Assessment of Immunogenicity and Efficacy of CV0501 mRNA-Based Omicron COVID-19 Vaccination in Small Animal Models
Abstract
1. Introduction
2. Methods and Materials
2.1. Vaccines
2.2. Animal Models
2.3. Dose Response and Booster Studies
2.4. Neutralizing Antibody Titers
2.5. Enzyme-Linked Immunosorbent Spot (ELISpot) Assay
2.6. ACE2 Binding Inhibition Assay Methodology
2.7. Hamster Challenge Model
2.7.1. Immunizations
2.7.2. Challenge Infection
2.7.3. Viral RNA Detection and Quantification
2.7.4. Surrogate ELISA
2.7.5. Neutralizing Antibody Titers
2.7.6. Statistical Analysis
3. Results
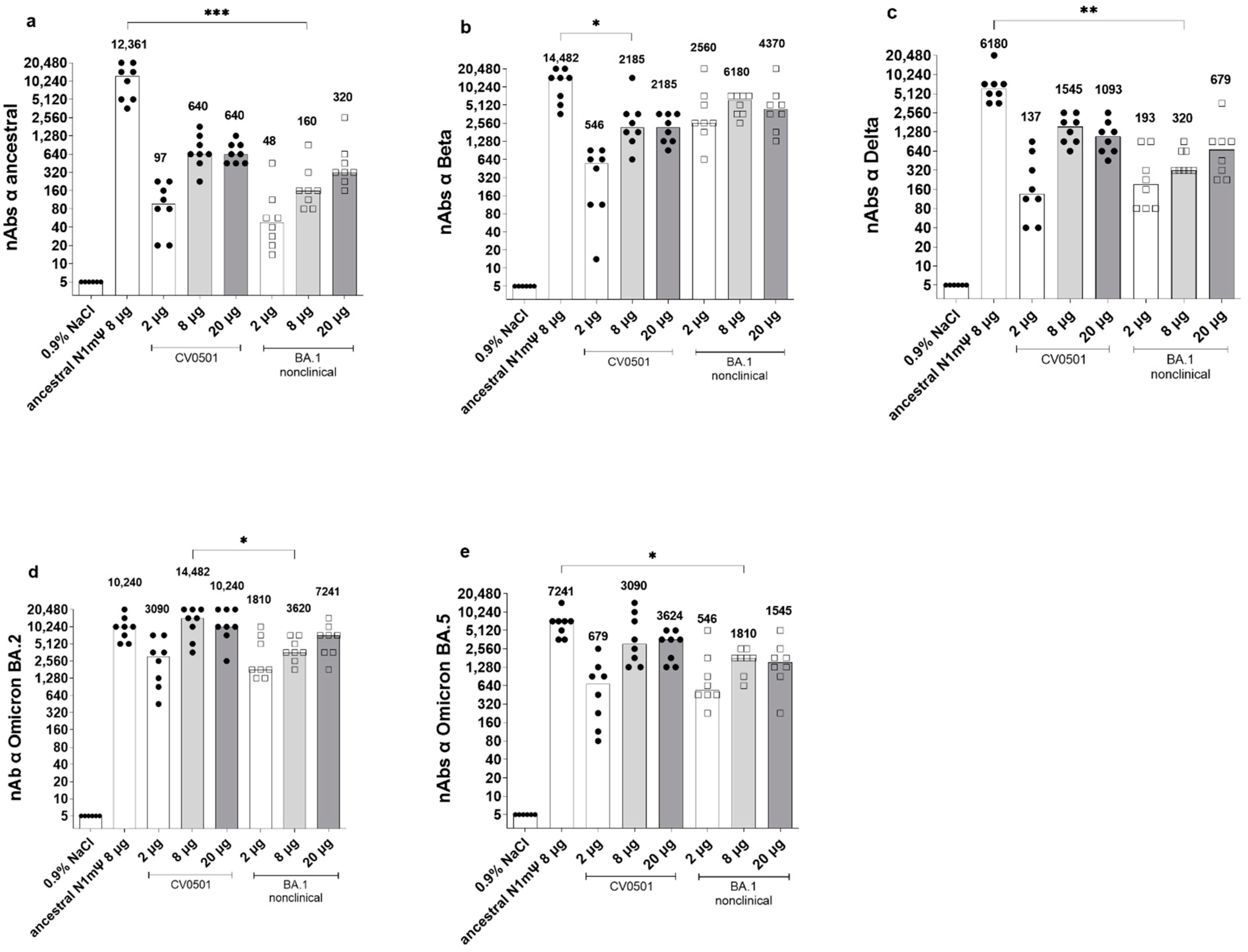
3.1. CV0501 Induces nAb Responses against SARS-CoV-2 Variants in Wistar Rats
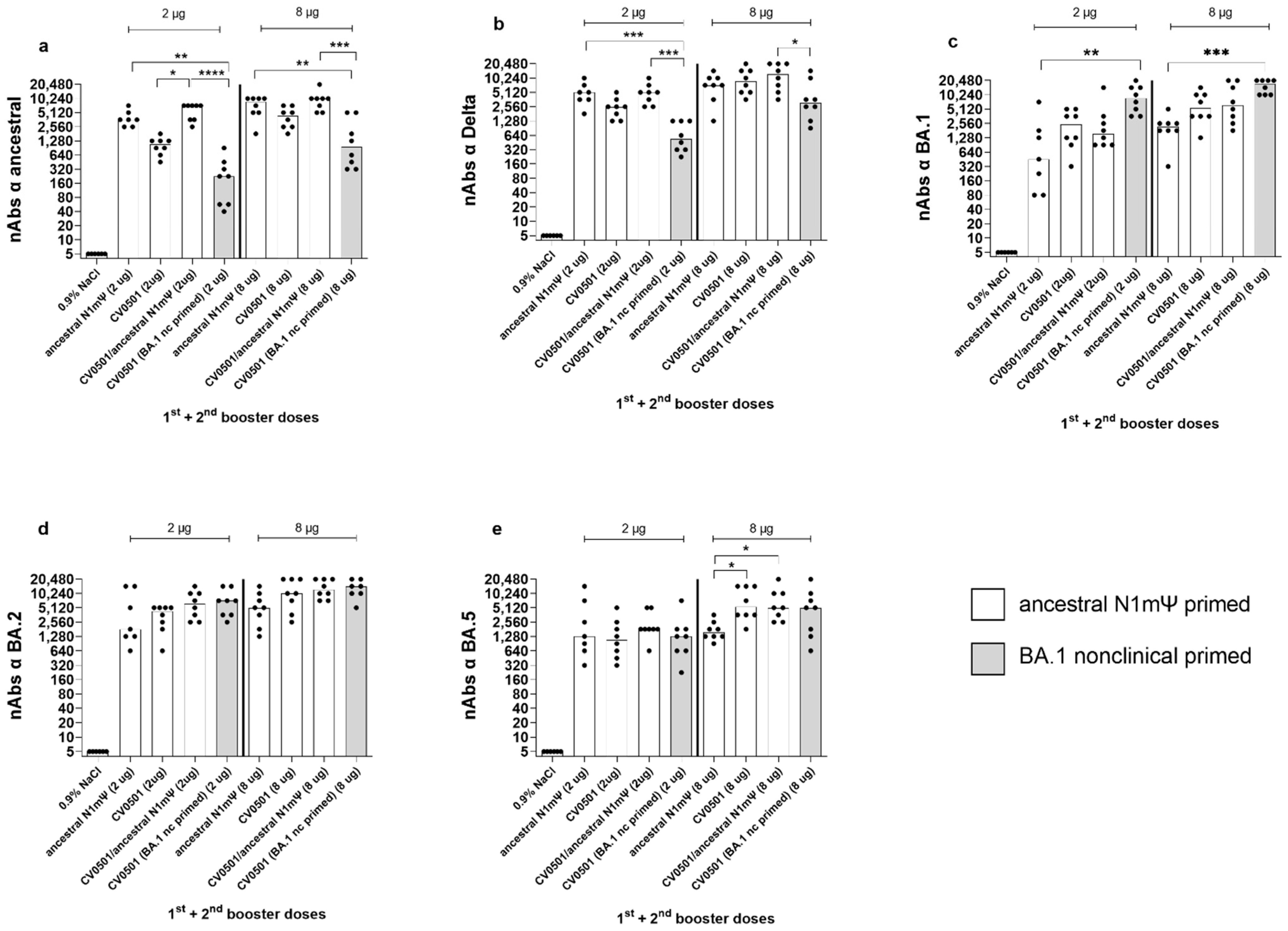
3.2. CV0501 Boosting Induces Cross-nAbs against SARS-CoV-2 Variants in Ancestral-Primed Wistar Rats
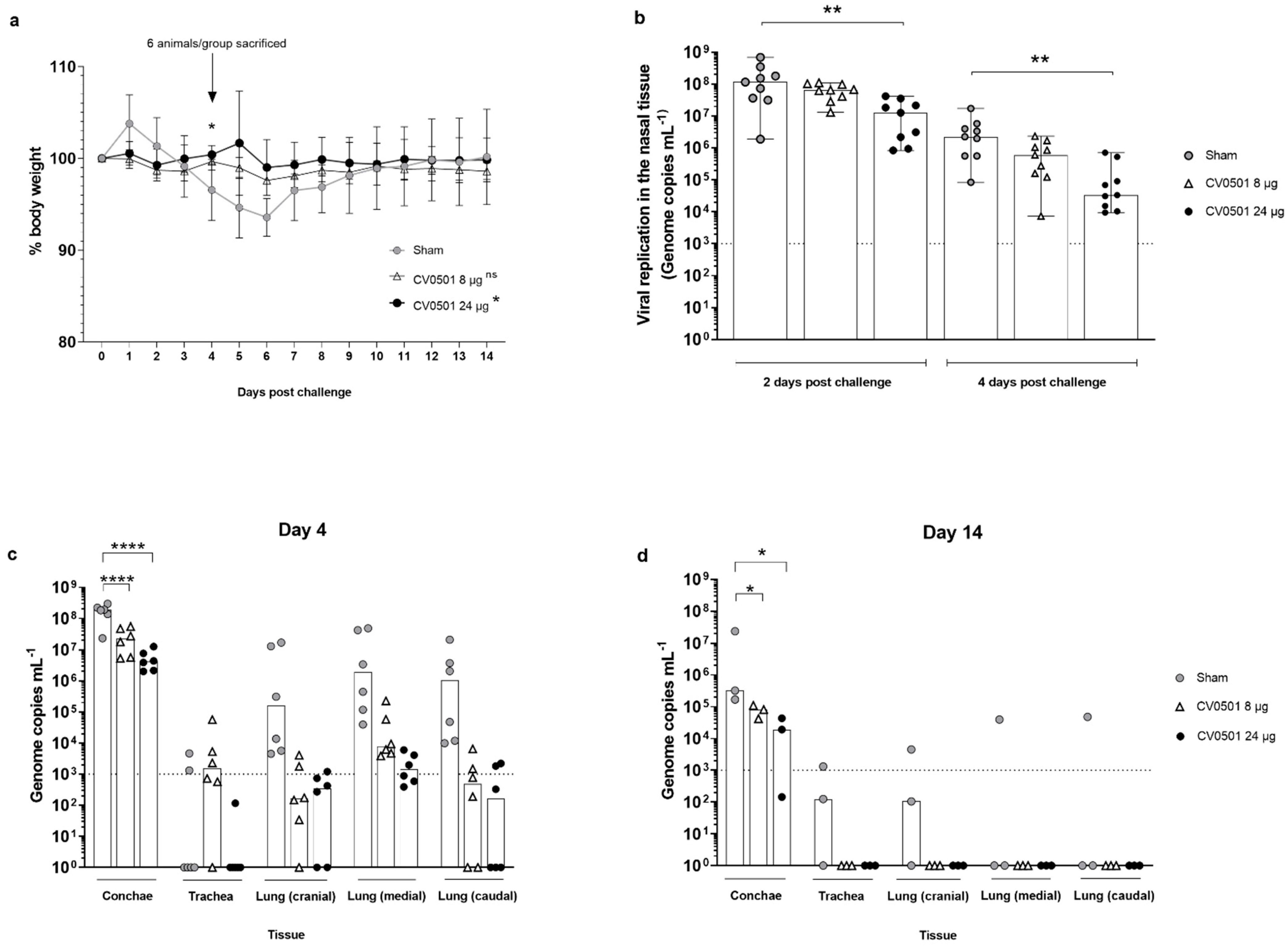
3.3. CV0501 Protects against Weight Loss and Viral Replication in the Respiratory Tract in Syrian Hamsters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 14 July 2022).
- World Health Organization. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants. (accessed on 3 January 2023).
- US FDA. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster (accessed on 3 January 2023).
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. bioRxiv 2022, 186, 279–286.e8. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, D.J.; Matthews-Juarez, P.; Smoot, D.; Hildreth, J.E.K.; Lamar, K.; Tabatabai, M.; Wilus, D.; Juarez, P.D. Breakthrough COVID-19 infections in the US: Implications for prolonging the pandemic. Vaccines 2022, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Ishak, A.; Dhawan, N.; Poudel, S.; Shrestha, P.S.; Singh, P.; Xie, E.; Tahir, P.; Marzaban, S.; Michel, J.; et al. Characteristics of COVID-19 breakthrough infections among vaccinated individuals and associated risk factors: A systematic review. Trop. Med. Infect. Dis. 2022, 7, 81. [Google Scholar] [CrossRef]
- Al-Otaiby, M.; Krissaane, I.; Al Seraihi, A.; Alshenaifi, J.; Qahtani, M.H.; Aljeri, T.; Zaatari, E.; Hassanain, M.; Algwizani, A.; Albarrag, A.; et al. SARS-CoV-2 reinfection rate and outcomes in Saudi Arabia: A national retrospective study. Int. J. Infect. Dis. 2022, 122, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, F.; Kochańczyk, M.; Lipniacki, T. The spread of SARS-CoV-2 variant Omicron with a doubling time of 2.0-3.3 days can be explained by immune evasion. Viruses 2022, 14, 294. [Google Scholar] [CrossRef]
- Shaheen, N.A.; Sambas, R.; Alenezi, M.; Alharbi, N.K.; Aldibasi, O.; Bosaeed, M. COVID-19 reinfection: A multicenter retrospective study in Saudi Arabia. Ann. Thorac. Med. 2022, 17, 81–86. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Schön, J.; Hoffmann, D.; Thran, M.; Thess, A.; Mueller, S.O.; Petsch, B.; Rauch, S. Optimised non-coding regions of mRNA SARS-CoV-2 vaccine CV2CoV improves homologous and heterologous neutralising antibody responses. Vaccines 2022, 10, 1251. [Google Scholar] [CrossRef]
- Gebre, M.S.; Rauch, S.; Roth, N.; Yu, J.; Chandrashekar, A.; Mercado, N.B.; He, X.; Liu, J.; McMahan, K.; Martinot, A.; et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 2022, 601, 410–414. [Google Scholar] [CrossRef]
- Hoffmann, D.; Corleis, B.; Rauch, S.; Roth, N.; Mühe, J.; Halwe, N.J.; Ulrich, L.; Fricke, C.; Schön, J.; Kraft, A.; et al. CVnCoV and CV2CoV protect human ACE2 transgenic mice from ancestral B BavPat1 and emerging B.1.351 SARS-CoV-2. Nat. Commun. 2021, 12, 4048. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef] [PubMed]
- Durovic, N.; Pillay, M.; Cox, P.H. The stability of 99mTc-DTPA and 99mTc-HIDA following ultrasonic nebulisation. Eur. J. Nucl. Med. 1988, 14, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Junker, D.; Dulovic, A.; Becker, M.; Wagner, T.R.; Kaiser, P.D.; Traenkle, B.; Kienzle, K.; Bunk, S.; Struemper, C.; Haeberle, H.; et al. COVID-19 patient serum less potently inhibits ACE2-RBD binding for various SARS-CoV-2 RBD mutants. Sci. Rep. 2022, 12, 7168. [Google Scholar] [CrossRef]
- Becker, M.; Strengert, M.; Junker, D.; Kaiser, P.D.; Kerrinnes, T.; Traenkle, B.; Dinter, H.; Häring, J.; Ghozzi, S.; Zeck, A.; et al. Exploring beyond clinical routine SARS-CoV-2 serology using MultiCoV-Ab to evaluate endemic coronavirus cross-reactivity. Nat. Commun. 2021, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Junker, D.; Becker, M.; Wagner, T.R.; Kaiser, P.D.; Maier, S.; Grimm, T.M.; Griesbaum, J.; Marsall, P.; Gruber, J.; Traenkle, B.; et al. Antibody binding and angiotensin-converting enzyme 2 binding inhibition Is significantly reduced for both the BA.1 and BA.2 Omicron variants. Clin. Infect. Dis. 2022, 19, ciac498. [Google Scholar] [CrossRef] [PubMed]
- Institut Pasteur. Protocol: Real-Time RT-PCR Assays for the Detection of SARS-CoV-2. Available online: https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2 (accessed on 3 January 2023).
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espie, E.; Feikin, D.R.; Knoll, M.D. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine effectiveness against the omicron (B.1.1.529). N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med Virol. 2022, 32, e2381. [Google Scholar] [CrossRef]
- Scheaffer, S.M.; Lee, D.; Whitener, B.; Ying, B.; Wu, K.; Liang, C.Y.; Jani, H.; Martin, P.; Amato, N.J.; Avena, L.E.; et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat. Med. 2022, 29, 247–257. [Google Scholar] [CrossRef]
- Muik, A.; Lui, B.G.; Bacher, M.; Wallisch, A.K.; Toker, A.; Couto, C.I.C.; Güler, A.; Mampilli, V.; Schmitt, G.J.; Mottl, J.; et al. Exposure to BA.4/5 S protein drives neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in vaccine-experienced humans and mice. Sci. Immunol. 2022, 7, eade9888. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Kuroda, M.; Maemura, T.; Chiba, S.; Armbrust, T.; Wright, R.; Balaram, A.; Florek, K.R.; Bateman, A.C.; Kawaoka, Y. Efficacy of vaccination and previous infection against the Omicron BA.1 variant in Syrian hamsters. Cell Rep. 2022, 39, 110688. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Schulz, J.E.; Adney, D.R.; Saturday, T.A.; Fischer, R.J.; Yinda, C.K.; Thakur, N.; Newman, J.; Ulaszewska, M.; Belij-Rammerstorfer, S.; et al. ChAdOx1 nCoV-19 (AZD1222) or nCoV-19-Beta (AZD2816) protect Syrian hamsters against Beta Delta and Omicron variants. Nat. Commun. 2022, 13, 4610. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.J.; van Doremalen, N.; Adney, D.R.; Yinda, C.K.; Port, J.R.; Holbrook, M.G.; Schulz, J.E.; Williamson, B.N.; Thomas, T.; Barbian, K.; et al. ChAdOx1 nCoV-19 (AZD1222) protects Syrian hamsters against SARS-CoV-2 B.1.351 and B.1.1.7. Nat. Commun. 2021, 12, 5868. [Google Scholar] [CrossRef]
- Corbett, K.S.; Werner, A.P.; Connell, S.O.; Gagne, M.; Lai, L.; Moliva, J.I.; Flynn, B.; Choi, A.; Koch, M.; Foulds, K.E.; et al. mRNA-1273 protects against SARS-CoV-2 beta infection in nonhuman primates. Nat. Immunol. 2021, 22, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sukhova, K.; McKay, P.F.; Kurshan, A.; Yau, Y.; Lechmere, T.; Brown, J.C.; Moshe, M.; Kugasathan, R.; Snell, L.B.; et al. Omicron breakthrough infections in vaccinated or previously infected hamsters. bioRxiv 2022, 05.20.492779. [Google Scholar] [CrossRef]
- Muñoz-Fontela, C.; Widerspick, L.; Albrecht, R.A.; Beer, M.; Carroll, M.W.; de Wit, E.; Diamond, M.S.; Dowling, W.E.; Funnell, S.G.P.; García-Sastre, A.; et al. Advances and gaps in SARS-CoV-2 infection models. PLoS Pathog. 2022, 18, e1010161. [Google Scholar] [CrossRef]
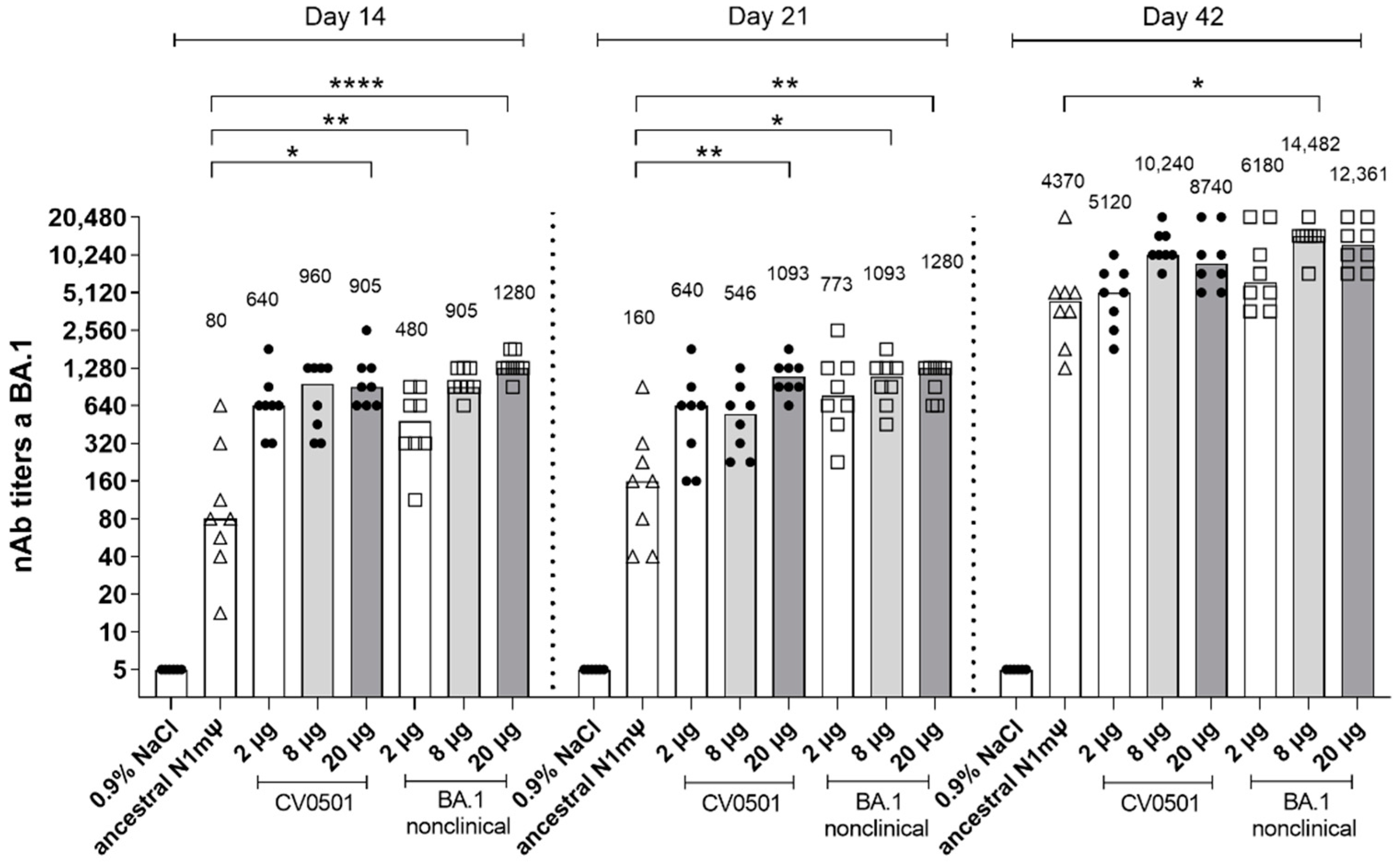



| Name | Encoded SARS-CoV-2 Variant | mRNA Modification | AA Mutations in the Spike Protein * (Compared with Ancestral) | AA Mutations Specific to the RBD Region (Compared with Ancestral) |
|---|---|---|---|---|
| CV0501 | BA.1 (12 RBD mutations) | N1mΨ | A67V, del69–70, T95I, G142D, del143–145, del211, L212I, ins214EPE, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K and L981F. | G339D, S371L, S373P, S375F, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H |
| BA.1 nonclinical | BA.1 (15 RBD mutations) | N1mΨ | A67V, del69–70, T95I, G142D, del143–145, del211, L212I, ins214EPE, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K and L981F. | As above but with 3 additional mutations: K417N, N440K and G446S |
| Ancestral | Ancestral | N1mΨ | - | - |
| Group | 1st Prime (Day 0) 2nd Prime (Day 21) | 1st Boost (Day 105) 2nd Boost (Day 189) | Dose Concentration | Median nAb Titers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 21 | Day 42 | Fold-Increase Days 21–42 | Day 105 | Day 133 | Fold-Increase Days 105–133 | Day 189 | Day 217 | Fold-Increase Days 189–217 | ||||
| 1 | Ancestral N1mΨ | CV0501 | 2 µg | 8 | 273 | 36 | 137 | 773 | 5.6 | 386 | 2450 | 6.3 |
| 8 µg | 34 | 2560 | 75 | 1810 | 2185 | 1.2 | 1810 | 5430 | 3.0 | |||
| 2 | Ancestral N1mΨ | CV0501/ ancestral N1mΨ | 2 µg | 12 | 1093 | 91 | 480 | 905 | 1.9 | 546 | 1545 | 2.8 |
| 8 µg | 113 | 7241 | 64 | 1920 | 3090 | 1.6 | 1545 | 6180 | 4.0 | |||
| 3 | Ancestral N1mΨ | Ancestral N1mΨ | 2 µg | 5 | 170 | 34 | 160 | 240 | 1.5 | 160 | 453 | 2.8 |
| 8 µg | 24 | 1810 | 75 | 960 | 1358 | 1.4 | 1092 | 2185 | 2.0 | |||
| 4 | BA.1 nonclinical | CV0501 | 2 µg | 773 | 7241 | 9 | 4370 | 5120 | 1.2 | 3620 | 8740 | 2.4 |
| 8 µg | 1280 | 12,361 | 10 | 4370 | 10,240 | 2.3 | 5120 | 17,481 | 3.4 | |||
| Median nAb Titers vs. SARS-CoV-2 Variants on Day 217 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 1st Prime (Day 0) 2nd Prime (Day 21) | 1st Boost (Day 105) | 2nd Boost (Day 189) | Dose Concentration | Ancestral | Delta | BA.1 | BA.2 | BA.5 |
| 1 | Ancestral N1mΨ | Ancestral N1mΨ | Ancestral N1mΨ | 2 µg | 3620 | 5120 | 452 | 1810 | 1280 |
| 8 µg | 8740 | 7241 | 2185 | 5120 | 1545 | ||||
| 2 | Ancestral N1mΨ | CV0501 | CV0501 | 2 µg | 1093 | 2560 | 2450 | 4370 | 1093 |
| 8 µg | 4370 | 8740 | 5431 | 10,240 | 5431 | ||||
| 3 | Ancestral N1mΨ | Bivalent CV0501/ancestral N1mΨ | Bivalent CV0501/ancestral N1mΨ | 2 µg | 7241 | 5120 | 1545 | 6180 | 1810 |
| 8 µg | 10,240 | 12,361 | 6180 | 12,361 | 5120 | ||||
| 4 | BA.1 nonclinical | CV0501 | CV0501 | 2 µg | 226 | 546 | 8740.4 | 7241 | 1280 |
| 8 µg | 960 | 3090 | 17,480.75 | 14,482 | 5120 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, N.; Gergen, J.; Kovacikova, K.; Mueller, S.O.; Ulrich, L.; Schön, J.; Halwe, N.J.; Fricke, C.; Corleis, B.; Dorhoi, A.; et al. Assessment of Immunogenicity and Efficacy of CV0501 mRNA-Based Omicron COVID-19 Vaccination in Small Animal Models. Vaccines 2023, 11, 318. https://doi.org/10.3390/vaccines11020318
Roth N, Gergen J, Kovacikova K, Mueller SO, Ulrich L, Schön J, Halwe NJ, Fricke C, Corleis B, Dorhoi A, et al. Assessment of Immunogenicity and Efficacy of CV0501 mRNA-Based Omicron COVID-19 Vaccination in Small Animal Models. Vaccines. 2023; 11(2):318. https://doi.org/10.3390/vaccines11020318
Chicago/Turabian StyleRoth, Nicole, Janina Gergen, Kristina Kovacikova, Stefan O. Mueller, Lorenz Ulrich, Jacob Schön, Nico Joel Halwe, Charlie Fricke, Björn Corleis, Anca Dorhoi, and et al. 2023. "Assessment of Immunogenicity and Efficacy of CV0501 mRNA-Based Omicron COVID-19 Vaccination in Small Animal Models" Vaccines 11, no. 2: 318. https://doi.org/10.3390/vaccines11020318
APA StyleRoth, N., Gergen, J., Kovacikova, K., Mueller, S. O., Ulrich, L., Schön, J., Halwe, N. J., Fricke, C., Corleis, B., Dorhoi, A., Hoffmann, D., Beer, M., Maione, D., Petsch, B., & Rauch, S. (2023). Assessment of Immunogenicity and Efficacy of CV0501 mRNA-Based Omicron COVID-19 Vaccination in Small Animal Models. Vaccines, 11(2), 318. https://doi.org/10.3390/vaccines11020318






