Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. SIV Infection and Sample Collection
2.3. Hematology
2.4. Plasma Isolation
2.5. Quantitative SIV Plasma Virus Load (PVL)
2.6. Quantification of Cytokines and Chemokines in Plasma
2.7. Flow Cytometry of Peripheral Blood Mononuclear Cells (PBMCs)
2.8. Statistical Analyses
3. Results
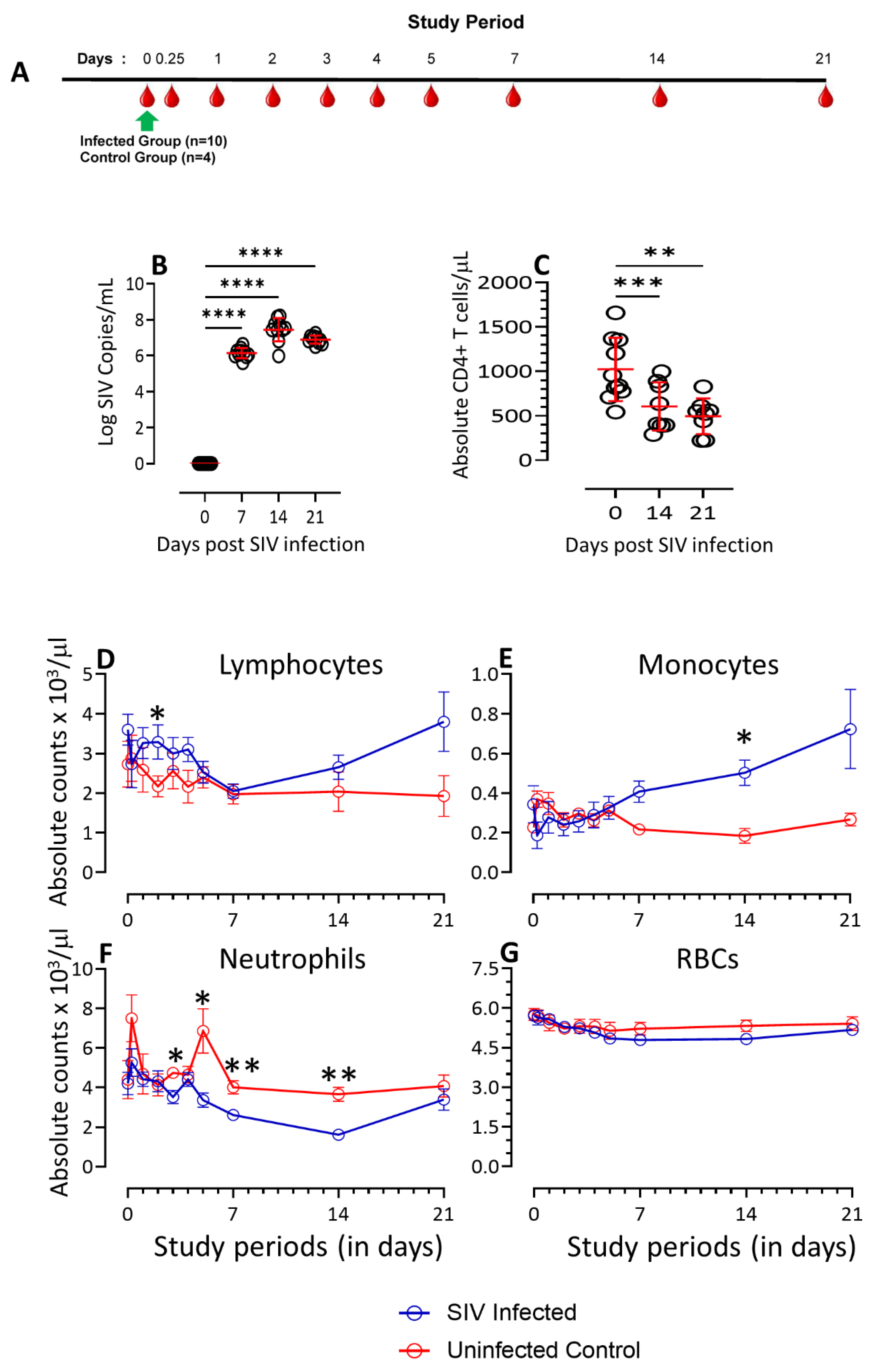
3.1. Dynamics of PVL, CD4, Lymphocytes, Monocytes, Neutrophils, and Red Blood Cells Count in SIV-Infected Macaques
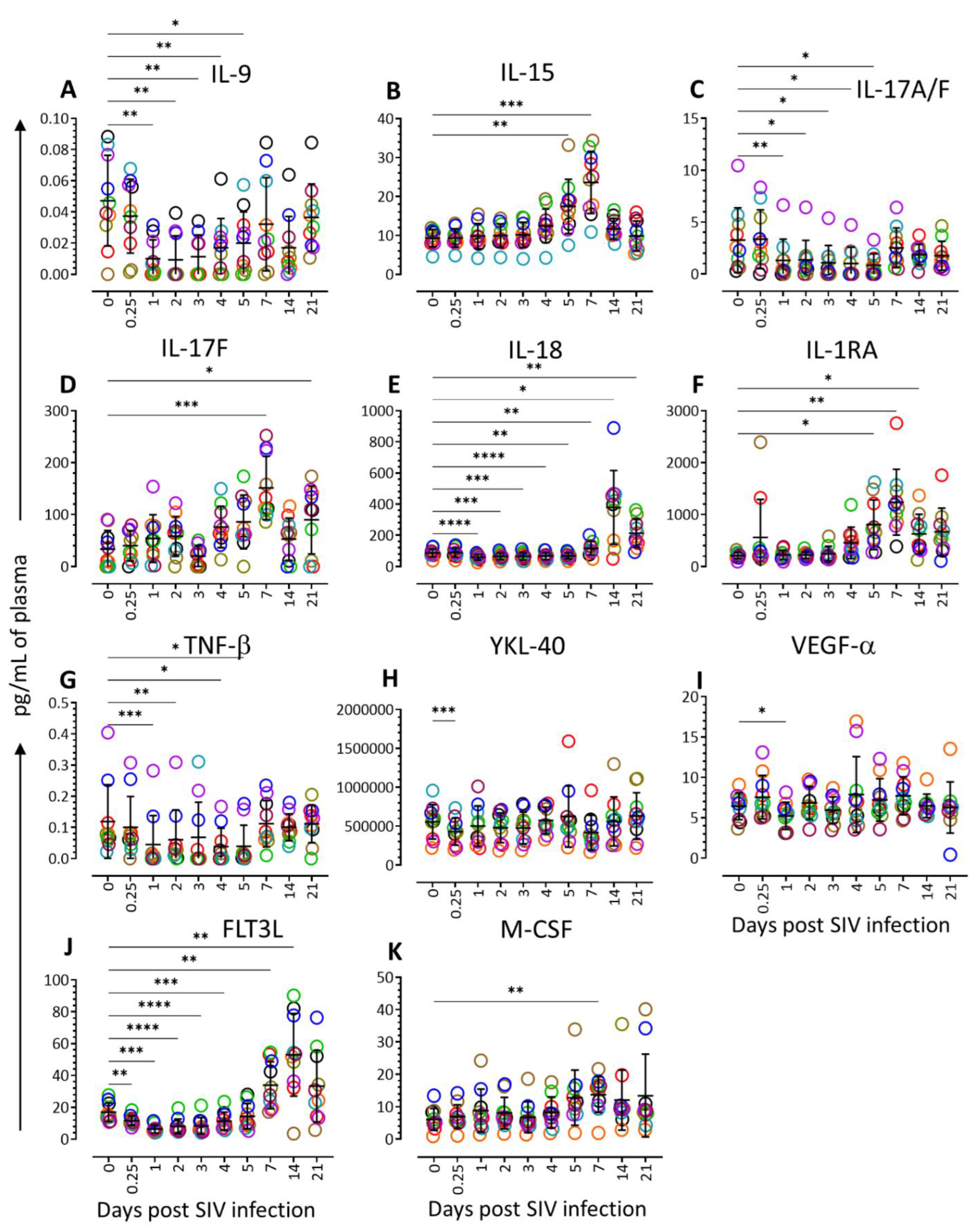
3.2. Cytokine Profiling during Acute SIV Infection
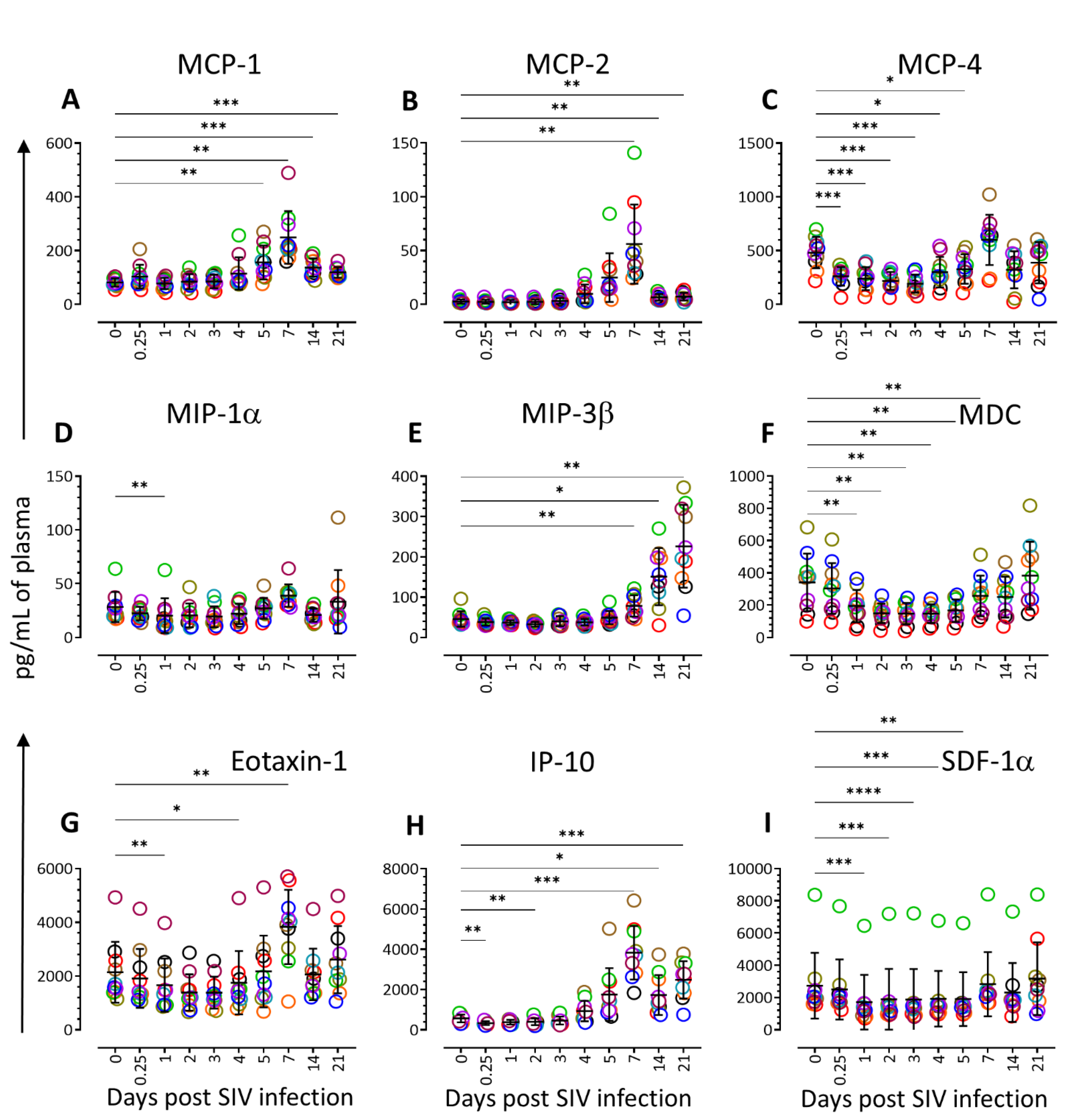
3.3. Chemokine Profiling during Acute SIV Infection
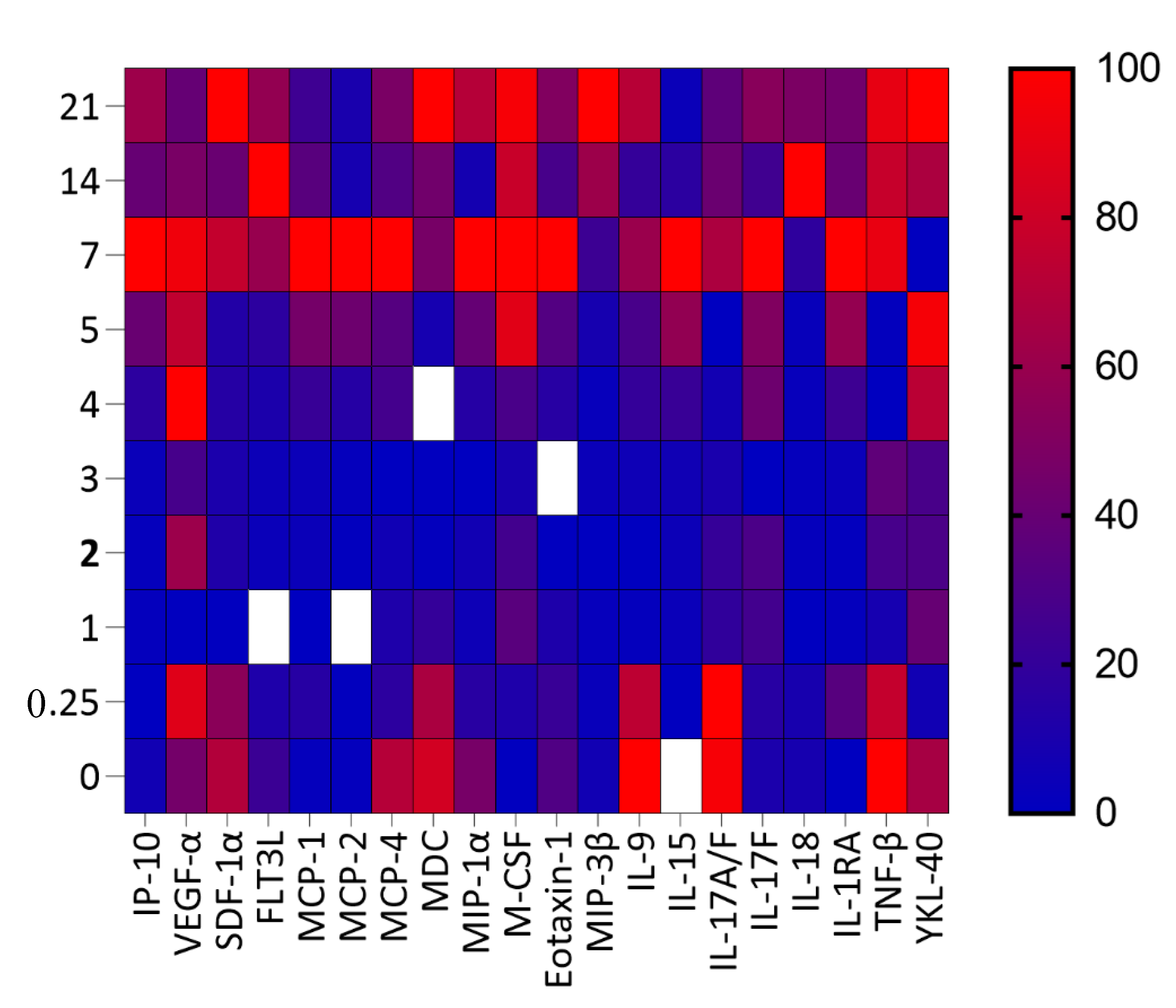
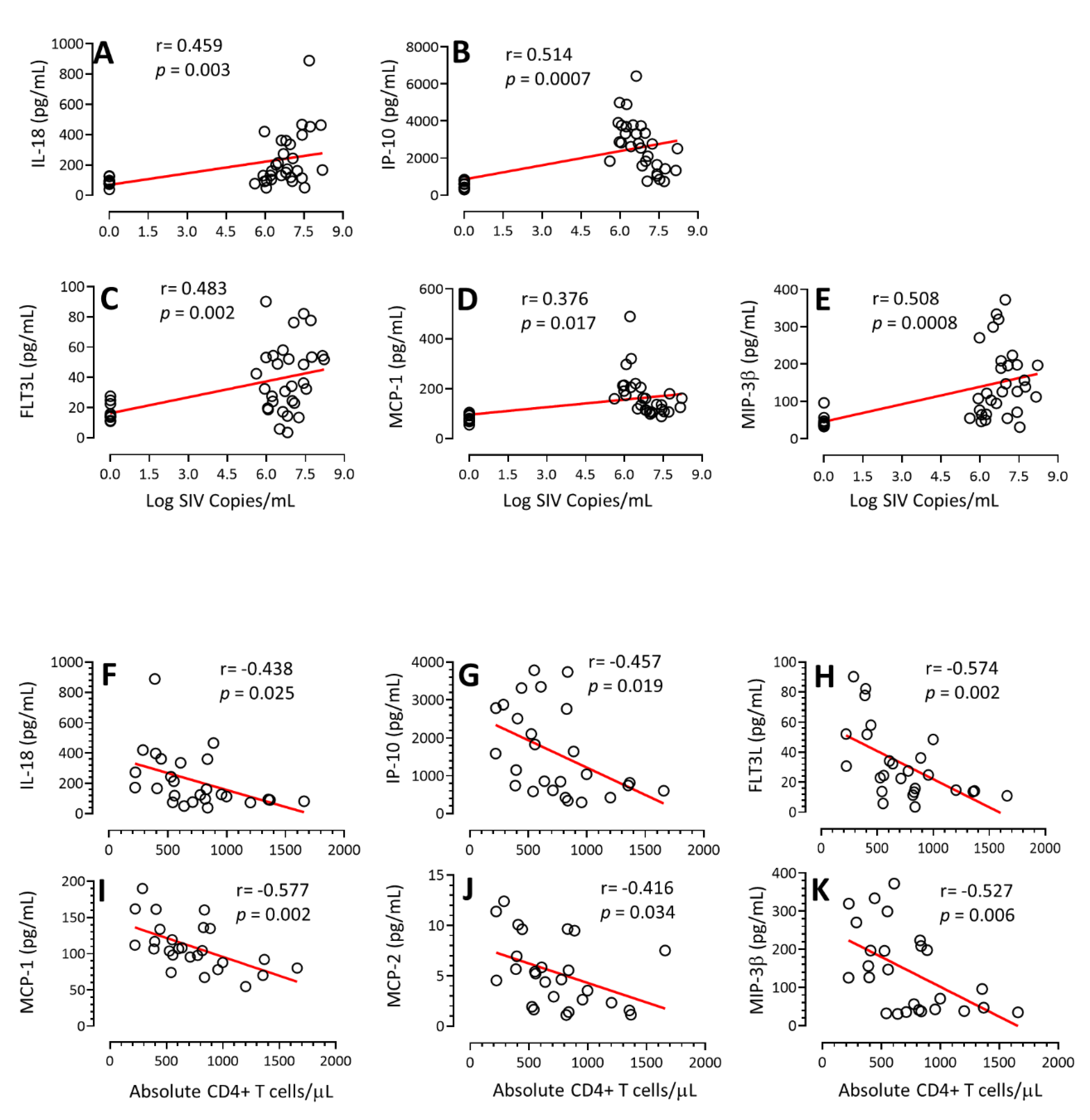
3.4. Correlation of Cytokines/Chemokines Concentration with PVL and Absolute CD4+ T Cell Count
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottlieb, M.S.; Centers for Disease, Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morb. Mortal. Wkly. Rep. 1981, 30, 250–252. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kenway-Lynch, C.S.; Das, A.; Lackner, A.A.; Pahar, B. Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 2014, 88, 9442–9457. [Google Scholar] [CrossRef]
- Kenway-Lynch, C.S.; Das, A.; Pan, D.; Lackner, A.A.; Pahar, B. Dynamics of cytokine/chemokine responses in intestinal CD4+ and CD8+ T Cells during Acute Simian Immunodeficiency Virus Infection. J. Virol. 2013, 87, 11916–11923. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Morici, L.A.; Pahar, B.; Veazey, R.S. Early divergent host responses in SHIVsf162P3 and SIVmac251 infected macaques correlate with control of viremia. PLoS ONE 2011, 6, e17965. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Passmore, J.A.; Williamson, C.; Little, F.; Bebell, L.M.; Mlisana, K.; Burgers, W.A.; van Loggerenberg, F.; Walzl, G.; Djoba Siawaya, J.F.; et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 2010, 24, 819–831. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, H.; Jiang, Y. Chemokines and Chemokine Receptors: Accomplices for Human Immunodeficiency Virus Infection and Latency. Front. Immunol. 2017, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Boby, N.; Cao, X.; Williams, K.; Gadila, S.K.G.; Shroyer, M.N.; Didier, P.J.; Srivastav, S.K.; Das, A.; Baker, K.; Sha, Q.; et al. Simian Immunodeficiency Virus Infection Mediated Changes in Jejunum and Peripheral SARS-CoV-2 Receptor ACE2 and Associated Proteins or Genes in Rhesus Macaques. Front. Immunol. 2022, 13, 835686. [Google Scholar] [CrossRef]
- Pan, D.; Kenway-Lynch, C.S.; Lala, W.; Veazey, R.S.; Lackner, A.A.; Das, A.; Pahar, B. Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection. J. Virol. 2014, 88, 13015–13028. [Google Scholar] [CrossRef]
- Pahar, B.; Lackner, A.A.; Veazey, R.S. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 2006, 36, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Felber, B.K.; Lu, Z.; Hu, X.; Valentin, A.; Rosati, M.; Remmel, C.A.L.; Weiner, J.A.; Carpenter, M.C.; Faircloth, K.; Stanfield-Oakley, S.; et al. Co-immunization of DNA and Protein in the Same Anatomical Sites Induces Superior Protective Immune Responses against SHIV Challenge. Cell Rep. 2020, 31, 107624. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Bjerkeli, V.; Yndestad, A.; Heggelund, L.; Waehre, T.; Damas, J.K.; Aukrust, P.; Froland, S.S. Raised serum levels of interleukin-18 is associated with disease progression and may contribute to virological treatment failure in HIV-1-infected patients. Clin. Exp. Immunol. 2003, 132, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, Y.; Zheng, D.; Wang, Z.; Pan, S.; Ji, T.; Shen, H.Y.; Wang, H. Elevation of YKL-40 in the CSF of Anti-NMDAR Encephalitis Patients Is Associated With Poor Prognosis. Front. Neurol. 2018, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Lista, S.; Cavedo, E.; Bonuccelli, U.; Hampel, H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev. Proteom. 2017, 14, 285–299. [Google Scholar] [CrossRef]
- Geindreau, M.; Bruchard, M.; Vegran, F. Role of Cytokines and Chemokines in Angiogenesis in a Tumor Context. Cancers 2022, 14, 2446. [Google Scholar] [CrossRef]
- Horiuchi, K.; Morioka, H.; Takaishi, H.; Akiyama, H.; Blobel, C.P.; Toyama, Y. Ectodomain shedding of FLT3 ligand is mediated by TNF-alpha converting enzyme. J. Immunol. 2009, 182, 7408–7414. [Google Scholar] [CrossRef]
- Noroozi, R.; Branicki, W.; Pyrc, K.; Labaj, P.P.; Pospiech, E.; Taheri, M.; Ghafouri-Fard, S. Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine 2020, 133, 155143. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, T.; Wang, R.; Zhang, H.; Huang, X.; Yin, J.; Zhang, L.; Xu, X.; Wu, H. Plasma IP-10 is associated with rapid disease progression in early HIV-1 infection. Viral Immunol. 2012, 25, 333–337. [Google Scholar] [CrossRef]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef]
- Boulware, D.R.; Hullsiek, K.H.; Puronen, C.E.; Rupert, A.; Baker, J.V.; French, M.A.; Bohjanen, P.R.; Novak, R.M.; Neaton, J.D.; Sereti, I.; et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J. Infect. Dis. 2011, 203, 1637–1646. [Google Scholar] [CrossRef]
- Stacey, A.R.; Norris, P.J.; Qin, L.; Haygreen, E.A.; Taylor, E.; Heitman, J.; Lebedeva, M.; DeCamp, A.; Li, D.; Grove, D.; et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009, 83, 3719–3733. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, C.; Gantt, K.R.; Vaccarezza, M.; Demarest, J.F.; Daucher, M.; Saag, M.S.; Shaw, G.M.; Quinn, T.C.; Cohen, O.J.; Welbon, C.C.; et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc. Natl. Acad. Sci. USA 1996, 93, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Biglino, A.; Sinicco, A.; Forno, B.; Pollono, A.M.; Sciandra, M.; Martini, C.; Pich, P.; Gioannini, P. Serum cytokine profiles in acute primary HIV-1 infection and in infectious mononucleosis. Clin. Immunol. Immunopathol. 1996, 78, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Kinter, A.L.; Fauci, A.S. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: Inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. USA 1994, 91, 108–112. [Google Scholar] [CrossRef]
- Nilsson, J.; Kinloch-de-Loes, S.; Granath, A.; Sonnerborg, A.; Goh, L.E.; Andersson, J. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS 2007, 21, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Lackner, A.A.; Simon, M.; Durand-Gasselin, I.; Galanaud, P.; Desrosiers, R.C.; Emilie, D. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 1997, 71, 1227–1236. [Google Scholar] [CrossRef]
- Benveniste, O.; Vaslin, B.; Le Grand, R.; Cheret, A.; Matheux, F.; Theodoro, F.; Cranage, M.P.; Dormont, D. Comparative interleukin (IL-2)/interferon IFN-gamma and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SICmac251 virus. Proc. Natl. Acad. Sci. USA 1996, 93, 3658–3663. [Google Scholar] [CrossRef]
- Naicker, D.D.; Werner, L.; Kormuth, E.; Passmore, J.A.; Mlisana, K.; Karim, S.A.; Ndung’u, T. Interleukin-10 promoter polymorphisms influence HIV-1 susceptibility and primary HIV-1 pathogenesis. J. Infect. Dis. 2009, 200, 448–452. [Google Scholar] [CrossRef]
- Lederer, S.; Favre, D.; Walters, K.A.; Proll, S.; Kanwar, B.; Kasakow, Z.; Baskin, C.R.; Palermo, R.; McCune, J.M.; Katze, M.G. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009, 5, e1000296. [Google Scholar] [CrossRef]
- Jacquelin, B.; Mayau, V.; Targat, B.; Liovat, A.S.; Kunkel, D.; Petitjean, G.; Dillies, M.A.; Roques, P.; Butor, C.; Silvestri, G.; et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Investig. 2009, 119, 3544–3555. [Google Scholar] [CrossRef] [PubMed]
- Bosinger, S.E.; Li, Q.; Gordon, S.N.; Klatt, N.R.; Duan, L.; Xu, L.; Francella, N.; Sidahmed, A.; Smith, A.J.; Cramer, E.M.; et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Investig. 2009, 119, 3556–3572. [Google Scholar] [CrossRef]
- Abel, K.; Rocke, D.M.; Chohan, B.; Fritts, L.; Miller, C.J. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 2005, 79, 12164–12172. [Google Scholar] [CrossRef]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Mueller, Y.M.; Villinger, F. The cytokine network of acute HIV infection: A promising target for vaccines and therapy to reduce viral set-point? PLoS Pathog. 2011, 7, e1002055. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Shebl, F.M.; Pinto, L.A.; Garcia-Pineres, A.; Lempicki, R.; Williams, M.; Harro, C.; Hildesheim, A. Comparison of mRNA and protein measures of cytokines following vaccination with human papillomavirus-16 L1 virus-like particles. Cancer Epidemiol. Biomark. Prev. 2010, 19, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Fenn, J.; Arriero, E.; Lowe, A.; Poulin, B.; MacColl, A.D.C.; Bradley, J.E. Relationships between immune gene expression and circulating cytokine levels in wild house mice. Ecol. Evol. 2020, 10, 13860–13871. [Google Scholar] [CrossRef]
- Estes, J.D.; Li, Q.; Reynolds, M.R.; Wietgrefe, S.; Duan, L.; Schacker, T.; Picker, L.J.; Watkins, D.I.; Lifson, J.D.; Reilly, C.; et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 2006, 193, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Boby, N.; Ransom, A.; Pace, B.T.; Williams, K.M.; Mabee, C.; Das, A.; Srivastav, S.K.; Porter, E.; Pahar, B. Enhanced Intestinal TGF-beta/SMAD-Dependent Signaling in Simian Immunodeficiency Virus Infected Rhesus Macaques. Cells 2021, 10, 806. [Google Scholar] [CrossRef]
- Platchek, M.; Lu, Q.; Tran, H.; Xie, W. Comparative Analysis of Multiple Immunoassays for Cytokine Profiling in Drug Discovery. SLAS Discov. 2020, 25, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Eberly, M.D.; Kader, M.; Hassan, W.; Rogers, K.A.; Zhou, J.; Mueller, Y.M.; Mattapallil, M.J.; Piatak, M., Jr.; Lifson, J.D.; Katsikis, P.D.; et al. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J. Immunol. 2009, 182, 1439–1448. [Google Scholar] [CrossRef]
- Dayer, J.M.; Oliviero, F.; Punzi, L. A Brief History of IL-1 and IL-1 Ra in Rheumatology. Front. Pharmacol. 2017, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, K.A.; Dayer, J.M.; Rockstroh, J.K.; Sauerbruch, T.; Spengler, U. The IL-1 system in HIV infection: Peripheral concentrations of IL-1beta, IL-1 receptor antagonist and soluble IL-1 receptor type II. Clin. Exp. Immunol. 1997, 109, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Iannello, A.; Samarani, S.; Debbeche, O.; Ahmad, R.; Boulassel, M.R.; Tremblay, C.; Toma, E.; Routy, J.P.; Ahmad, A. Potential role of interleukin-18 in the immunopathogenesis of AIDS: Involvement in fratricidal killing of NK cells. J. Virol. 2009, 83, 5999–6010. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; King, S.R.; Bock, P.J.; Strieter, R.M.; Coffey, M.J.; Markovitz, D.M. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 2003, 307, 122–134. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, T.; Ma, M.; Zhang, Z.; Fu, Y.; Liu, J.; Xu, J.; Ding, H.; Han, X.; Chu, Z.; et al. Elevated interferon-gamma-induced protein 10 and its receptor CXCR3 impair NK cell function during HIV infection. J. Leukoc. Biol. 2017, 102, 163–170. [Google Scholar] [CrossRef]
- Ramirez, L.A.; Arango, T.A.; Thompson, E.; Naji, M.; Tebas, P.; Boyer, J.D. High IP-10 levels decrease T cell function in HIV-1-infected individuals on ART. J. Leukoc. Biol. 2014, 96, 1055–1063. [Google Scholar] [CrossRef]
- Valverde-Villegas, J.M.; de Medeiros, R.M.; Ellwanger, J.H.; Santos, B.R.; Melo, M.G.; Almeida, S.E.M.; Chies, J.A.B. High CXCL10/IP-10 levels are a hallmark in the clinical evolution of the HIV infection. Infect. Genet. Evol. 2018, 57, 51–58. [Google Scholar] [CrossRef]
- Lee, S.; Chung, Y.S.; Yoon, C.H.; Shin, Y.; Kim, S.; Choi, B.S.; Kim, S.S. Interferon-inducible protein 10 (IP-10) is associated with viremia of early HIV-1 infection in Korean patients. J. Med. Virol. 2015, 87, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Mhandire, K.; Mlambo, T.; Zijenah, L.S.; Duri, K.; Mateveke, K.; Tshabalala, M.; Mhandire, D.Z.; Musarurwa, C.; Wekare, P.T.; Mazengera, L.R.; et al. Plasma IP-10 Concentrations Correlate Positively with Viraemia and Inversely with CD4 Counts in Untreated HIV Infection. Open AIDS J. 2017, 11, 24–31. [Google Scholar] [CrossRef]
- Pham, T.N.Q.; Meziane, O.; Miah, M.A.; Volodina, O.; Colas, C.; Beland, K.; Li, Y.; Dallaire, F.; Keler, T.; Guimond, J.V.; et al. Flt3L-Mediated Expansion of Plasmacytoid Dendritic Cells Suppresses HIV Infection in Humanized Mice. Cell Rep. 2019, 29, 2770–2782. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. CCL2 increases X4-tropic HIV-1 entry into resting CD4+ T cells. J. Biol. Chem. 2008, 283, 30745–30753. [Google Scholar] [CrossRef] [PubMed]
- Megra, B.W.; Eugenin, E.A.; Berman, J.W. The Role of Shed PrP(c) in the Neuropathogenesis of HIV Infection. J. Immunol. 2017, 199, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Coll, B.; Tous, M.; Ferre, N.; Alonso-Villaverde, C.; Parra, S.; Camps, J. The influence of HIV infection on the correlation between plasma concentrations of monocyte chemoattractant protein-1 and carotid atherosclerosis. Clin. Chim. Acta 2006, 368, 114–119. [Google Scholar] [CrossRef]
- Floris-Moore, M.; Fayad, Z.A.; Berman, J.W.; Mani, V.; Schoenbaum, E.E.; Klein, R.S.; Weinshelbaum, K.B.; Fuster, V.; Howard, A.A.; Lo, Y.; et al. Association of HIV viral load with monocyte chemoattractant protein-1 and atherosclerosis burden measured by magnetic resonance imaging. AIDS 2009, 23, 941–949. [Google Scholar] [CrossRef]
- Blanpain, C.; Migeotte, I.; Lee, B.; Vakili, J.; Doranz, B.J.; Govaerts, C.; Vassart, G.; Doms, R.W.; Parmentier, M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood 1999, 94, 1899–1905. [Google Scholar] [CrossRef]
- Rom, S.; Rom, I.; Passiatore, G.; Pacifici, M.; Radhakrishnan, S.; Del Valle, L.; Pina-Oviedo, S.; Khalili, K.; Eletto, D.; Peruzzi, F. CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J. 2010, 24, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Suhre, K.; Sarwath, H.; Engelke, R.; Sohail, M.U.; Cho, S.J.; Whalen, W.; Alvarez-Mulett, S.; Krumsiek, J.; Choi, A.M.K.; Schmidt, F. Identification of Robust Protein Associations With COVID-19 Disease Based on Five Clinical Studies. Front. Immunol. 2021, 12, 781100. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Farmaki, E.; Chavez, B.; Cai, C.; Kaza, V.; Zhang, Y.; Soltanmohammadi, E.; Daneshvar, N.; Chatzistamou, I.; Kiaris, H. Beneficial effects of CCL8 inhibition at lipopolysaccharide-induced lung injury. iScience 2022, 25, 105520. [Google Scholar] [CrossRef]
- Anderson, J.L.; Cheong, K.; Lee, A.K.; Saleh, S.; da Fonseca Pereira, C.; Cameron, P.U.; Lewin, S.R. Entry of HIV in primary human resting CD4(+) T cells pretreated with the chemokine CCL19. AIDS Res. Hum. Retrovir. 2014, 30, 207–208. [Google Scholar] [CrossRef]
- Saleh, S.; Lu, H.K.; Evans, V.; Harisson, D.; Zhou, J.; Jaworowski, A.; Sallmann, G.; Cheong, K.Y.; Mota, T.M.; Tennakoon, S.; et al. HIV integration and the establishment of latency in CCL19-treated resting CD4(+) T cells require activation of NF-kappaB. Retrovirology 2016, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Armani-Tourret, M.; Zhou, Z.; Gasser, R.; Staropoli, I.; Cantaloube-Ferrieu, V.; Benureau, Y.; Garcia-Perez, J.; Perez-Olmeda, M.; Lorin, V.; Puissant-Lubrano, B.; et al. Mechanisms of HIV-1 evasion to the antiviral activity of chemokine CXCL12 indicate potential links with pathogenesis. PLoS Pathog. 2021, 17, e1009526. [Google Scholar] [CrossRef]
- Chan, L.; Karimi, N.; Morovati, S.; Alizadeh, K.; Kakish, J.E.; Vanderkamp, S.; Fazel, F.; Napoleoni, C.; Alizadeh, K.; Mehrani, Y.; et al. The Roles of Neutrophils in Cytokine Storms. Viruses 2021, 13, 2318. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Foucault, C.; Mokhtari, M.; Tamalet, C. Severe transient neutropenia associated with acute human immunodeficiency virus type 1 infection. Eur. J. Intern. Med. 2005, 16, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Skiest, D.J.; King, M.E. Granulocytopenia secondary to acute infection with the human immunodeficiency virus. J. Infect. 1994, 28, 315–318. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boby, N.; Srivastav, A.; Srivastav, S.K.; Pahar, B. Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model. Vaccines 2023, 11, 264. https://doi.org/10.3390/vaccines11020264
Boby N, Srivastav A, Srivastav SK, Pahar B. Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model. Vaccines. 2023; 11(2):264. https://doi.org/10.3390/vaccines11020264
Chicago/Turabian StyleBoby, Nongthombam, Apurv Srivastav, Sudesh K. Srivastav, and Bapi Pahar. 2023. "Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model" Vaccines 11, no. 2: 264. https://doi.org/10.3390/vaccines11020264
APA StyleBoby, N., Srivastav, A., Srivastav, S. K., & Pahar, B. (2023). Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model. Vaccines, 11(2), 264. https://doi.org/10.3390/vaccines11020264





