A Bibliometric Visualization Analysis on Vaccine Development of Coronavirus Disease 2019 (COVID-19)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Search Strategy
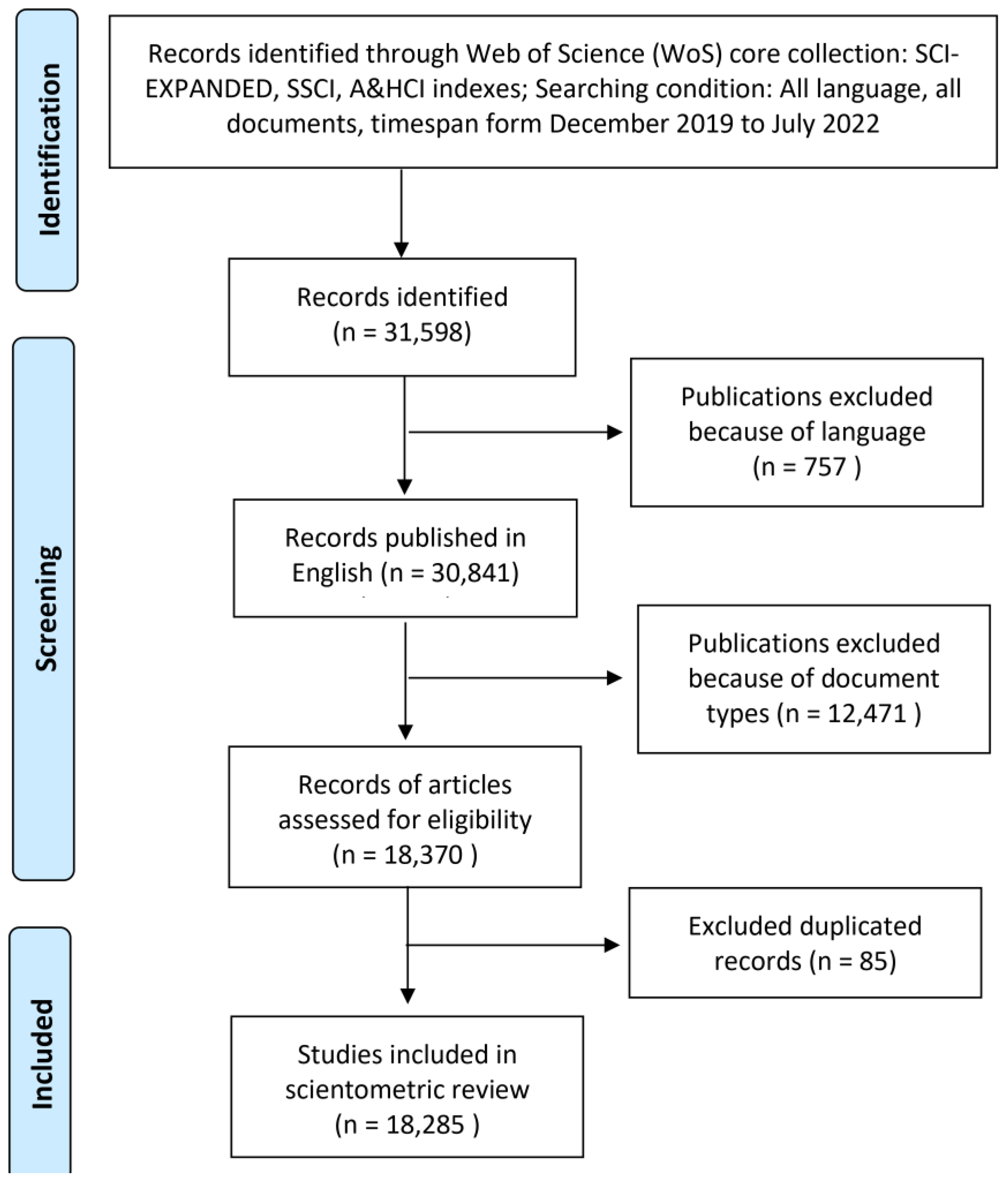
2.4. Selection Criteria and Collection
3. Data Analysis
4. Results
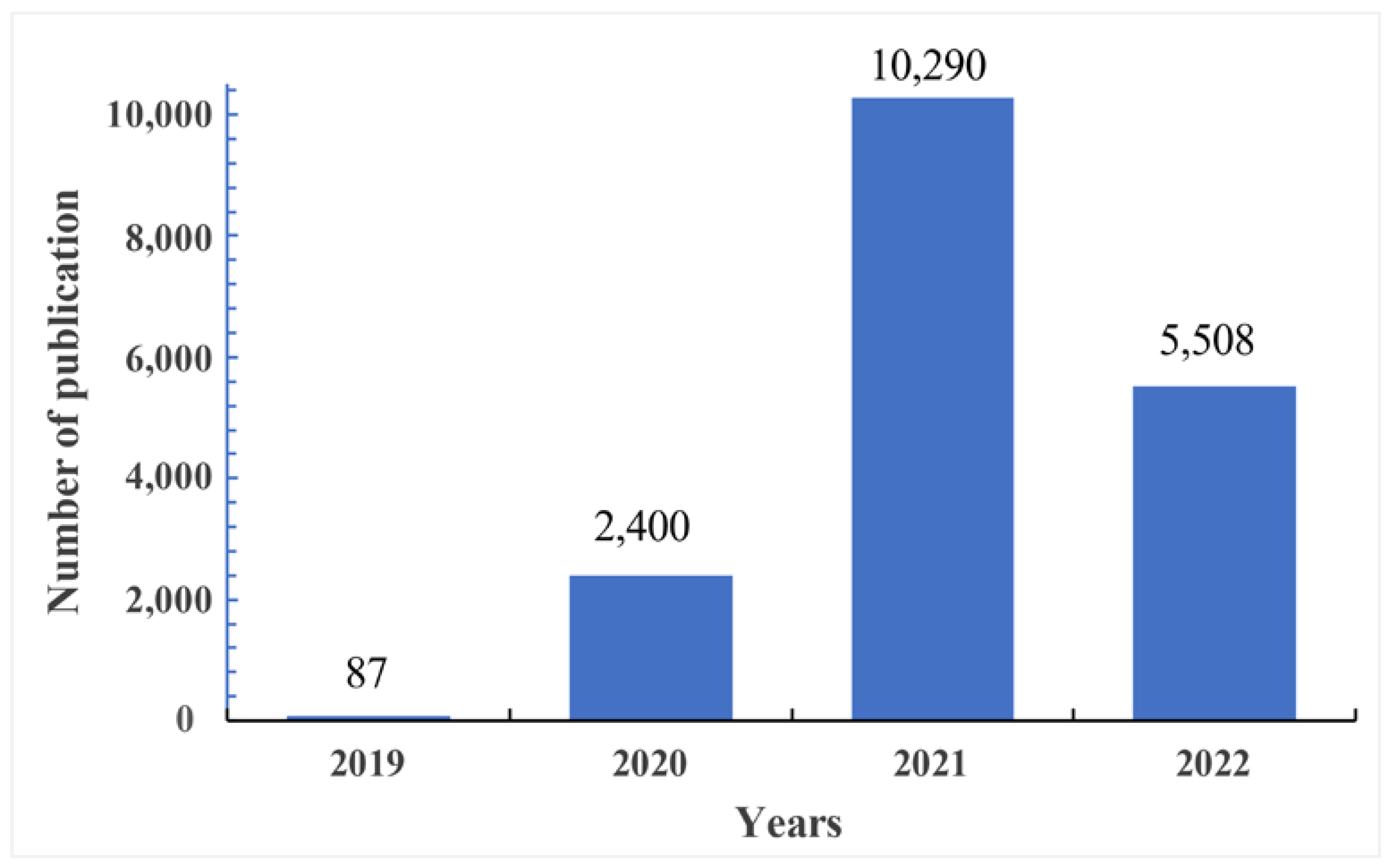
4.1. Publication Outputs
4.2. Main Publication of Countries, Institutions, Authors and Journals
4.3. Visualization Map of Co-Authorship Countries and Institutions
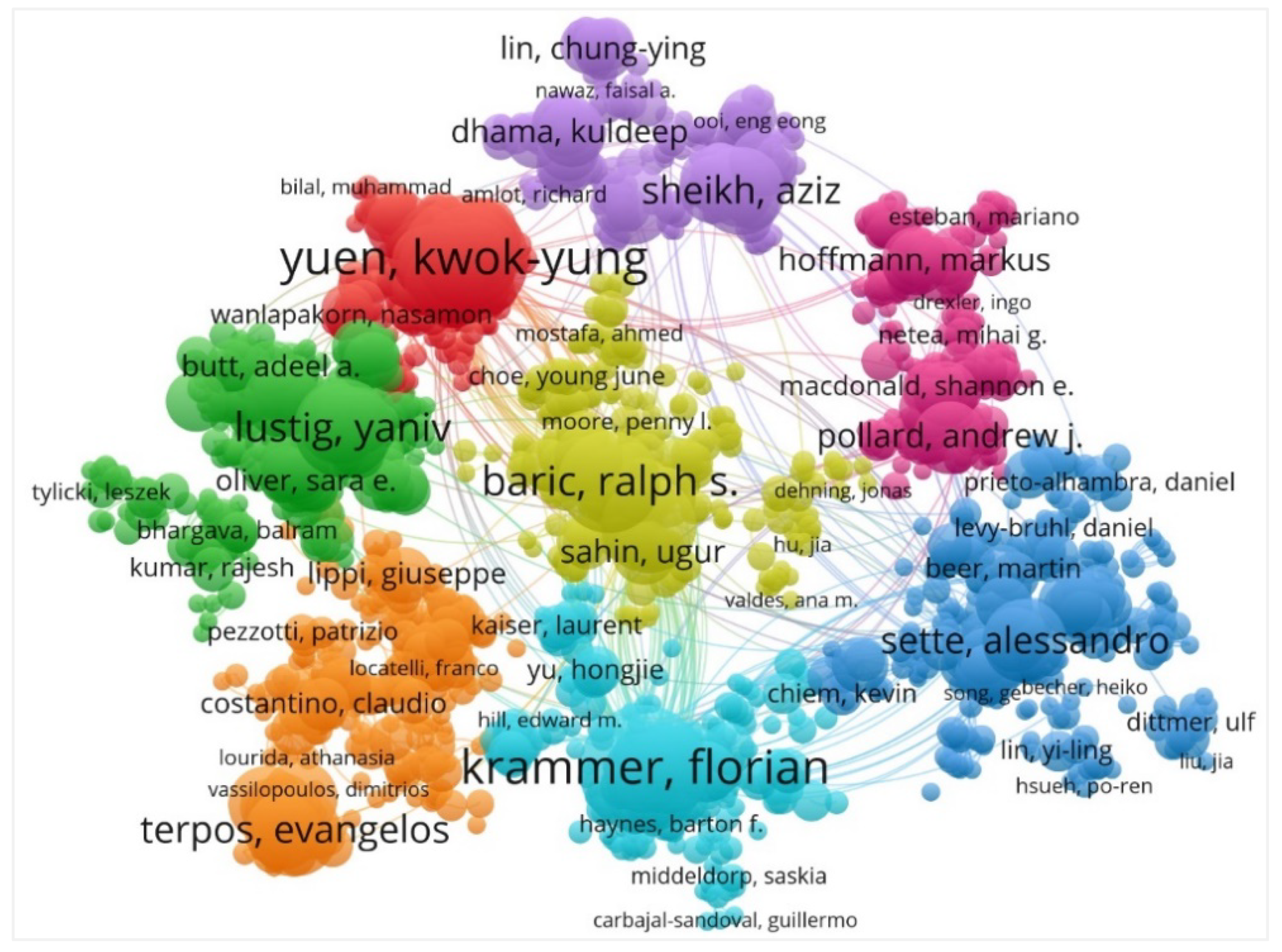
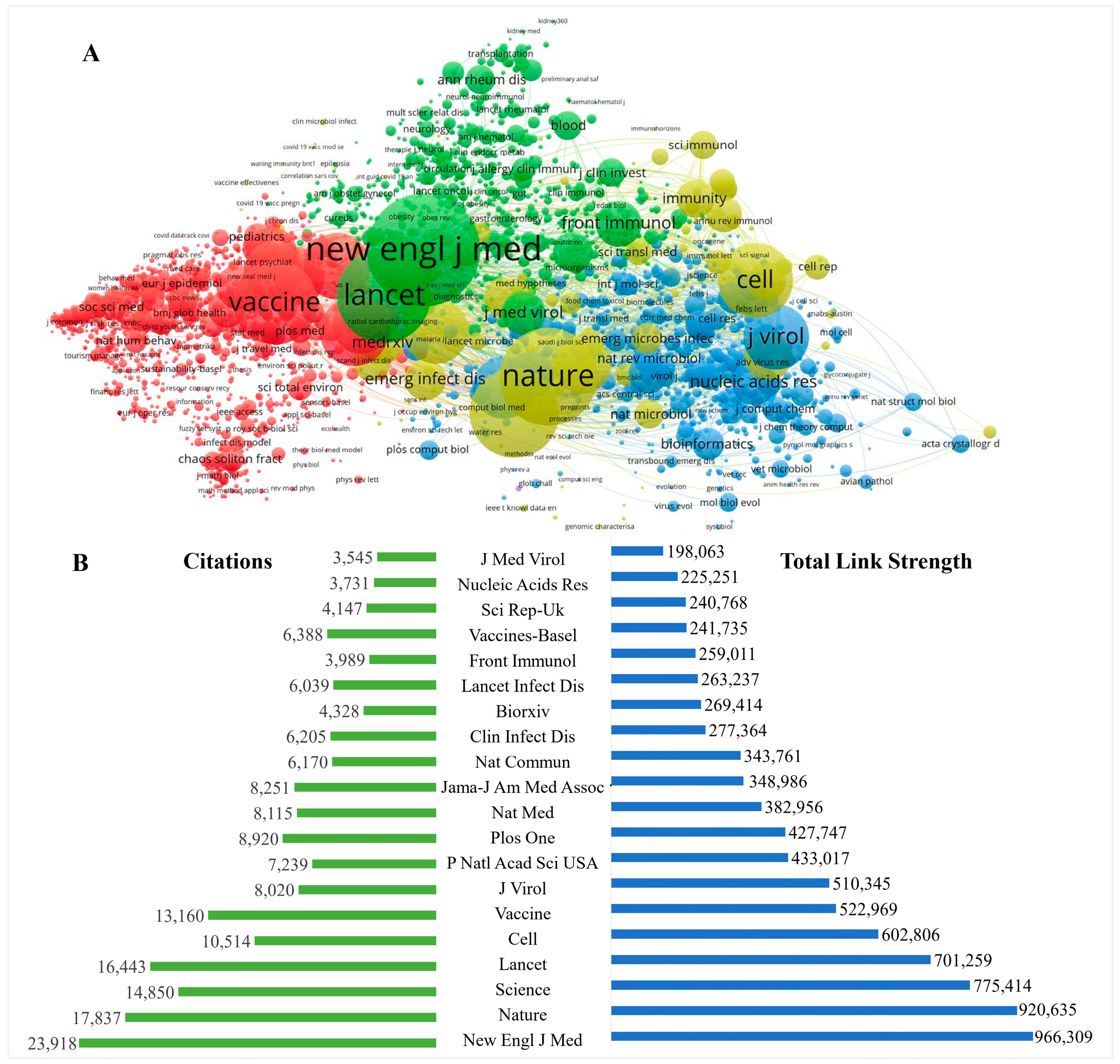
4.4. Visualization Map of Active Authors and Journals
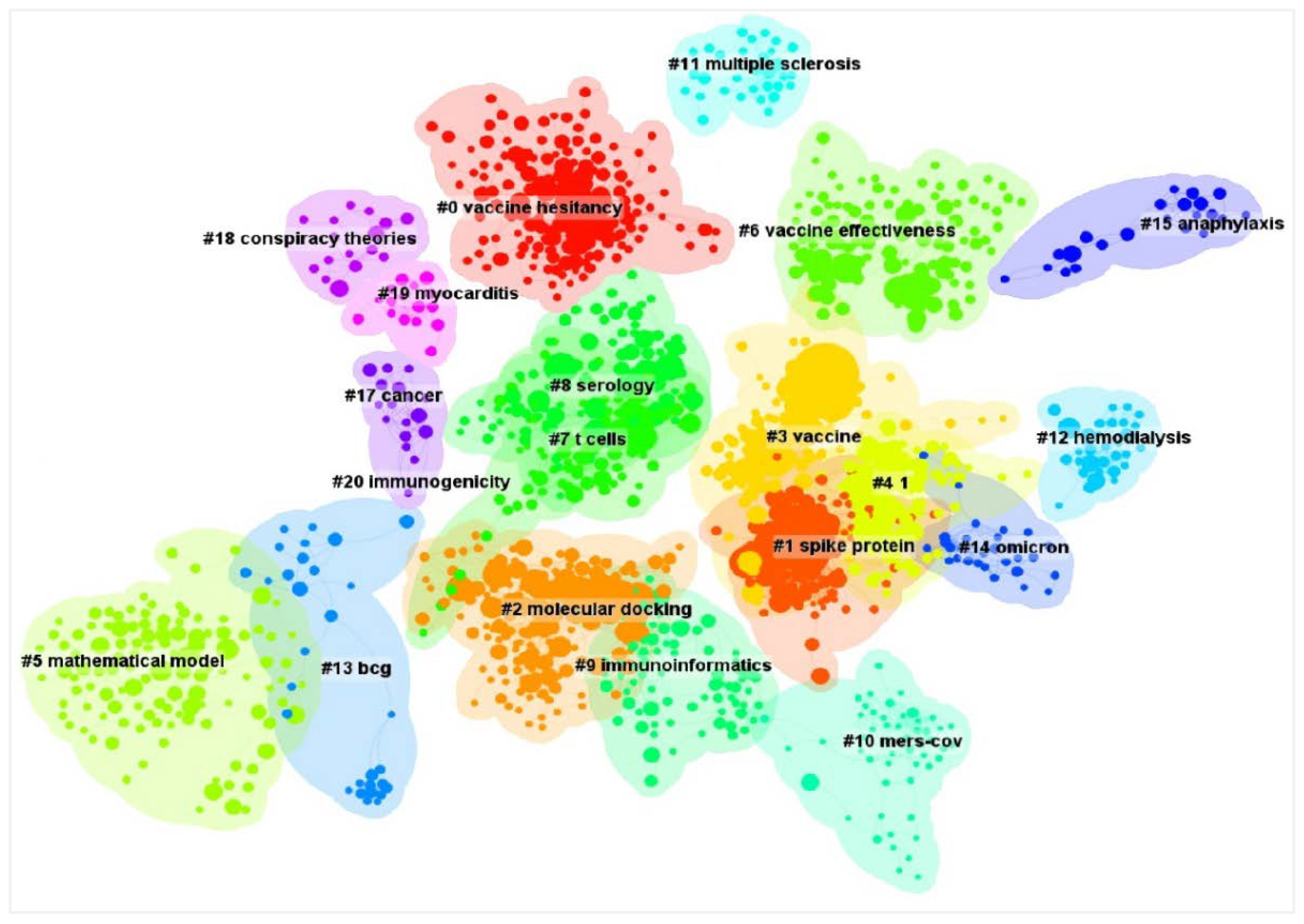
4.5. Visualization Map of Co-Cited References Cluster
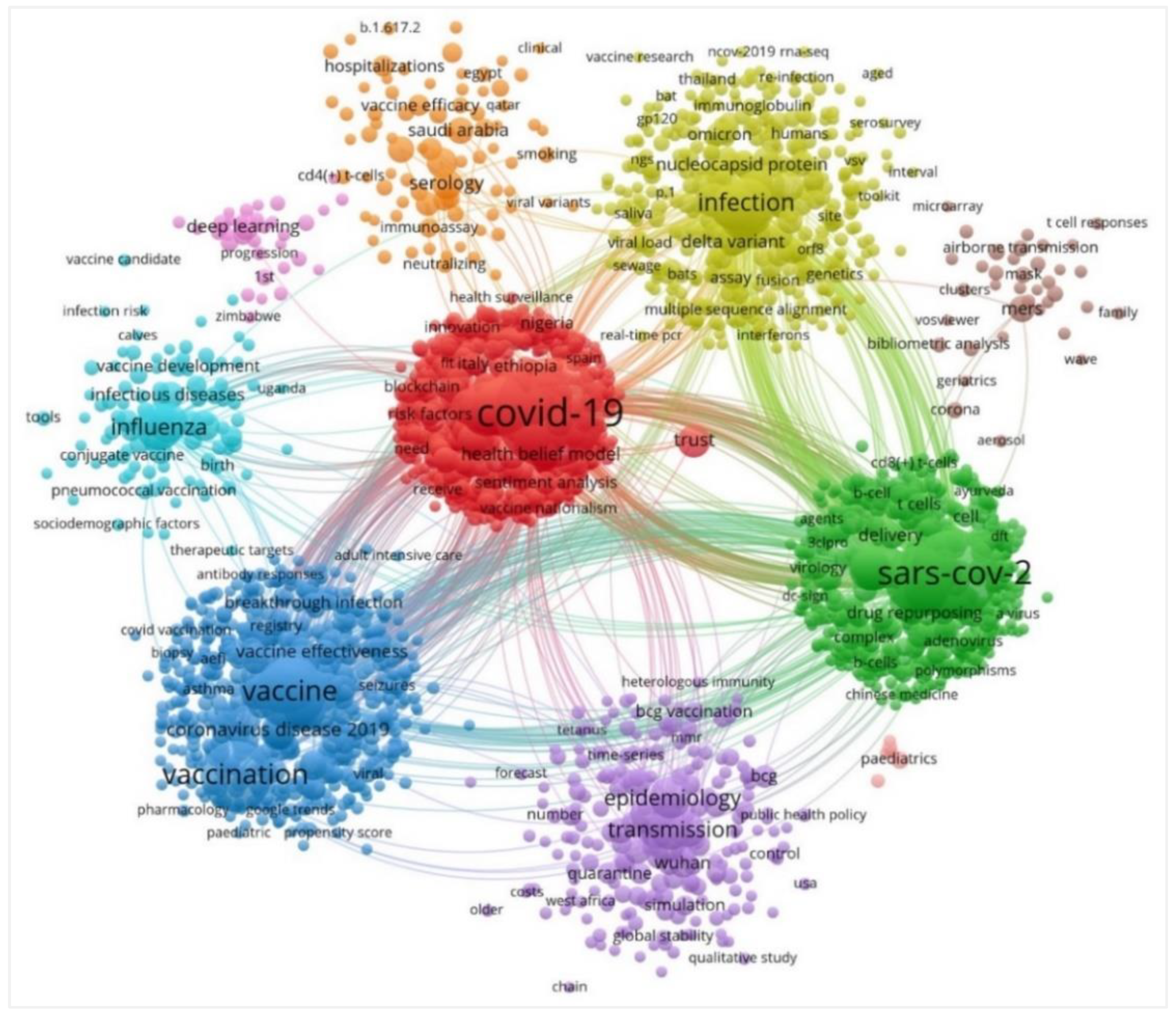
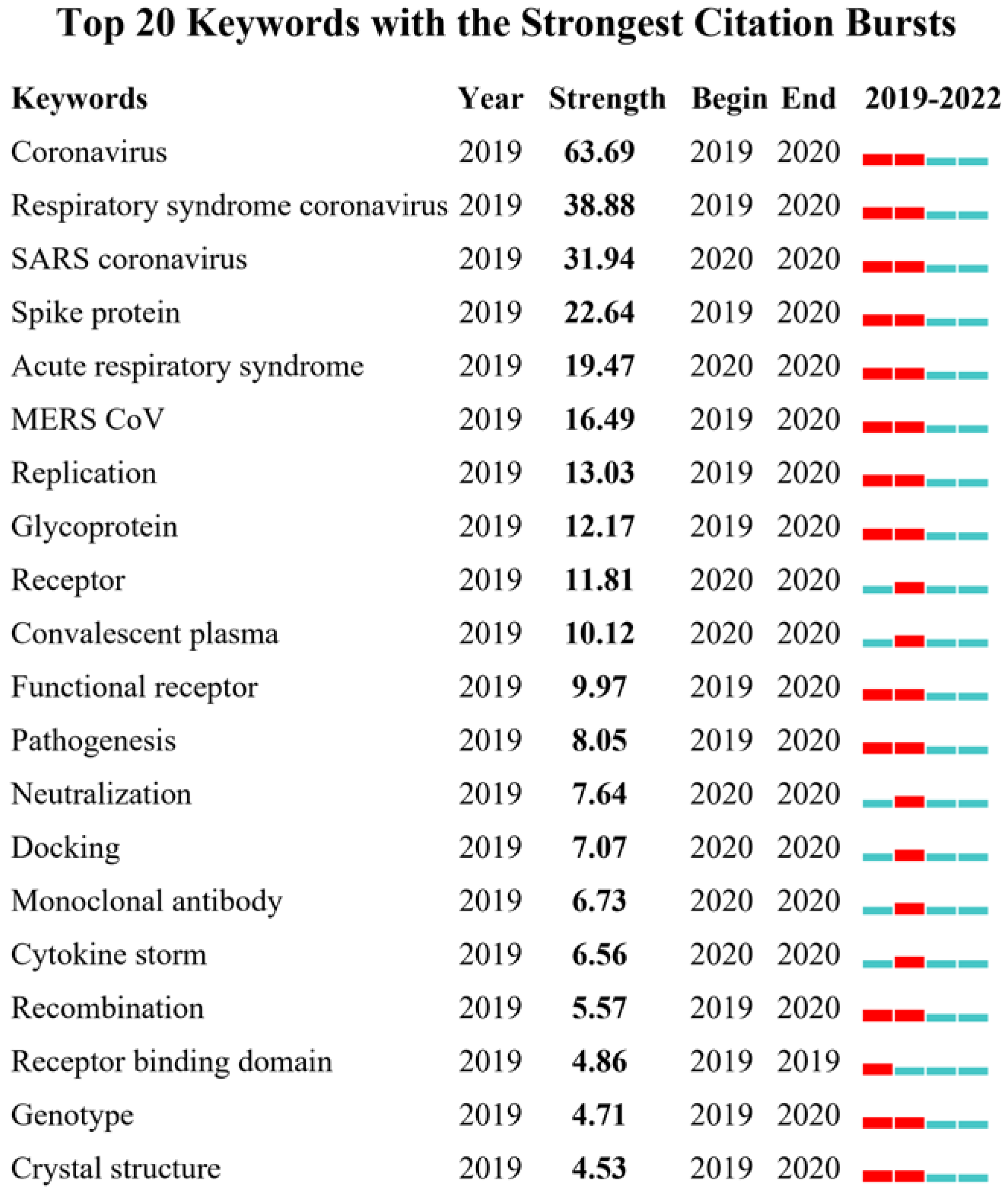
4.6. Visualization Map of Co-Occurrence Keywords and Burst Keywords
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz-Fresneda, M.A.; Jiménez-Contreras, E.; Ruiz-Fresneda, C.; Ruiz-Pérez, R. Bibliometric analysis of international scientific production on pharmacologic treatments for SARS-CoV-2/COVID-19 During 2020. Front. Public Health 2022, 9, 778203. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, E.K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal. Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon, L.L.M.; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.; Wang, Q.; Lv, J.; Zhao, B.; Hu, Q.; Du, H.; Gong, W.; Zhao, Z.; Xu, J.; Zhu, Y.; et al. The transmission dynamics of middle east respiratory syndrome coronavirus. Travel. Med. Infect. Dis. 2022, 45, 102243. [Google Scholar] [CrossRef]
- Lin, X.; Chen, P.; Lin, F. Mapping global research trends in diabetes and COVID-19 outbreak in the past year: A bibliometric analysis. Ann. Palliat. Med. 2022, 11, 1241–1252. [Google Scholar] [CrossRef]
- Guarner, J. Three emerging coronaviruses in two decades: The story of SARS, MERS, and now COVID-19. Am. J. Clin. Pathol. 2020, 153, 420–421. [Google Scholar] [CrossRef]
- Qamar, N.; Rukh, G.; Khan, S.N. Vaccines for COVID-19: An insight on their effectiveness and adverse effects. J. Med. Virol. 2022, 94, 3554–3560. [Google Scholar] [CrossRef]
- Orenstein, W.A.; Ahmed, R. Simply put: Vaccination saves lives. Proc. Natl. Acad. Sci. USA 2017, 114, 4031–4033. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 10 August 2022).
- World Health Organization. COVID-19 Vaccines WHO Emergency Use Listing (EUL) Issued. Available online: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (accessed on 10 August 2022).
- Ahmad, T.; Murad, M.A.; Baig, M.; Hui, J. Research trends in COVID-19 vaccine: A bibliometric analysis. Hum. Vaccines Immunother. 2021, 17, 2367–2372. [Google Scholar] [CrossRef]
- Xu, Z.; Qu, H.; Ren, Y.; Gong, Z.; Ri, H.J.; Zhang, F.; Chen, X.; Zhu, W.; Shao, S.; Chen, X. Update on the COVID-19 vaccine research trends: A bibliometric analysis. Infect. Drug. Resist. 2021, 14, 4237–4247. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101, 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientifc literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Tu, S.J.; Jin, C.; Chen, B.T.; Xu, A.Y.; Luo, C.; Wang, X.H. Study on the fusion of sports and medicine in China from 2012 to 2021: A bibliometric analysis via CiteSpace. Front. Public Health 2022, 10, 939557. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, D.; Zhang, L.; Yu, L.; Wang, Z.; Li, L.; Zhang, J.; Yao, R.; Huang, L.; Shao, M. Knowledge domain and emerging trends in diabetic cardiomyopathy: A scientometric review based on CiteSpace analysis. Front. Cardiovasc. Med. 2022, 9, 891428. [Google Scholar] [CrossRef]
- Li, M.N.; Porter, A.L.; Wang, Z.L. Evolutionary trend analysis of nanogenerator research based on a novel perspective of phased bibliographic coupling. Nano Energy 2017, 34, 93–102. [Google Scholar] [CrossRef]
- Zhong, D.; Li, Y.; Huang, Y.; Hong, X.; Li, J.; Jin, R. Molecular mechanisms of exercise on cancer: A bibliometrics study and visualization analysis via CiteSpace. Front. Mol. Biosci. 2021, 8, 797902. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Wang, J.; Luo, Y. Knowledge domain and emerging trends on echinococcosis research: A scientometric analysis. Int. J. Environ. Res. Public Health 2019, 16, 842. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Li, W.H.; Hadizadeh, M.; Yusof, A.; Naharudin, M.N. analysis of research trends on elbow pain in overhead sports: A bibliometric study based on Web of Science Database and VOSviewer. Healthcare 2022, 10, 2242. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual. Available online: https://www.vosviewer.com/getting-started#vosviewer-manual (accessed on 10 August 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Radanliev, P.; De-Roure, D.; Walton, R. Data mining and analysis of scientific research data records on COVID-19 mortality, immunity, and vaccine development—In the first wave of the COVID-19 pandemic. Diabetes Metab. Syndr. 2020, 14, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Y.; Zhang, Z.; Gu, Z.; Zhong, H.; Zha, Q.; Yang, L.; Zhu, C.; Chen, E. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann. Transl. Med. 2020, 8, 816. [Google Scholar] [CrossRef] [PubMed]
- Hannah, R.; Edouard, M.; Lucas, R.G.; Cameron, A.; Charlie, G.; Esteban, O.O.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 10 August 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard: Situation by Region, Country, Territory & Area. Available online: https://covid19.who.int/table (accessed on 10 August 2022).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Reyes, I.E.; Hernández-García, F.; Mejia, C.R. COVID-19 and diabetes: Analysis of the scientific production indexed in Scopus. Diabetes Metab. Syndr. 2021, 15, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; El-Dahiyat, F.; Jairoun, M.; Shahwan, M.; Al-Ani, M.; Habeb, M.; Babar, Z.-U. Assessing public knowledge, attitudes and determinants of third COVID-19 vaccine booster dose acceptance: Current scenario and future perspectives. J. Pharm. Policy Pract. 2022, 15, 26. [Google Scholar] [CrossRef]
- Elhadi, M.; Alsoufi, A.; Alhadi, A.; Hmeida, A.; Alshareea, E.; Dokali, M.; Abodabos, S.; Alsadiq, O.; Abdelkabir, M.; Ashini, A.; et al. Knowledge, attitude, and acceptance of healthcare workers and the public regarding the COVID-19 vaccine: A cross-sectional study. BMC Public Health 2021, 21, 955. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 10 August 2022).
- Majid, U.; Ahmad, M.; Zain, S.; Akande, A.; Ikhlaq, F. COVID-19 vaccine hesitancy and acceptance: A comprehensive scoping review of global literature. Health Promot. Int. 2022, 37, daac078. [Google Scholar] [CrossRef]
- Peters, M.D.J. Addressing vaccine hesitancy and resistance for COVID-19 vaccines. Int. J. Nurs. Stud. 2022, 131, 104241. [Google Scholar] [CrossRef]
- Robertson, D.A.; Mohr, K.S.; Barjaková, M.; Lunn, P.D. A lack of perceived benefits and a gap in knowledge distinguish the vaccine hesitant from vaccine accepting during the COVID-19 pandemic. Psychol. Med. 2021, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; You, Q.; Wu, J.; Jin, L. Factors influencing the knowledge gap regarding influenza and influenza vaccination in the context of COVID-19 pandemic: A cross-sectional survey in China. Vaccines 2022, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Samrat, S.K.; Tharappel, A.M.; Li, Z.; Li, H. Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development. Virus Res. 2020, 288, 198141. [Google Scholar] [CrossRef]
- Wu, F.; Liu, M.; Wang, A.; Lu, L.; Wang, Q.; Gu, C.; Chen, J.; Wu, Y.; Xia, S.; Ling, Y.; et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern. Med. 2020, 180, 1356–1362. [Google Scholar] [CrossRef]
- Au, W.Y.; Cheung, P.P. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: Living systematic review with network meta-analysis. BMJ 2022, 377, e069989. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S. Development of COVID 19 vaccine: A summarized review on global trials, efficacy, and effectiveness on variants. Diabetes Metab. Syndr. 2022, 16, 102482. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Effectiveness and safety of COVID-19 vaccine among pregnant women in real-world studies: A systematic review and meta-analysis. Vaccines 2022, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Polat, T.G.; Duman, O.; Tunç, S. Agar/κ-carrageenan/montmorillonite nanocomposite hydrogels for wound dressing applications. Int. J. Biol. Macromol. 2020, 164, 4591–4602. [Google Scholar] [CrossRef]
- De la Garza-Ramos, R.; Benvenutti-Regato, M.; Caro-Osorio, E. The 100 most-cited articles in spinal oncology. J. Neurosurg. Spine 2016, 24, 810–823. [Google Scholar] [CrossRef]
- Murray, M.R.; Wang, T.; Schroeder, G.D.; Hsu, W.K. The 100 most cited spine articles. Eur. Spine J. 2012, 21, 2059–2069. [Google Scholar] [CrossRef]
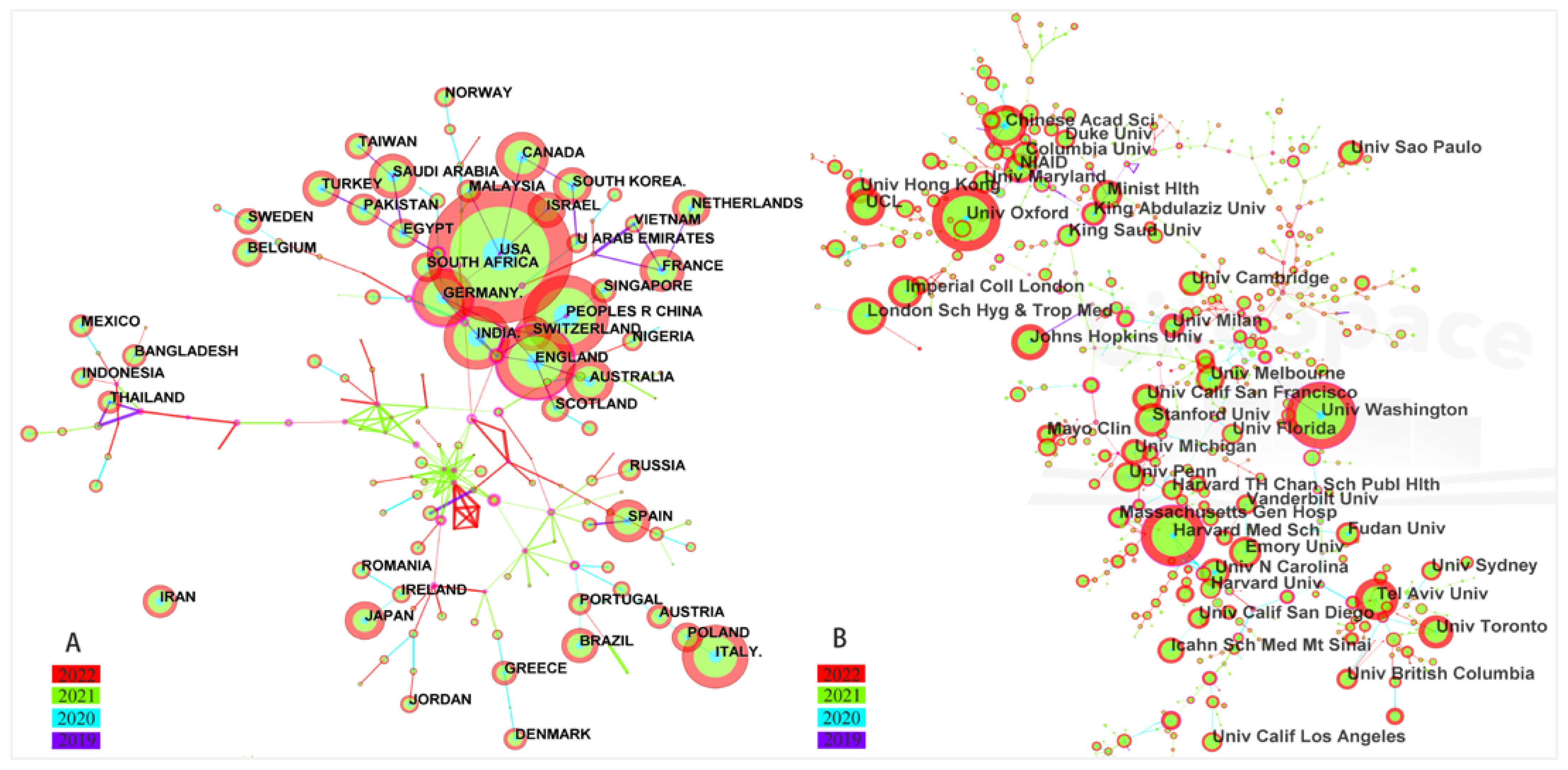
| Ranking | Countries | Records | Percentage (%) | Centrality |
|---|---|---|---|---|
| 1 | USA | 5711 | 31.23 | 0.05 |
| 2 | UK | 2020 | 11.05 | 0.23 |
| 3 | China | 1819 | 9.95 | 0.02 |
| 4 | Italy | 1253 | 6.85 | 0.01 |
| 5 | India | 1193 | 6.52 | 0.01 |
| 6 | Germany | 985 | 5.39 | 0.18 |
| 7 | Canada | 773 | 4.23 | 0.03 |
| 8 | Australia | 652 | 3.57 | 0.01 |
| 9 | Saudi Arabia | 583 | 3.19 | 0.02 |
| 10 | France | 567 | 3.10 | 0.02 |
| Ranking | Institutions | Records | Percentage (%) | Centrality |
|---|---|---|---|---|
| 1 | University of Washington | 340 | 1.86 | 0.22 |
| 2 | University of Oxford | 337 | 1.84 | 0.02 |
| 3 | Harvard Medical School | 309 | 1.69 | 0.38 |
| 4 | Tel Aviv University | 214 | 1.17 | 0.27 |
| 5 | Chinese Academy of Sciences | 210 | 1.15 | 0.05 |
| 6 | University College London (UCL) | 192 | 1.05 | 0.05 |
| 7 | The London School of Hygiene & Tropical Medicine | 191 | 1.04 | 0.02 |
| 8 | Johns Hopkins University | 184 | 1.01 | 0.01 |
| 9 | Stanford University | 174 | 0.95 | 0.01 |
| 10 | University of Toronto | 171 | 0.94 | 0.04 |
| Ranking | Authors | Records | Citations | TLS |
|---|---|---|---|---|
| 1 | Krammer F | 32 | 3217 | 198 |
| 2 | Yuen KY | 32 | 3142 | 271 |
| 3 | To KK | 25 | 3014 | 234 |
| 4 | Baric RS | 25 | 954 | 163 |
| 5 | Lustig Y | 24 | 875 | 167 |
| 6 | Shi PY | 23 | 3071 | 154 |
| 7 | Terpos E | 22 | 179 | 166 |
| 8 | Diamond MS | 21 | 1634 | 166 |
| 9 | Sheikh A | 21 | 235 | 125 |
| 10 | Sette A | 20 | 3704 | 109 |
| Ranking | Journals | Records | Percentage (%) | IF |
|---|---|---|---|---|
| 1 | Vaccines | 965 | 5.3 | 4.96 |
| 2 | Vaccine | 365 | 1.9 | 4.16 |
| 3 | Plos One | 327 | 1.8 | 3.75 |
| 4 | International Journal of Environmental Research and Public Health | 320 | 1.7 | 4.61 |
| 5 | Human Vaccines Immunotherapeutics | 294 | 1.6 | 4.52 |
| 6 | Frontiers in Immunology | 276 | 1.5 | 8.78 |
| 7 | Scientific Reports | 270 | 1.4 | 4.99 |
| 8 | Viruses Basel | 225 | 1.2 | 5.82 |
| 9 | Frontiers in Public Health | 169 | 0.9 | 6.41 |
| 10 | Clinical Infectious Diseases | 157 | 0.8 | 20.99 |
| Ranking | Citation Counts | References and Research Topics | Cluster ID |
|---|---|---|---|
| 1 | 2521 | [24] Polack et al. evaluated the safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine with a multinational, placebo-controlled, observer-blinded trial which was published in the Journal of N Engl J Med in 2020. | 3 (#3) |
| 2 | 1610 | [25] Baden et al. conducted a phase 3 randomized controlled trial and investigated the efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, which published in the Journal of N Engl J Med in 2021. | 3 (#3) |
| 3 | 995 | [26] Zhu et al. isolated a novel coronavirus from patients in China, named 2019-nCoV, which is the seventh member of the family of coronaviruses that infect humans and published in the Journal of N Engl J Med in 2020. | 2 (#2) |
| 4 | 958 | [27] Voysey et al. evaluated the safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2 with an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, which was published in The Lancet in 2021. | 3 (#3) |
| 5 | 940 | [28] Hoffmann et al. demonstrated that SARS-CoV-2 uses the SARS-CoV receptor ACE2 for entry and the serine protease TMPRSS2 for S protein priming, which is a potential target for antiviral intervention, and published their report in the journal Cell in 2020. | 1 (#1) |
| 6 | 893 | [29] Walls et al. determined cryo-EM structures of the SARS-CoV-2 S ectodomain trimer, providing a blueprint for the design of vaccines and inhibitors of viral entry, and published in the journal Cell in 2020. | 1 (#1) |
| 7 | 852 | [30] Wrapp et al. determined a 3.5-angstrom-resolution cryo-electron microscopy structure of the 2019-nCoV S trimer in the prefusion conformation to facilitate medical countermeasure development, and published in the journal Science in 2020. | 1 (#1) |
| 8 | 755 | [31] Huang et al. reported the epidemiological, clinical, laboratory, and radiological characteristics and treatment and clinical outcomes of these patients infected with 2019 novel coronavirus in Wuhan, and published in The Lancet in 2020. | 2 (#2) |
| 9 | 638 | [32] Lazarus et al. surveyed 13,426 people in 19 countries to determine the potential acceptance rates and factors influencing acceptance of a COVID-19 vaccine, and published in the Journal of Natural Medicines in 2021. | 0 (#0) |
| 10 | 614 | [33] Zhou et al. confirmed that 2019-nCoV is 96% identical at the whole-genome level to a bat coronavirus, and using the same cell entry receptor-angiotensin converting enzyme II (ACE2)-as SARS-CoV, and published in the journal Nature in 2020. | 1 (#1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, D.; Wang, J.; Xiao, B.; Zhang, H.; Ma, X. A Bibliometric Visualization Analysis on Vaccine Development of Coronavirus Disease 2019 (COVID-19). Vaccines 2023, 11, 295. https://doi.org/10.3390/vaccines11020295
Zeng D, Wang J, Xiao B, Zhang H, Ma X. A Bibliometric Visualization Analysis on Vaccine Development of Coronavirus Disease 2019 (COVID-19). Vaccines. 2023; 11(2):295. https://doi.org/10.3390/vaccines11020295
Chicago/Turabian StyleZeng, Dequan, Jie Wang, Bin Xiao, Hao Zhang, and Xingming Ma. 2023. "A Bibliometric Visualization Analysis on Vaccine Development of Coronavirus Disease 2019 (COVID-19)" Vaccines 11, no. 2: 295. https://doi.org/10.3390/vaccines11020295
APA StyleZeng, D., Wang, J., Xiao, B., Zhang, H., & Ma, X. (2023). A Bibliometric Visualization Analysis on Vaccine Development of Coronavirus Disease 2019 (COVID-19). Vaccines, 11(2), 295. https://doi.org/10.3390/vaccines11020295





