The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein
Abstract
:1. Introduction
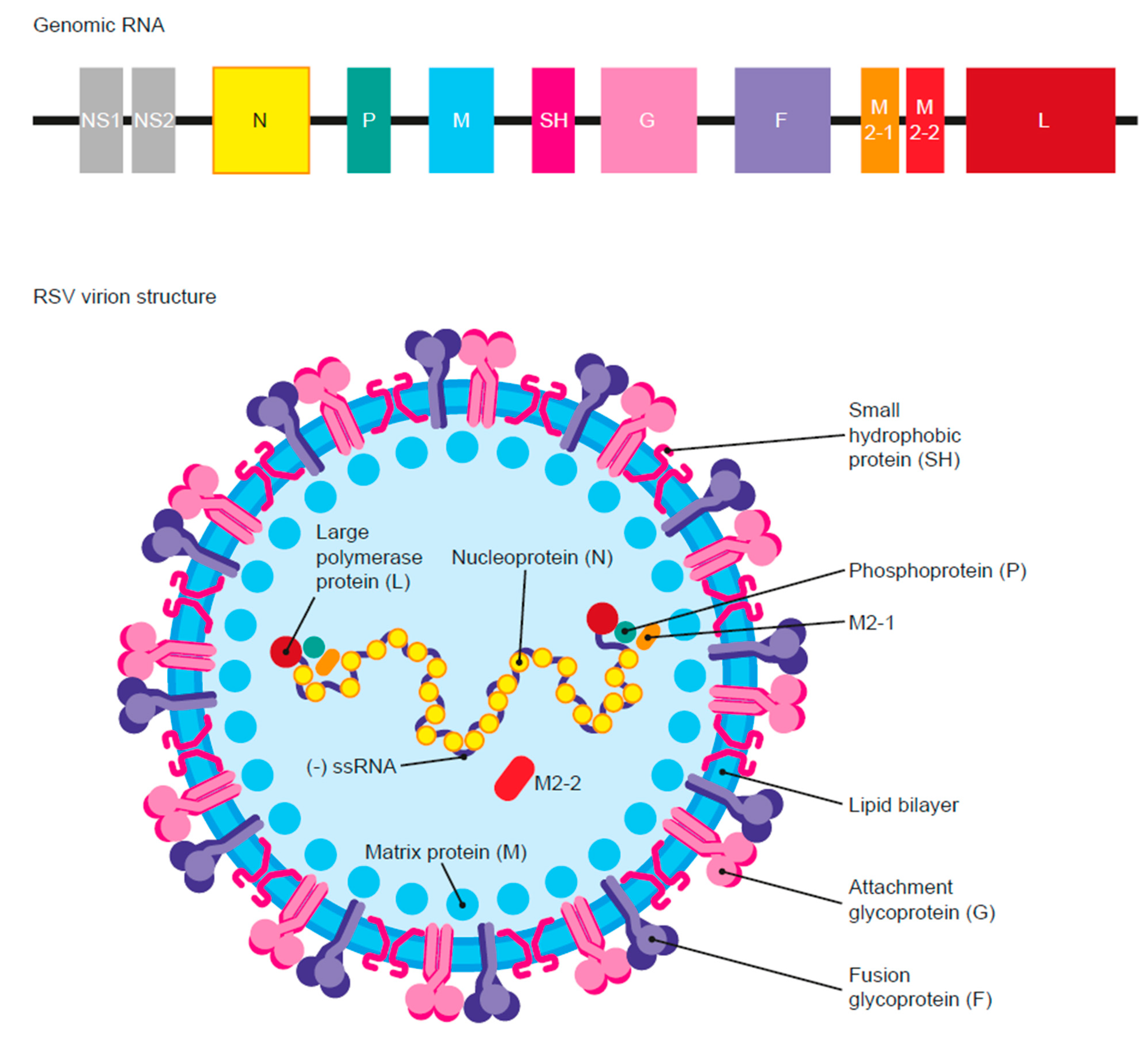
2. RSV Pathogenesis
2.1. Virus Structure
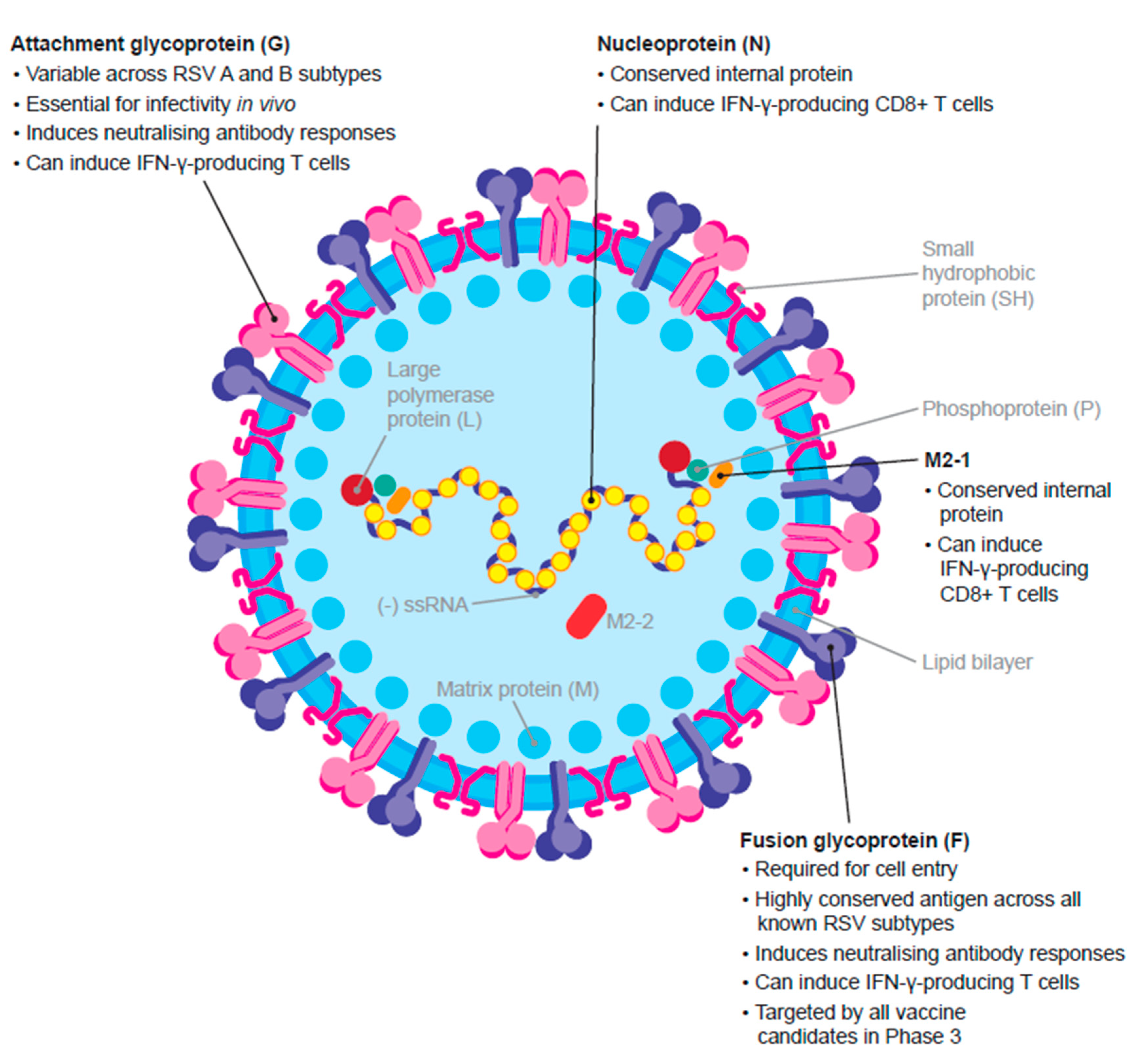
2.2. RSV F Antigen and Approved Prophylactic Therapies
3. Considerations for an Effective RSV Vaccine in Older Adults: RSV Target Antigens and the Immune Response
3.1. The F Protein
| Vaccine Candidate, Sponsor | Formulation, Antigen | Clinical Trial Phase | Populations Assessed | N | Neutralising Antibody * GMFRs at up to 1 Month vs. Baseline | Neutralising Antibody * GMFRs at ≥12 Months vs. Baseline | Vaccine Efficacy (VE) * |
|---|---|---|---|---|---|---|---|
| RSVpreF, Pfizer [37,38,55] | Bivalent subunit, Prefusion F | Phase 1/2 | Adults aged 18–49 years | 618 | RSV A: 10.6–16.9 RSV B: 10.3–19.8 | RSV A: 3.9–5.2 RSV B: 3.7–5.1 | – |
| Phase 1/2 | Older adults aged 65–85 years | 317 | RSV A: 4.8–11.6 RSV B: 4.5–14.1 | RSV A: 2.1–3.5 RSV B: 2.2–4.3 | – | ||
| Phase 2a human challenge trial | Adults aged 18–50 years | 62 | RSV A: 20.5 (95% CI, 16.6–25.3) RSV B: 20.3 (95% CI, 15.6–26.4) | – | VE against RT-PCR-confirmed symptomatic RSV infection with ≥1 symptom: † 86.7% (95% CI, 53.8–96.5) VE against quantitative culture-confirmed RSV infection with ≥1 symptom: ‡ 100.0% (95% CI, 67.7–100.0) | ||
| Phase 3 | Older adults aged ≥60 years | 34,284 (n = 1050) | – | – | VE against RSV-associated LRTD: 66.7 (96.66% CI, 28.8–85.8%)–85.7% (96.66% CI, 32.0–98.7) VE against RSV-associated ARI: 62.1% (95% CI, 37.1–77.9) | ||
| GSK3844766A, GSK [39,44,45,56] | Recombinant subunit, Prefusion F + AS01E adjuvant | Phase 1/2 | Adults aged 18–40 years | 48 | RSV A: 7.5–13.7 | – | – |
| Phase 1/2 | Older adults aged 60–80 years | 1005 | RSV A: 5.6–9.9 | RSV A: 2.7–4.4 | – | ||
| Phase 3 | Older adults aged ≥60 years | 1653 (n = 566) | RSV A: 10.5 RSV B: 7.8 | RSV A: § 4.4 RSV B: § 3.5 | |||
| Phase 3 | Older adults aged ≥60 years | 26,664 (n = 24,960) | – | – | VE against RSV-associated LRTD: 82.6 (96.95% CI, 57.9–94.1)–94.1% (95% CI, 62.4–99.9) VE against RSV-associated ARI: 71.7% (95% CI, 56.2–82.3) | ||
| Ad26.RSV.preF, Janssen [41,47,57,58,59] | Adenoviral vector 26, Prefusion F | Phase 1 | Older adults aged >60 years | 72 | RSV A and B: Increased compared with baseline ¶ | Maintained above baseline levels 2 years after vaccination | – |
| Phase 2a human challenge trial | Adults aged 18–50 years | 63 | RSV A: 5.8 | – | VE against RT-PCR-confirmed RSV infection ≥1 symptom: ** 51.9% (95% CI, –7.4–83.2) VE against quantitative culture-confirmed RSV infection with ≥1 symptom: ‡ 55.1% (95% CI, 9.4–82.2) | ||
| Adeno 26 viral vector Prefusion F + Prefusion F subunit | Phase 2b | Older adults aged ≥65 years | 5782 | RSV neutralising antibodies: †† 13.5 RSV pre-F-specific antibodies: †† 8.6 | RSV neutralising antibodies: 2.8 RSV pre-F-specific antibodies: 2.1 | VE against RSV-associated LRTD: up to 80% (95% CI, 52.2–92.9; p < 0.001) VE against RSV-associated ARI: 69.8% (95% CI, 42.7–85.1) VE against RT-PCR-confirmed RSV-associated LRTD at 3-year follow-up: 78.7% (95% CI, 57.3–90.4) VE against RT-PCR-confirmed RSV-associated ARI at 3-year follow-up: 65.7% (95% CI, 43.5–79.9) | |
| mRNA-1345, Moderna [49,60] | mRNA, Prefusion F | Phase 1 | Older adults aged 65–79 years ‡‡ | n = 298 | RSV A: 9.9–16.6 RSV B: 5.3–12.6 | RSV A: †† 3.1–5.8 RSV B: †† 2.9–5.5 | – |
| Phase 3 | Older adults aged >60 years | ~37,000 | – | – | VE against RSV-associated LRTD: 83.7% (95.88% CI, 66.1–92.2)–82.4% (96.36% CI, 34.8–95.3) | ||
| MVA-BN-RSV, Bavarian Nordic [61,62,63,64,65,66] | Bivalent vector-based, RSV F, G (A), G (B), M2-1 and N | Phase 1 | Adults aged 18–49 years | 63 | 1.2–1.9 §§,¶¶ | – | – |
| Older adults aged 50–65 years | 1.0–2.0 §§,¶¶ | – | – | ||||
| Phase 2 | Older adults aged ≥55 years | 420 | 1.3–3.4 ††,§§ | Maintained above baseline levels 1 year after vaccination | – | ||
| Phase 2a human challenge trial | Adults aged 18–50 years | 61 | RSV A: 2.2 †† RSV B: 1.6 †† | RSV A: 1.7 § RSV B: 1.3 § | VE against RT-PCR-confirmed RSV infection with ≥1 symptom: *** 79.3% (95% CI, 13.4–95.1) VE against quantitative culture-confirmed RSV infection with ≥1 symptom: ††† 88.5% (95% CI, 14.8–98.5) |
3.2. The G Protein
3.3. Other Immune Mechanisms of Protection
| Vaccine Candidate, Sponsor | Formulation, Antigen | Clinical Trial Phase | Populations Assessed | N | RSV-Specific IFN-γ-Secreting T Cells GMFRs vs. Baseline * | RSV-Specific IFN-γ-Secreting T Cells GMFRs ≥1-Year Post-Vaccination * |
|---|---|---|---|---|---|---|
| RSVpreF, Pfizer [38] | Bivalent subunit, Prefusion F | Phase 1/2 | Older adults aged 65–85 years | 317 (n = 64) | Month 1: T cell responses declined but remained above baseline | – |
| GSK3844766A, GSK [39,56] | Recombinant subunit, Prefusion F + AS01E adjuvant | Phase 1/2 | Adults aged 18–40 years | 48 | Day 31: CD4+ 2.2–3.2 | – |
| Phase 1/2 | Older adults aged 60–80 years | 1005 | Day 31: CD4+ 1.7–3.2 | – | ||
| Phase 3 | Older adults aged ≥60 years | 1653 (n = 566) | Day 31: CD4+ increased from 191 to 1339 events/106 PBMC; CD8+ no detectable response Month 6: CD4+ declined to 666 events/106 PBMC; CD8+ no detectable response | – | ||
| Ad26.RSV.preF, Janssen [41,57,59] | Adenoviral vector 26, Prefusion F | Phase 1 | Older adults aged ≥60 years | 72 | Day 28: ≥2.1 † | ≥2.3-fold 28 days after second vaccination at 1 year †; maintained above baseline 2 years after vaccination † |
| Adeno 26 viral vector Prefusion F + Prefusion F subunit | Phase 2b | Older adults aged ≥65 years | 5782 (n = 195) | Day 14: increased from 34 to 444 SFC/106 PBMC† | 1.5 years: 143 SFC/106 PBMC | |
| MVA-BN-RSV, Bavarian Nordic [61,62] | Bivalent vector-based, RSV F, G (A), G (B), M2-1 and N | Phase 1 | Adults aged 18–49 years | 63 | 1.8–3.7 ‡ | – |
| Older adults aged 50–65 years | 2.4–3.8 ‡ | – | ||||
| Phase 2 | Older adults aged ≥55 years | 420 (n = 113) | Week 1: § 5.4–9.7 Week 5: ¶ 2.4–4.7 | Maintained above baseline 1 year after initial vaccination |
4. MVA-BN-RSV: A Late-Stage Vaccine Candidate Targeting Multiple RSV Antigens
5. Other Vaccines in Early Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Efstathiou, C.; Abidi, S.H.; Harker, J.; Stevenson, N.J. Revisiting respiratory syncytial virus’s interaction with host immunity, towards novel therapeutics. Cell. Mol. Life Sci. 2020, 77, 5045–5058. [Google Scholar] [CrossRef]
- Taylor, S.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Fleming, D.M. Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open 2016, 6, e009337. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Taylor, S. Modelling estimates of the burden of respiratory syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect. Dis. 2015, 15, 443. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.-Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: A systematic review and meta-analysis. J. Infect. Dis. 2020, 222, S577–S583. [Google Scholar] [CrossRef]
- Jozwik, A.; Habibi, M.S.; Paras, A.; Zhu, J.; Guvenel, A.; Dhariwal, J.; Almond, M.; Wong, E.H.C.; Sykes, A.; Maybeno, M.; et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015, 6, 10224. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The human immune response to respiratory syncytial virus infection. Clin Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- Fonseca, W.; Lukacs, N.W.; Ptaschinski, C. Factors affecting the immunity to respiratory syncytial virus: From epigenetics to microbiome. Front. Immunol. 2018, 9, 226. [Google Scholar] [CrossRef]
- Stephens, L.M.; Varga, S.M. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines 2021, 9, 624. [Google Scholar] [CrossRef]
- Synagis (Palivizumab). Summary of Product Characteristics. European Medicines Agency. Updated July 2022. Available online: https://www.ema.europa.eu/documents/product-information/synagis-epar-product-information_en.pdf (accessed on 24 November 2022).
- Synagis (Palivizumab). Prescribing Information. U.S. Food and Drug Administration. Updated November 2021. Available online: https://www.synagis.com/synagis.pdf (accessed on 24 November 2022).
- Ananworanich, J.; Heaton, P.M. Bringing preventive RSV monoclonal antibodies to infants in low- and middle-income countries: Challenges and opportunities. Vaccines 2021, 9, 961. [Google Scholar] [CrossRef]
- Sanofi. Press Release: European Commission Grants First Approval Worldwide of Beyfortus® (Nirsevimab) for Prevention of RSV Disease in Infants. Available online: https://www.sanofi.com/en/media-room/press-releases/2022/2022-11-04-07-00-00-2548492 (accessed on 24 November 2022).
- Lambert, L.; Sagfors, A.M.; Openshaw, P.J.M.; Culley, F.J. Immunity to RSV in early-life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Chiu, C. Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol. 2013, 3, 468–474. [Google Scholar] [CrossRef]
- Kiss, G.; Holl, J.M.; Williams, G.M.; Alonas, E.; Vanover, D.; Lifland, A.W.; Gudheti, M.; Guerrero-Ferreira, R.C.; Nair, V.; Yi, H.; et al. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J. Virol. 2014, 88, 7602–7617. [Google Scholar] [CrossRef]
- Laham, F.R.; Mansbach, J.M.; Piedra, P.A.; Hasegawa, K.; Sullivan, A.F.; Espinola, J.A.; Camargo, C.A., Jr. Clinical profiles of respiratory syncytial virus subtypes A AND B among children hospitalized with bronchiolitis. Pediatr. Infect. Dis. J. 2017, 36, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.E.; Kim, T.H.; Lee, H.K. Contribution of dendritic cells in protective immunity against respiratory syncytial virus infection. Viruses 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Ray, W.C.; Peeples, M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104. [Google Scholar] [PubMed]
- Fuentes, S.; Hahn, M.; Chilcote, K.; Chemaly, R.F.; Shah, D.P.; Ye, X.; Avadhanula, V.; Piedra, P.A.; Golding, H.; Khurana, S. Antigenic fingerprinting of respiratory syncytial virus (RSV)-A–infected hematopoietic cell transplant recipients reveals importance of mucosal anti–RSV G antibodies in control of RSV infection in humans. J. Infect. Dis. 2019, 221, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Ascough, S.; Paterson, S.; Chiu, C. Induction and subversion of human protective immunity: Contrasting influenza and respiratory syncytial virus. Front. Immunol. 2018, 9, 323. [Google Scholar] [CrossRef]
- Tang, A.; Chen, Z.; Cox, K.S.; Su, H.-P.; Callahan, C.; Fridman, A.; Zhang, L.; Patel, S.B.; Cejas, P.J.; Swoyer, R.; et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 2019, 10, 4153. [Google Scholar] [CrossRef]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef]
- Anderson, E.J.; Carosone-Link, P.; Yogev, R.; Yi, J.; Simões, E.A.F. Effectiveness of palivizumab in high-risk infants and children: A propensity score weighted regression analysis. Pediatr. Infect. Dis. J. 2017, 36, 699–704. [Google Scholar] [CrossRef]
- NCT04540627. Exploratory Study to Estimate the Prophylactic Efficacy of Palivizumab in Healthy Adult Participants Inoculated with RSV. Available online: https://clinicaltrials.gov/ct2/show/NCT04540627 (accessed on 24 November 2022).
- Sanofi. CHMP Recommends Approval of Beyfortus (Nirsevimab) for Prevention of RSV Disease in Infants. Available online: https://www.sanofi.com/en/media-room/press-releases/2022/2022-09-16-06-00-00-2517378 (accessed on 24 November 2022).
- European Medicines Agency. Meeting Highlights from the Committee for Medicinal Products for Human Use 12–15 September 2022. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-12-15-september-2022 (accessed on 24 November 2022).
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Griffin, M.P.; Khan, A.A.; Esser, M.T.; Jensen, K.; Takas, T.; Kankam, M.K.; Villafana, T.; Dubovsky, F. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob. Agents Chemother 2017, 61, e01714–e01716. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Xu, X.-N.; Akbar, A.N. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front. Immunol. 2020, 11, 583019. [Google Scholar] [CrossRef]
- Wagner, A.; Weinberger, B. Vaccines to prevent infectious diseases in the older population: Immunological challenges and future perspectives. Front. Immunol. 2020, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Gilman, M.S.A.; Castellanos, C.A.; Chen, M.; Ngwuta, J.O.; Goodwin, E.; Moin, S.M.; Mas, V.; Melero, J.A.; Wright, P.F.; Graham, B.S.; et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci. Immunol. 2016, 1, eaaj1879. [Google Scholar] [CrossRef]
- Falloon, J.; Yu, J.; Esser, M.T.; Villafana, T.; Yu, L.; Dubovsky, F.; Takas, T.; Levin, M.J.; Falsey, A.R. An adjuvanted, postfusion F protein–based vaccine did not prevent respiratory syncytial virus illness in older adults. J. Infect. Dis. 2017, 216, 1362–1370. [Google Scholar] [CrossRef]
- Novavax. Novavax Announces Topline RSV F Vaccine Data from Two Clinical Trials in Older Adults. Available online: https://ir.novavax.com/2016-09-25-Novavax-Announces-Topline-RSV-F-Vaccine-Data-from-Two-Clinical-Trials-in-Older-Adults (accessed on 12 January 2023).
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A randomized Phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef]
- Baber, J.; Arya, M.; Moodley, Y.; Jaques, A.; Jiang, Q.; Swanson, K.A.; Cooper, D.; Maddur, M.S.; Loschko, J.; Gurtman, A.; et al. A phase 1/2 study of a respiratory syncytial virus prefusion F vaccine with and without adjuvant in healthy older adults. J. Infect. Dis. 2022, 226, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Davis, M.G.; Steenackers, K.; Essink, B.; Vandermeulen, C.; Fogarty, C.; Andrews, C.P.; Kerwin, E.; David, M.P.; Fissette, L.; et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: Phase I/II randomized clinical trial. J. Infect. Dis. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Guiñazú, J.R.; Tica, J.; Andrews, C.P.; Davis, M.G.; De Smedt, P.; Essink, B.; Fogarty, C.; Kerwin, E.; Leroux-Roels, I.; Vandermeulen, C.; et al. 121. A respiratory syncytial virus prefusion F protein (RSVPreF3) candidate vaccine administered in older adults in a Phase I/II randomized clinical trial is immunogenic. Open Forum Infect. Dis. 2020, 7, S188–S189. [Google Scholar] [CrossRef]
- Williams, K.; Bastian, A.R.; Feldman, R.A.; Omoruyi, E.; de Paepe, E.; Hendriks, J.; van Zeeburg, H.; Godeaux, O.; Langedijk, J.P.M.; Schuitemaker, H.; et al. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J. Infect. Dis. 2020, 222, 979–988. [Google Scholar] [CrossRef] [PubMed]
- GSK. GSK’s Respiratory Syncytial Virus Older Adult Vaccine Candidate Granted Priority Review by US FDA. Available online: https://www.gsk.com/en-gb/media/press-releases/gsk-s-rsv-oa-vaccine-candidate-granted-priority-review-by-us-fda/ (accessed on 24 November 2022).
- GSK. GSK’s Older Adult Respiratory Syncytial Virus (RSV) Vaccine Candidate Shows 94.1% Reduction in Severe RSV Disease and Overall Vaccine Efficacy of 82.6% in Pivotal Trial. Available online: https://www.gsk.com/en-gb/media/press-releases/gsk-s-older-adult-respiratory-syncytial-virus-rsv-vaccine-candidate/ (accessed on 24 November 2022).
- Rizkalla, B.; GSK RSV OA Candidate Vaccine Clinical Development. Advisory Committee on Immunization Practices (ACIP) meeting 20 October 2022. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/02-RSV-Adults-Rizkalla-508.pdf (accessed on 24 November 2022).
- Ison, M.G.; Papi, A.; Langley, J.M.; Dong-Gun, L.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; Van Zyl-Smit, R.N.; Dezutter, N.; De Schrevel, N.; et al. LB745–Respiratory syncytial virus (RSV) prefusion F protein candidate vaccine (RSVPreF3 OA) is efficacious in adults ≥ 60 years of age (YOA). Presented at ID Week, Washington, DC, USA, 19–23 October 2022. [Google Scholar]
- Pfizer. Pfizer Granted FDA Breakthrough Therapy Designation for Respiratory Syncytial Virus Vaccine Candidate for the Prevention of RSV in Older Adults. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-granted-fda-breakthrough-therapy-designation-0 (accessed on 24 November 2022).
- Walsh, E.E.; Polack, F.; Zareba, A.; Falsey, A.R.; Perez Marc, G.; Jiang, Q.; Schneider, K.; Cooper, D.; Maddalena Lino, M.; Anderson, A.S.; et al. LB748–Efficacy and safety of bivalent respiratory syncytial virus (RSVpreF) vaccine in older adults. Presented at ID Week, Washington, DC, USA, 19–23 October 2022. [Google Scholar]
- Gurtman, A. Safety and Efficacy of Bivalent RSV Prefusion F Vaccine in Adults ≥ 60 Years of Age. Advisory Committee on Immunization Practices (ACIP) Meeting 20 October 2022. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/03-RSV-Adults-Gurtma-508.pdf (accessed on 24 November 2022).
- Moderna. Moderna Announces mRNA-1345, an Investigational Respiratory Syncytial Virus (RSV) Vaccine, as Met Primary Efficacy Endpoints in Phase 3 Trial in Older Adults. Available online: https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx (accessed on 18 January 2023).
- Simões, E.A.F.; Forleo-Neto, E.; Geba, G.P.; Kamal, M.; Yang, F.; Cicirello, H.; Houghton, M.R.; Rideman, R.; Zhao, Q.; Benvin, S.L.; et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin. Infect. Dis. 2020, 73, e4400–e4408. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, X.; Wei, C.; Li, S.; Zhao, J.; Zheng, Y.; Liu, X.; Zeng, X.; Yuan, W.; Peng, S. Molecular evolutionary characteristics of SARS-CoV-2 emerging in the United States. J. Med. Virol. 2022, 94, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-M.; Fu, Y.-H.; Peng, X.-L.; Zheng, Y.-P.; He, J.-S. Genetic diversity and molecular evolution of human respiratory syncytial virus A and B. Sci. Rep. 2021, 11, 12941. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e2379. [Google Scholar] [CrossRef]
- Mas, V.; Nair, H.; Campbell, H.; Melero, J.A.; Williams, T.C. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine 2018, 36, 6660–6673. [Google Scholar] [CrossRef]
- Schmoele-Thoma, B.; Zareba, A.M.; Jiang, Q.; Maddur, M.S.; Danaf, R.; Mann, A.; Eze, K.; Fok-Seang, J.; Kabir, G.; Catchpole, A.; et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2022, 386, 2377–2386. [Google Scholar] [CrossRef]
- Schwarz, T.F.; Hwang, S.-J.; Ylisastigui, P.P.; Liu, C.-S.; Takazawa, K.; Yono, M.; Ervin, J.E.; Andrews, C.; Fogarty, C.; Eckermann, T.; et al. A candidate respiratory syncytial virus (RSV) prefusion F protein investigational vaccine (RSVPreF3 OA) is immunogenic when administered in adults ≥ 60 years of age: Results at 6 months after vaccination. Presented at ID Week, Washington, DC, USA, 19–23 October 2022. [Google Scholar]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.A.; Ervin, J.E.; Bastian, A.R.; Menten, J.; De Paepe, E.; de Boer, H.; Vandenberghe, S.; et al. LB14. Efficacy and immunogenicity of an Ad26.RSV.preF-based vaccine in the prevention of RT-PCR-confirmed RSV-mediated lower respiratory tract disease in adults aged ≥65 years: A randomized, placebo-controlled, phase 2b study. Open Forum Infect. Dis. 2021, 8, S812. [Google Scholar] [CrossRef]
- Sadoff, J.; De Paepe, E.; DeVincenzo, J.; Gymnopoulou, E.; Menten, J.; Murray, B.; Rosemary Bastian, A.; Vandebosch, A.; Haazen, W.; Noulin, N.; et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J. Infect. Dis. 2021, 226, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.R.; Comeaux, C.; Falsey, A.R.; Williams, K.; Bart, S.A.; Ervin, J.E.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.; et al. 2321–Long-term immunogenicity of Ad26.RSV.preF/RSV preF protein vaccine against RSV in a phase 2b study by age and risk level. Presented at ID Week, Washington, DC, USA, 19–23 October 2022. [Google Scholar]
- Chen, G.L.; Mithani, R.; Kapoor, A.; Lu, S.; El Asmar, L.; Panozzo, C.A.; Shaw, C.A.; Stoszek, S.K.; August, A. Safety and immunogenicity of mRNA-1345, an mRNA-based vaccine against RSV in older adults through 6 month follow-up. Presented at 12th International RSV Symposium (RSV 2022), Belfast, UK, 29 September–2 October 2022. [Google Scholar]
- Samy, N.; Reichhardt, D.; Schmidt, D.; Chen, L.M.; Silbernagl, G.; Vidojkovic, S.; Meyer, T.P.H.; Jordan, E.; Adams, T.; Weidenthaler, H.; et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: A randomized phase I clinical trial. Vaccine 2020, 38, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Lawrence, S.J.; Meyer, T.P.H.; Schmidt, D.; Schultz, S.; Mueller, J.; Stroukova, D.; Koenen, B.; Gruenert, R.; Silbernagl, G.; et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J. Infect. Dis. 2021, 223, 1062–1072. [Google Scholar] [CrossRef]
- Bavarian Nordic. MVA-BN-RSV Human Challenge Trial Results. Available online: https://www.bavarian-nordic.com/media/309881/210901-mva-bn-hct-results-en.pdf (accessed on 24 November 2022).
- Weidenthaler, H.; Schultz, S.; Sanos, S.; Silbernagl, G.; Schmidt, D.; Kabir, G.; Jordan, E.; Jenkins, V.; DeMoerlooze, L. Efficacy, safety and immunogenicity of the recombinant MVA-BN-RSV vaccine against respiratory syncytial virus (RSV) infection in a human challenge trial (HCT) in healthy adult participants. Presented at 6th ReSViNET Conference, Virtual, 10–12 November 2021. [Google Scholar]
- NCT04752644. Phase 2a Study of MVA-BN-RSV Vaccination and RSV Challenge in Healthy Adults. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04752644 (accessed on 24 November 2022).
- Jordan, E.; Kabir, G.; Schultz, S.; Silbernagl, G.; Schmidt, D.; Jenkins, V.A.; Weidenthaler, H.; Stroukova, D.; Martin, B.K.; De Moerlooze, L. Decreased viral load, symptom reduction, and prevention of respiratory syncytial virus infection with MVA-BN-RSV vaccine. J. Infect. Dis. 2022. submitted. [Google Scholar]
- Fuentes, S.; Crim, R.L.; Beeler, J.; Teng, M.N.; Golding, H.; Khurana, S. Development of a simple, rapid, sensitive, high-throughput luciferase reporter based microneutralization test for measurement of virus neutralizing antibodies following respiratory syncytial virus vaccination and infection. Vaccine 2013, 31, 3987–3994. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Rosenberg, H.F. Respiratory syncytial virus infection: Immune response, immunopathogenesis, and treatment. Clin. Microbiol. Rev. 1999, 12, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Bingham, P.; Hierholzer, J.C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 1988, 62, 4232–4238. [Google Scholar] [CrossRef]
- Tripp, R.A.; Power, U.F.; Openshaw, P.J.M.; Kauvar, L.M. Respiratory syncytial virus: Targeting the G protein provides a new approach for an old problem. J. Virol. 2018, 92, e01302–e01317. [Google Scholar] [CrossRef]
- Capella, C.; Chaiwatpongsakorn, S.; Gorrell, E.; Risch, Z.A.; Ye, F.; Mertz, S.E.; Johnson, S.M.; Moore-Clingenpeel, M.; Ramilo, O.; Mejias, A.; et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J. Infect. Dis. 2017, 216, 1398–1406. [Google Scholar] [CrossRef]
- Volkman, A.; Endt, K.; Cheminay, C.; Kalla, M.; Steigerwald, R.; Wennier, S.T.; Gilbert, B.E.; Chaplin, P. A novel multi-insert MVA-BN based RSV vaccine provides improved protection in mice and cotton rats. Presented at 5th ReSViNET Conference, Accra, Ghana, 12–14 November 2019. [Google Scholar]
- Fuentes, S.; Coyle, E.M.; Beeler, J.; Golding, H.; Khurana, S. Antigenic fingerprinting following primary RSV infection in young children identifies novel antigenic sites and reveals unlinked evolution of human antibody repertoires to fusion and attachment glycoproteins. PLoS Pathog. 2016, 12, e1005554. [Google Scholar] [CrossRef]
- Walsh, E.E.; Peterson, D.R.; Falsey, A.R. Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 2004, 189, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, R.; Higgins, D.; Hosken, N. RSV neutralization assays–Use in immune response assessment. Vaccine 2021, 39, 4591–4597. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Klenow, L.; Coyle, E.M.; Golding, H.; Khurana, S. Protective antigenic sites in respiratory syncytial virus G attachment protein outside the central conserved and cysteine noose domains. PLoS Pathog. 2018, 14, e1007262. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, Y.-T.; Kim, K.-H.; Ko, E.-J.; Lee, Y.; Kwon, Y.-M.; Kang, S.-M. Virus-like particle vaccine primes immune responses preventing inactivated-virus vaccine-enhanced disease against respiratory syncytial virus. Virology 2017, 511, 142–151. [Google Scholar] [CrossRef]
- Tripp, R.A.; Power, U.F. Original antigenic sin and respiratory syncytial virus vaccines. Vaccines 2019, 7, 107. [Google Scholar] [CrossRef]
- Hall, C.B.; Walsh, E.E.; Long, C.E.; Schnabel, K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 1991, 163, 693–698. [Google Scholar] [CrossRef]
- Habibi, M.S.; Jozwik, A.; Makris, S.; Dunning, J.; Paras, A.; DeVincenzo, J.P.; de Haan, C.A.M.; Wrammert, J.; Openshaw, P.J.M.; Chiu, C. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2015, 191, 1040–1049. [Google Scholar] [CrossRef]
- Bagga, B.; Cehelsky, J.E.; Vaishnaw, A.; Wilkinson, T.; Meyers, R.; Harrison, L.M.; Roddam, P.L.; Walsh, E.E.; DeVincenzo, J.P. Effect of preexisting serum and mucosal antibody on experimental respiratory syncytial virus (RSV) challenge and infection of adults. J. Infect. Dis. 2015, 212, 1719–1725. [Google Scholar] [CrossRef]
- Cherukuri, A.; Patton, K.; Gasser Robert, A.; Zuo, F.; Woo, J.; Esser Mark, T.; Tang Roderick, S. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin. Vaccine Immunol. 2013, 20, 239–247. [Google Scholar] [CrossRef]
- Guvenel, A.; Jozwik, A.; Ascough, S.; Ung, S.K.; Paterson, S.; Kalyan, M.; Gardener, Z.; Bergstrom, E.; Kar, S.; Habibi, M.S.; et al. Epitope-specific airway-resident CD4+ T cell dynamics during experimental human RSV infection. J. Clin. Investig. 2020, 130, 523–538. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Varga, S.M. Cytokines and CD8 T cell immunity during respiratory syncytial virus infection. Cytokine 2020, 133, 154481. [Google Scholar] [CrossRef]
- Bavarian Nordic. Bavarian Nordic Announces Grant of Prime Eligibility from the European Medicines Agency for Its RSV Vaccine Candidate for the Prevention of Respiratory Syncytial Virus in Older Adults. Available online: https://www.bavarian-nordic.com/investor/news/news.aspx?news=6589 (accessed on 24 November 2022).
- Endt, K.; Wollmann, Y.; Haug, J.; Bernig, C.; Feigl, M.; Heiseke, A.; Kalla, M.; Hochrein, H.; Suter, M.; Chaplin, P.; et al. A recombinant MVA-based RSV vaccine induces T-cell and antibody responses that cooperate in the protection against RSV infection. Front. Immunol. 2022, 13, 841471. [Google Scholar] [CrossRef] [PubMed]
- PATH. RSV Vaccine and mAB Snapshot. Available online: https://media.path.org/documents/RSV_Snapshot_Nov_2022.pdf?_gl=1*1f0eszm*_ga*MzE4NjgzMjMzLjE2NzM1MjE1ODA.*_ga_YBSE7ZKDQM*MTY3MzUzMjM0MS4yLjAuMTY3MzUzMjM0OC4wLjAuMA (accessed on 12 January 2023).
- Cheng, X.; Zhao, G.; Dong, A.; He, Z.; Wang, J.; Jiang, B.; Wang, B.; Wang, M.; Huai, X.; Zhang, S.; et al. A first in human trial to evaluate the safety and immunogenicity of a G protein based recombinant respiratory syncytial virus vaccine in healthy adults 18–45 years. medRxiv 2022. [Google Scholar]
- Advaccine. Advaccine Announces First Participants Dosed in Phase 2 Study of ADV110 Evaluating Respiratory Syncytial Virus (RSV) Vaccine Candidate in Australia. Available online: https://www.prnewswire.com/news-releases/advaccine-announces-first-participants-dosed-in-phase-2-study-of-adv110-evaluating-respiratory-syncytial-virus-rsv-vaccine-candidate-in-australia-301305992.html (accessed on 12 January 2023).
- Langley, J.M.; MacDonald, L.D.; Weir, G.M.; MacKinnon-Cameron, D.; Ye, L.; McNeil, S.; Schepens, B.; Saelens, X.; Stanford, M.M.; Halperin, S.A. A respiratory syncytial virus vaccine based on the small hydrophobic protein ectodomain presented with a novel lipid-based formulation is highly immunogenic and safe in adults: A first-in-humans study. J. Infect. Dis. 2018, 218, 378–387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkins, V.A.; Hoet, B.; Hochrein, H.; De Moerlooze, L. The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein. Vaccines 2023, 11, 382. https://doi.org/10.3390/vaccines11020382
Jenkins VA, Hoet B, Hochrein H, De Moerlooze L. The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein. Vaccines. 2023; 11(2):382. https://doi.org/10.3390/vaccines11020382
Chicago/Turabian StyleJenkins, Victoria A., Bernard Hoet, Hubertus Hochrein, and Laurence De Moerlooze. 2023. "The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein" Vaccines 11, no. 2: 382. https://doi.org/10.3390/vaccines11020382
APA StyleJenkins, V. A., Hoet, B., Hochrein, H., & De Moerlooze, L. (2023). The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein. Vaccines, 11(2), 382. https://doi.org/10.3390/vaccines11020382





