Global Cross-Sectional Study Evaluating the Attitudes towards a COVID-19 Vaccine in Pregnant and Postpartum Women
Abstract
:1. Introduction
2. Methods
2.1. Ethics Approval
2.2. Survey Participants
2.3. Recruitment
2.4. Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
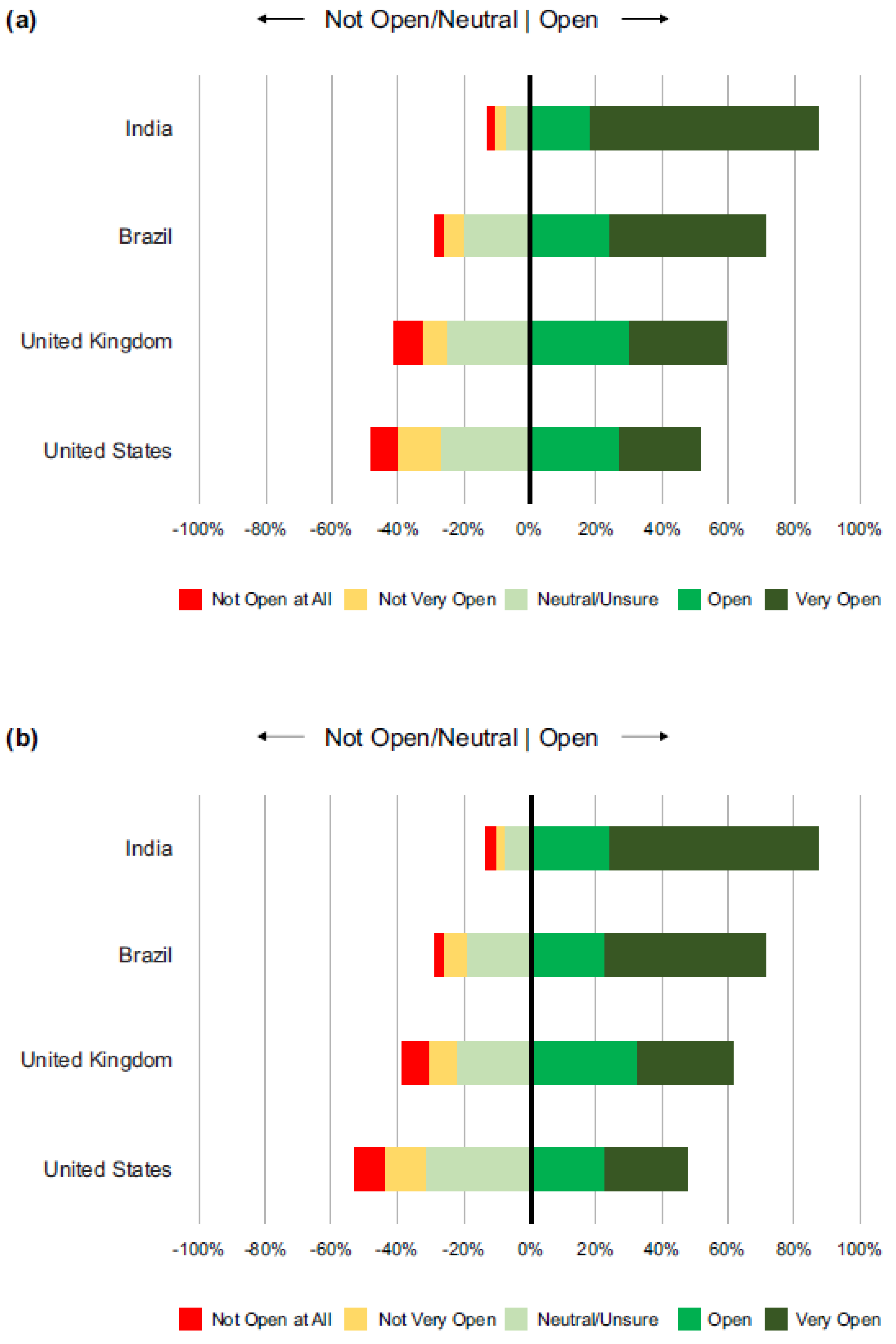
3.2. Openness to Self Receiving a COVID-19 Vaccine
3.3. Reasons for Vaccine Hesitancy
3.4. Openness to Children/Other Family Members Receiving a COVID-19 Vaccine
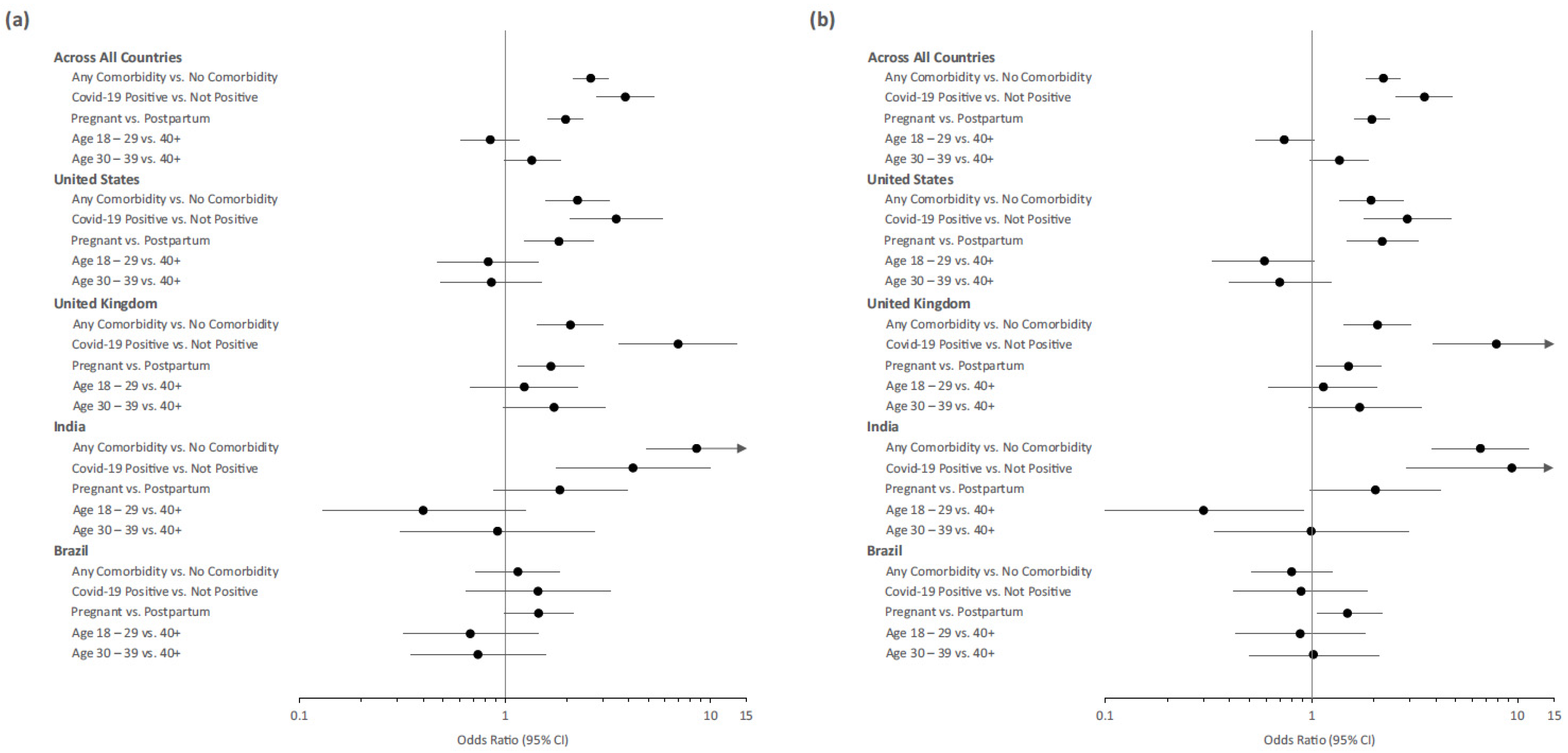
3.5. Predictors of Vaccine Acceptance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, E.O.; Haapala, J.; DeSilva, M.; Vazquez-Benitez, G.; Vesco, K.K.; Naleway, A.L.; Lipkind, H.S. Spontaneous Abortion Following COVID-19 Vaccination During Pregnancy. JAMA 2021, 326, 1629–1631. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Medina Baez, A.; Shook, L.L.; Cvrk, D.; James, K.; et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Shanes, E.D.; Otero, S.; Mithal, L.B.; Mupanomunda, C.A.; Miller, E.S.; Goldstein, J.A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination in Pregnancy: Measures of Immunity and Placental Histopathology. Obstet. Gynecol. 2021, 138, 281–283. [Google Scholar] [CrossRef]
- Prabhu, M.; Murphy, E.A.; Sukhu, A.C.; Yee, J.; Singh, S.; Eng, D.; Zhao, Z.; Riley, L.E.; Yang, Y.J. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage into Cord Blood. Obstet. Gynecol. 2021, 138, 278–280. [Google Scholar] [CrossRef]
- Goldshtein, I.; Steinberg, D.M.; Kuint, J.; Chodick, G.; Segal, Y.; Shapiro Ben David, S.; Ben-Tov, A. Association of BNT162b2 COVID-19 Vaccination during Pregnancy with Neonatal and Early Infant Outcomes. JAMA Pediatr. 2022, 176, 470–477. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Effectiveness and Safety of COVID-19 Vaccine among Pregnant Women in Real-World Studies: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 246. [Google Scholar] [CrossRef]
- Rawal, S.; Tackett, R.L.; Stone, R.H.; Young, H.N. COVID-19 Vaccination among Pregnant People in the U.S.: A Systematic Review. Am. J. Obstet. Gynecol. MFM 2022, 4, 100616. [Google Scholar] [CrossRef] [PubMed]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Kalafat, E.; Blakeway, H.; Townsend, R.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Ladhani, S.; et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022, 13, 2414. [Google Scholar] [CrossRef]
- Lamptey, E.; Senkyire, E.K.; Banoya, M.T.; Yaidoo, S. COVID-19 vaccination in pregnancy: A review of maternal and infant benefits. Gynecol. Obstet. Clinc. Med. 2022, 2, 124–128. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine during Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months—17 States, July 2021–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J. Pregnancy, Postpartum Care, and COVID-19 Vaccination in 2021. JAMA 2021, 325, 1099–1100. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. ACOG and SMFM Recommend COVID-19 Vaccination for Pregnant Individuals. Available online: https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals (accessed on 24 March 2022).
- Centers for Disease Control and Prevention. COVID-19 Vaccination for Pregnant People to Prevent Serious Illness, Deaths, and Adverse Pregnancy Outcomes from COVID-19. Available online: https://emergency.cdc.gov/han/2021/han00453.asp (accessed on 24 March 2022).
- World Health Organization. Questions and Answers: COVID-19 Vaccines and Pregnancy. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-FAQ-Pregnancy-Vaccines-2022.1 (accessed on 24 March 2022).
- Berman Institute of Bioethics & Center for Immunization Research, Johns Hopkins University. COVID-19 Maternal Immunization Tracker (COMIT). Available online: https://www.comitglobal.org/ (accessed on 5 July 2022).
- Shamshirsaz, A.A.; Hessami, K.; Morain, S.; Afshar, Y.; Nassr, A.A.; Arian, S.E.; Asl, N.M.; Aagaard, K. Intention to Receive COVID-19 Vaccine during Pregnancy: A Systematic Review and Meta-analysis. Am. J. Perinatol. 2021, 39, 492–500. [Google Scholar] [CrossRef]
- Carbone, L.; Di Girolamo, R.; Mappa, I.; Saccone, G.; Raffone, A.; Di Mascio, D.; De Vivo, V.; D’Antonio, F.; Guida, M.; Rizzo, G.; et al. Worldwide beliefs among pregnant women on SARS-CoV-2 vaccine: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 268, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Nikpour, M.; Sepidarkish, M.; Omidvar, S.; Firouzbakht, M. Global prevalence of acceptance of COVID-19 vaccines and associated factors in pregnant women: A systematic review and meta-analysis. Expert Rev. Vaccines 2022, 21, 843–851. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Stefanizzi, P.; Di Gioia, M.C.; Brescia, N.; Lattanzio, S.; Tafuri, S. COVID-19 vaccination hesitancy in pregnant and breastfeeding women and strategies to increase vaccination compliance: A systematic review and meta-analysis. Expert Rev. Vaccines 2022, 21, 1443–1454. [Google Scholar] [CrossRef]
- Bhattacharya, O.; Siddiquea, B.N.; Shetty, A.; Afroz, A.; Billah, B. COVID-19 vaccine hesitancy among pregnant women: A systematic review and meta-analysis. BMJ Open 2022, 12, e061477. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Nasirkandy, M.P.; Esmaeili Gouvarchin Ghaleh, H.; Ranjbar, R. COVID-19 vaccine acceptance among pregnant women worldwide: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272273. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Siskou, O.; Konstantakopoulou, O.; Katsiroumpa, A.; Kaitelidou, D. Uptake of COVID-19 Vaccines among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Januszek, S.M.; Faryniak-Zuzak, A.; Barnaś, E.; Łoziński, T.; Góra, T.; Siwiec, N.; Szczerba, P.; Januszek, R.; Kluz, T. The Approach of Pregnant Women to Vaccination Based on a COVID-19 Systematic Review. Medicina 2021, 57, 977. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Malhotra, S.; Krishnan, N.M.A.; Sharma, A. The profiles of first and second SARS-CoV-2 waves in the top ten COVID-19 affected countries. J. Glob. Health Rep. 2021, 5, e2021082. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 Vaccines While Pregnant or Breastfeeding. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (accessed on 17 January 2023).
- World Health Organization. Update on WHO Interim Recommendations on COVID-19 Vaccination of Pregnant and Lactating Women. Available online: https://cdn.who.int/media/docs/default-source/2021-dha-docs/update-on-who-interim-recommendations-on-c-19-vaccination-for-pregnant-and-lactating-women-70-.pdf?sfvrsn=2c1d9ac8_1&download=true (accessed on 17 January 2023).
- UK Health Security Agency. COVID-19 Vaccination: A Guide on Pregnancy and Breastfeeding. Available online: https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-on-pregnancy-and-breastfeeding#:~:text=Coronavirus%20(COVID%2D19)%20vaccine,protect%20you%20and%20your%20baby (accessed on 17 January 2023).
- Battarbee, A.N.; Stockwell, M.S.; Varner, M.; Newes-Adeyi, G.; Daugherty, M.; Gyamfi-Bannerman, C.; Tita, A.T.; Vorwaller, K.; Vargas, C.; Subramaniam, A.; et al. Attitudes Toward COVID-19 Illness and COVID-19 Vaccination among Pregnant Women: A Cross-Sectional Multicenter Study during August–December 2020. Am. J. Perinatol. 2022, 39, 75–83. [Google Scholar] [CrossRef]
- Carbone, L.; Mappa, I.; Sirico, A.; Di Girolamo, R.; Saccone, G.; Di Mascio, D.; Donadono, V.; Cuomo, L.; Gabrielli, O.; Migliorini, S.; et al. Pregnant women’s perspectives on severe acute respiratory syndrome coronavirus 2 vaccine. Am. J. Obstet. Gynecol. MFM 2021, 3, 100352. [Google Scholar] [CrossRef]
- Ceulemans, M.; Foulon, V.; Panchaud, A.; Winterfeld, U.; Pomar, L.; Lambelet, V.; Cleary, B.; O’Shaughnessy, F.; Passier, A.; Richardson, J.L.; et al. Vaccine Willingness and Impact of the COVID-19 Pandemic on Women’s Perinatal Experiences and Practices-A Multinational, Cross-Sectional Study Covering the First Wave of the Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 3367. [Google Scholar] [CrossRef]
- Goncu Ayhan, S.; Oluklu, D.; Atalay, A.; Menekse Beser, D.; Tanacan, A.; Moraloglu Tekin, O.; Sahin, D. COVID-19 vaccine acceptance in pregnant women. Int. J. Gynaecol. Obstet. 2021, 154, 291–296. [Google Scholar] [CrossRef]
- Levy, A.T.; Singh, S.; Riley, L.E.; Prabhu, M. Acceptance of COVID-19 vaccination in pregnancy: A survey study. Am. J. Obstet. Gynecol. MFM 2021, 3, 100399. [Google Scholar] [CrossRef]
- Mohan, S.; Reagu, S.; Lindow, S.; Alabdulla, M. COVID-19 vaccine hesitancy in perinatal women: A cross sectional survey. J. Perinat. Med. 2021, 49, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Hoang, M.T.; Nguyen, L.D.; Ninh, L.T.; Nguyen, H.T.T.; Nguyen, A.D.; Vu, L.G.; Vu, G.T.; Doan, L.P.; Latkin, C.A.; et al. Acceptance and willingness to pay for COVID-19 vaccines among pregnant women in Vietnam. Trop. Med. Int. Health 2021, 26, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Oluklu, D.; Goncu Ayhan, S.; Menekse Beser, D.; Uyan Hendem, D.; Ozden Tokalioglu, E.; Turgut, E.; Sahin, D. Factors affecting the acceptability of COVID-19 vaccine in the postpartum period. Hum. Vaccin. Immunother. 2021, 17, 4043–4047. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Jouzová, A.; Üstün, B.; Lagová, E.; Hruban, L.; Janků, P.; Pokorná, A.; Klugarová, J.; Koščík, M.; Klugar, M. COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 13373. [Google Scholar] [CrossRef] [PubMed]
- Schaal, N.K.; Zöllkau, J.; Hepp, P.; Fehm, T.; Hagenbeck, C. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch. Gynecol. Obstet. 2021, 306, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Stuckelberger, S.; Favre, G.; Ceulemans, M.; Nordeng, H.; Gerbier, E.; Lambelet, V.; Stojanov, M.; Winterfeld, U.; Baud, D.; Panchaud, A.; et al. SARS-CoV-2 Vaccine Willingness among Pregnant and Breastfeeding Women during the First Pandemic Wave: A Cross-Sectional Study in Switzerland. Viruses 2021, 13, 1199. [Google Scholar] [CrossRef]
- Sutton, D.; D’Alton, M.; Zhang, Y.; Kahe, K.; Cepin, A.; Goffman, D.; Staniczenko, A.; Yates, H.; Burgansky, A.; Coletta, J.; et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am. J. Obstet. Gynecol. MFM 2021, 3, 100403. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women (accessed on 15 November 2022).
- UK Health Security Agency. COVID-19 Vaccine Surveillance Report: Week 44. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115385/Vaccine_surveillance_report___week-44.pdf (accessed on 15 November 2022).
- Cabar, F.R.; Francisco, R.P.V. Reflections on the need for a vaccine strategy against COVID-19 for pregnant and postpartum women. Clinics 2021, 76, e3471. [Google Scholar] [CrossRef]
- de Freitas Paganoti, C.; Alkmin da Costa, R.; Papageorghiou, A.T.; da Silva Costa, F.; Quintana, S.M.; Graziela de Godoi, L.; Adriana Jimenez Monroy, N.; Sacramento Rodrigues, A.; Pulcineli Vieira Francisco, R. COVID-19 Vaccines Confer Protection in Hospitalized Pregnant and Postpartum Women with Severe COVID-19: A Retrospective Cohort Study. Vaccines 2022, 10, 749. [Google Scholar] [CrossRef]
- Naqvi, S.; Saleem, S.; Naqvi, F.; Billah, S.M.; Nielsen, E.; Fogleman, E.; Peres-da-Silva, N.; Figueroa, L.; Mazariegos, M.; Garces, A.L.; et al. Knowledge, attitudes, and practices of pregnant women regarding COVID-19 vaccination in pregnancy in 7 low- and middle-income countries: An observational trial from the Global Network for Women and Children’s Health Research. BJOG 2022, 129, 2002–2009. [Google Scholar] [CrossRef]
- Ministry of Health and Family Welfare. Pregnant Women Now Eligible for COVID-19 Vaccination. Available online: https://www.pib.gov.in/PressReleasePage.aspx?PRID=1732312 (accessed on 17 May 2022).
- Tagoe, E.T.; Sheikh, N.; Morton, A.; Nonvignon, J.; Sarker, A.R.; Williams, L.; Megiddo, I. COVID-19 Vaccination in Lower-Middle Income Countries: National Stakeholder Views on Challenges, Barriers, and Potential Solutions. Front. Public Health 2021, 9, 709127. [Google Scholar] [CrossRef]
- Assistant Secretary for Planning and Evaluation Office of Science & Data Policy. Disparities in COVID-19 Vaccination Rates across Racial and Ethnic Minority Groups in the United States. Available online: https://aspe.hhs.gov/sites/default/files/private/pdf/265511/vaccination-disparities-brief.pdf (accessed on 11 July 2022).
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 15 April 2022).
- Wilson, R.J.; Paterson, P.; Jarrett, C.; Larson, H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine 2015, 33, 6420–6429. [Google Scholar] [CrossRef]
- Myers, K.L. Predictors of maternal vaccination in the United States: An integrative review of the literature. Vaccine 2016, 34, 3942–3949. [Google Scholar] [CrossRef]
- Chervenak, F.A.; McCullough, L.B.; Grünebaum, A. Reversing physician hesitancy to recommend COVID-19 vaccination for pregnant patients. Am. J. Obstet. Gynecol. 2022, 226, 805–812. [Google Scholar] [CrossRef]

| Total (N = 2010) | United States (N = 500) | United Kingdom (N = 500) | India (N = 510) | Brazil (N = 500) | |
|---|---|---|---|---|---|
| N (%) | |||||
| Age (years) | |||||
| 18–29 | 703 (35) | 226 (45) | 166 (33) | 90 (18) | 221 (44) |
| 30–39 | 1103 (55) | 211 (42) | 276 (55) | 381 (75) | 235 (47) |
| 40–49 | 180 (9) | 49 (10) | 54 (11) | 37 (7) | 40 (8) |
| 50–59 | 24 (1) | 14 (3) | 4 (1) | 2 (0) | 4 (1) |
| Stage | |||||
| 1st trimester | 366 (18) | 76 (15) | 61 (12) | 149 (29) | 80 (16) |
| 2nd trimester | 649 (32) | 162 (32) | 146 (29) | 213 (42) | 128 (26) |
| 3rd trimester | 375 (19) | 112 (22) | 92 (18) | 99 (19) | 72 (14) |
| Postpartum | 620 (31) | 150 (30) | 201 (40) | 49 (10) | 220 (44) |
| COVID-19 test † | |||||
| Ever positive | 357 (28) | 89 (30) | 95 (35) | 137 (30) | 36 (15) |
| Not positive | 900 (72) | 205 (70) | 174 (65) | 320 (70) | 201 (85) |
| Comorbidity | |||||
| Any | 934 (46) | 221 (44) | 224 (45) | 376 (74) | 113 (23) |
| None | 1076 (54) | 279 (56) | 276 (55) | 134 (26) | 387 (77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, N.D.; Pairman, S.; Fisher, A.C.; Cheng, R.-f.J.; Sylvester, S. Global Cross-Sectional Study Evaluating the Attitudes towards a COVID-19 Vaccine in Pregnant and Postpartum Women. Vaccines 2023, 11, 390. https://doi.org/10.3390/vaccines11020390
Hernandez ND, Pairman S, Fisher AC, Cheng R-fJ, Sylvester S. Global Cross-Sectional Study Evaluating the Attitudes towards a COVID-19 Vaccine in Pregnant and Postpartum Women. Vaccines. 2023; 11(2):390. https://doi.org/10.3390/vaccines11020390
Chicago/Turabian StyleHernandez, Natalie D., Sally Pairman, Alan C. Fisher, Ru-fong J. Cheng, and Shirley Sylvester. 2023. "Global Cross-Sectional Study Evaluating the Attitudes towards a COVID-19 Vaccine in Pregnant and Postpartum Women" Vaccines 11, no. 2: 390. https://doi.org/10.3390/vaccines11020390
APA StyleHernandez, N. D., Pairman, S., Fisher, A. C., Cheng, R.-f. J., & Sylvester, S. (2023). Global Cross-Sectional Study Evaluating the Attitudes towards a COVID-19 Vaccine in Pregnant and Postpartum Women. Vaccines, 11(2), 390. https://doi.org/10.3390/vaccines11020390






