, 572–577. [Google Scholar] [CrossRef]
- Günthard, H.F.; Wong, J.K.; Spina, C.A.; Ignacio, C.; Kwok, S.; Christopherson, C.; Hwang, J.; Haubrich, R.; Havlir, D.; Richman, D.D. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J. Infect. Dis. 2000, 181, 522–531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).