Evaluation of the Effect of CD70 Co-Expression on CD8 T Cell Response in Protein-Prime MVA-Boost Vaccination in Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Generation and Production of MVA Vectors
2.2. Evaluation of Gene Expression in Cell Culture
2.3. Ethical Statement
2.4. Experimental Animals
2.5. Heterologous Protein-Prime MVA-Boost Vaccination
2.6. Isolation of Lymphocytes from the Spleen and Liver
2.7. Detection of HBV Core-Specific CD8 T Cells by Multimers and Intracellular Cytokine Staining
2.8. Serological Analysis
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Generation of Recombinant MVA Encoding CD70
3.2. CD70 Co-Expression during Boost Vaccination Exclusively Increases the Number of Transgene-Specific CD8 T Cells in wt Mice
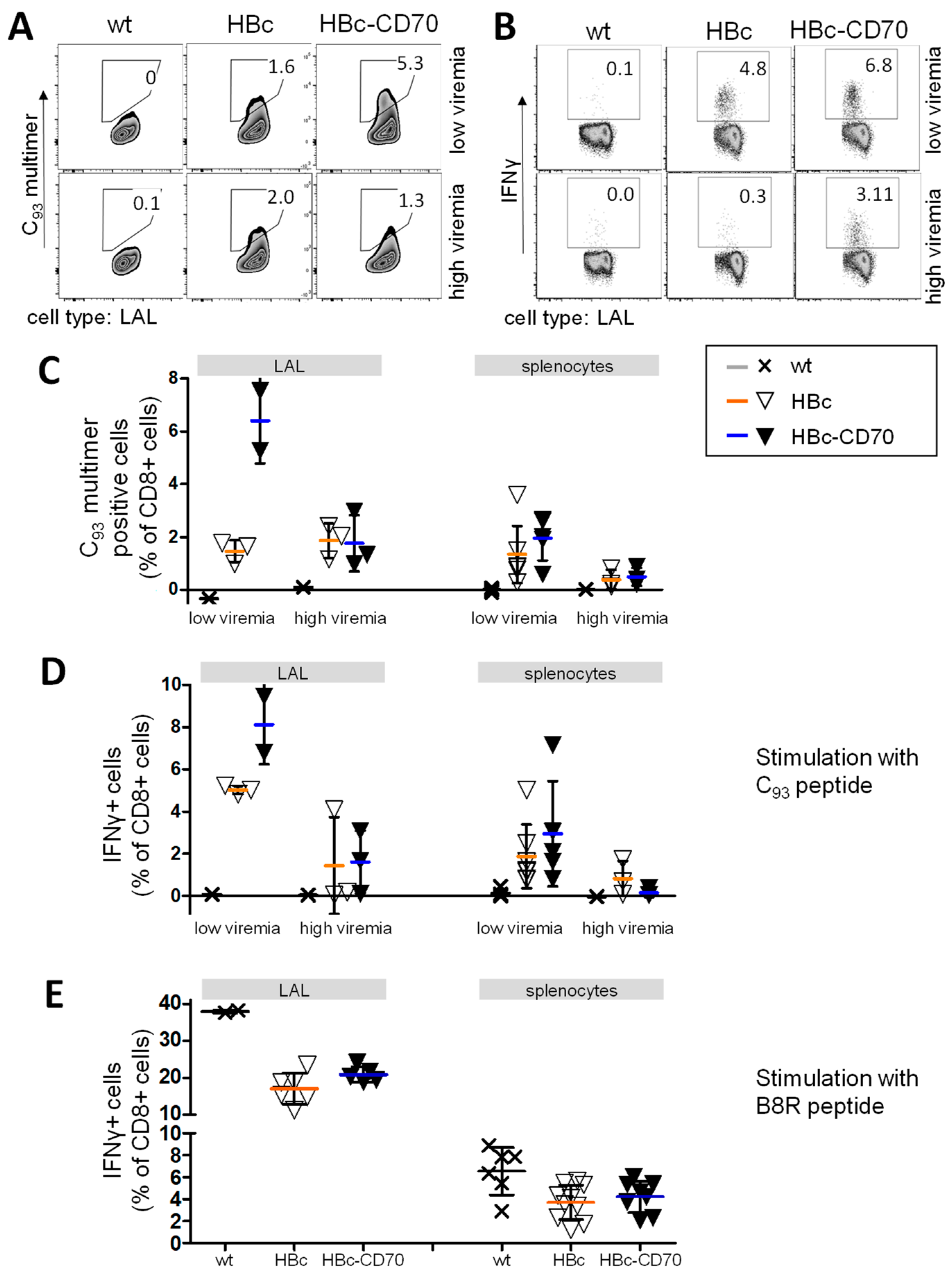
3.3. Transgene-Specific CD8 T Cells Gain Functionality by Co-Expression with CD70
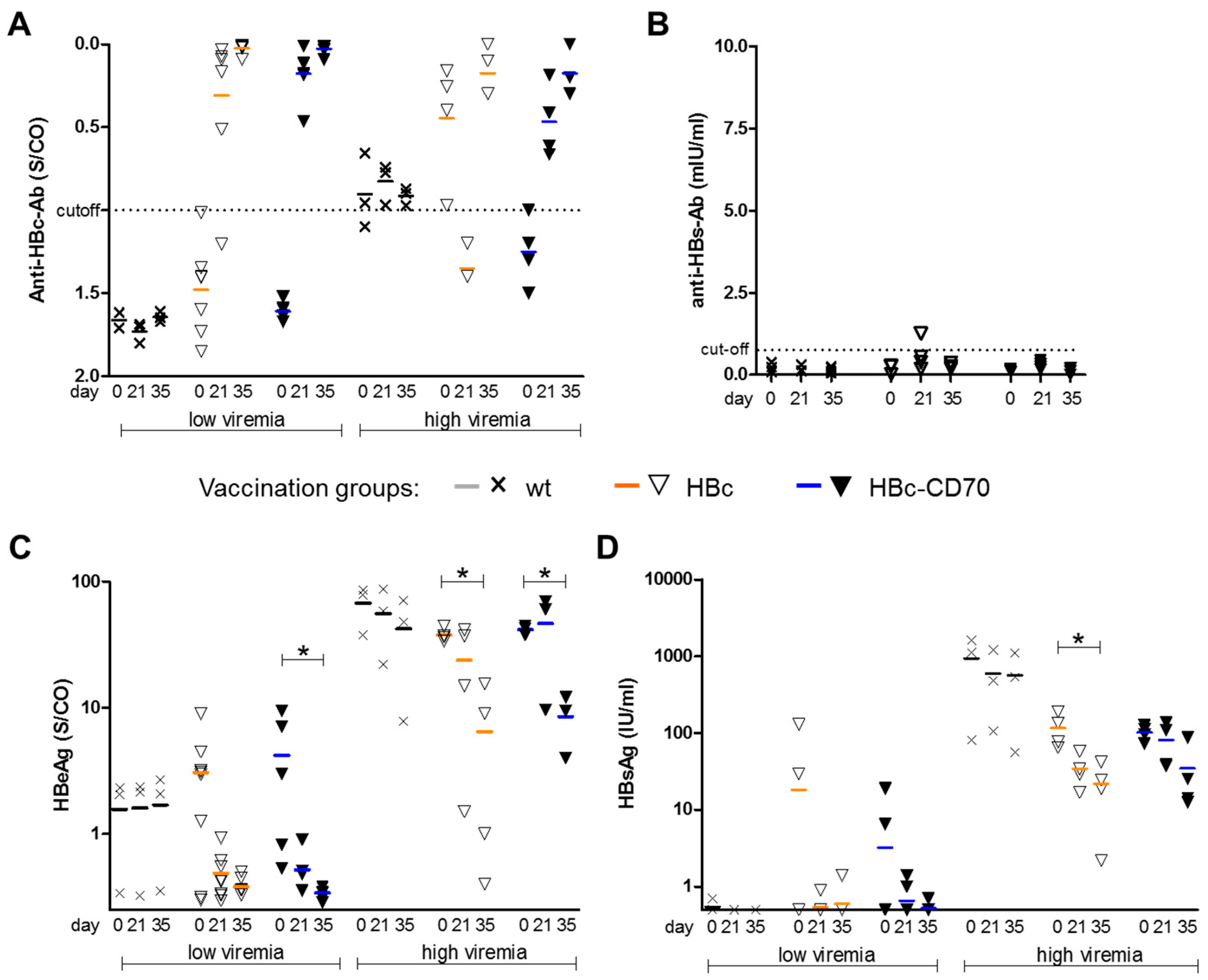
3.4. CD70 Co-Expression during Boost Vaccination Does Not Affect Humoral Immunity in a Therapeutic Vaccination Setting
3.5. CD70 Co-Expression during Boost Vaccination Has No Influence on HBeAg and HBsAg Levels
3.6. CD70 Co-Expression during Boost Vaccination Enhances the Number of Transgene-Specific CD8 T Cells in a Therapeutic Vaccination Setting Using rMVA-HBc-CD70
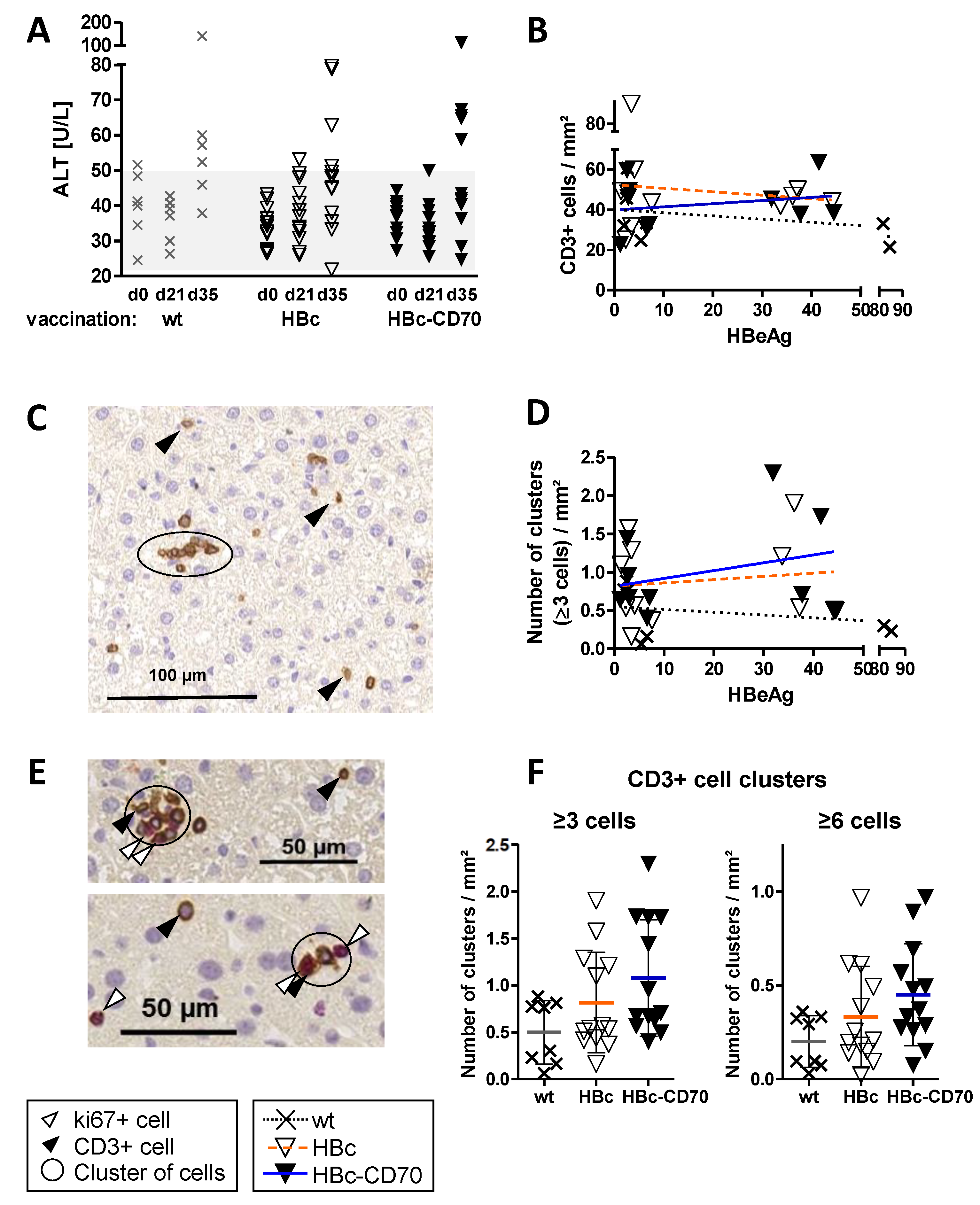
3.7. Co-Expression by CD70 during Boost Vaccination Increases the Number of T Cell Clusters in Livers of Vaccinated HBV1.3tg Mice without Promoting Immunopathology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, P.O. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 15 December 2022).
- EASL. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Sandmann, L.; Protzer, U.; Niederau, C.; Tacke, F.; Berg, T.; Glebe, D.; Jilg, W.; Wedemeyer, H.; Wirth, S.; et al. S3-Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) zur Prophylaxe, Diagnostik und Therapie der Hepatitis-B-Virusinfektion–(AWMF-Register-Nr. 021-11). Z. Fur Gastroenterol. 2021, 59, 691–776. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Zoulim, F.; Dusheiko, G.; Ghany, M.G. Hepatitis B cure: From discovery to regulatory approval. J. Hepatol. 2017, 67, 847–861. [Google Scholar] [CrossRef]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef]
- Chisari, F.V.; Ferrari, C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1995, 13, 29–60. [Google Scholar] [CrossRef]
- Chisari, F.V.; Isogawa, M.; Wieland, S.F. Pathogenesis of hepatitis B virus infection. Pathol. -Biol. 2010, 58, 258–266. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Robidoux, M.P.; Schwarz, T.; Heydmann, L.; Cheney, J.A.; Kvistad, D.; Aneja, J.; Melgaço, J.G.; Fernandes, C.A.; Chung, R.T.; et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 2019, 68, 893–904. [Google Scholar] [CrossRef]
- Maini, M.K.; Boni, C.; Ogg, G.S.; King, A.S.; Reignat, S.; Lee, C.K.; Larrubia, J.R.; Webster, G.J.; McMichael, A.J.; Ferrari, C.; et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 1999, 117, 1386–1396. [Google Scholar] [CrossRef]
- Thimme, R.; Wieland, S.; Steiger, C.; Ghrayeb, J.; Reimann, K.A.; Purcell, R.H.; Chisari, F.V. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 2003, 77, 68–76. [Google Scholar] [CrossRef]
- Boni, C.; Fisicaro, P.; Valdatta, C.; Amadei, B.; Di Vincenzo, P.; Giuberti, T.; Laccabue, D.; Zerbini, A.; Cavalli, A.; Missale, G.; et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007, 81, 4215–4225. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Thompson, A.J.; Visvanathan, K.; Kent, S.J.; Cameron, P.U.; Wightman, F.; Desmond, P.; Locarnini, S.A.; Lewin, S.R. The phenotype of hepatitis B virus-specific T cells differ in the liver and blood in chronic hepatitis B virus infection. Hepatology 2007, 46, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Hoare, M.; Davies, N.; Lopes, A.R.; Dunn, C.; Kennedy, P.T.; Alexander, G.; Finney, H.; Lawson, A.; Plunkett, F.J.; et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J. Exp. Med. 2008, 205, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Hill, A. T cells and viral persistence: Lessons from diverse infections. Nat. Immunol. 2005, 6, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Maini, M.K.; Boni, C.; Lee, C.K.; Larrubia, J.R.; Reignat, S.; Ogg, G.S.; King, A.S.; Herberg, J.; Gilson, R.; Alisa, A.; et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 2000, 191, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.T.; Loggi, E.; Boni, C.; Chia, A.; Gehring, A.J.; Sastry, K.S.; Goh, V.; Fisicaro, P.; Andreone, P.; Brander, C.; et al. Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J. Virol. 2008, 82, 10986–10997. [Google Scholar] [CrossRef]
- Webster, G.J.; Reignat, S.; Brown, D.; Ogg, G.S.; Jones, L.; Seneviratne, S.L.; Williams, R.; Dusheiko, G.; Bertoletti, A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: Implications for immunotherapy. J. Virol. 2004, 78, 5707–5719. [Google Scholar] [CrossRef]
- Barnes, E. Therapeutic vaccines in HBV: Lessons from HCV. Med. Microbiol. Immunol. 2015, 204, 79–86. [Google Scholar] [CrossRef]
- Cargill, T.; Barnes, E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin. Exp. Immunol. 2021, 205, 106–118. [Google Scholar] [CrossRef]
- Dembek, C.; Protzer, U.; Roggendorf, M. Overcoming immune tolerance in chronic hepatitis B by therapeutic vaccination. Curr. Opin. Virol. 2018, 30, 58–67. [Google Scholar] [CrossRef]
- Liu, J.; Kosinska, A.; Lu, M.; Roggendorf, M. New therapeutic vaccination strategies for the treatment of chronic hepatitis B. Virol. Sin. 2014, 29, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, A.D.; Liu, J.; Lu, M.; Roggendorf, M. Therapeutic vaccination and immunomodulation in the treatment of chronic hepatitis B: Preclinical studies in the woodchuck. Med. Microbiol. Immunol. 2015, 204, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Schurich, A.; Khanna, P.; Lopes, A.R.; Han, K.J.; Peppa, D.; Micco, L.; Nebbia, G.; Kennedy, P.T.; Geretti, A.M.; Dusheiko, G.; et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011, 53, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Backes, S.; Jäger, C.; Dembek, C.J.; Kosinska, A.D.; Bauer, T.; Stephan, A.S.; Dišlers, A.; Mutwiri, G.; Busch, D.H.; Babiuk, L.A.; et al. Protein-prime/modified vaccinia virus Ankara vector-boost vaccination overcomes tolerance in high-antigenemic HBV-transgenic mice. Vaccine 2016, 34, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Michler, T.; Kosinska, A.D.; Festag, J.; Bunse, T.; Su, J.; Ringelhan, M.; Imhof, H.; Grimm, D.; Steiger, K.; Mogler, C.; et al. Knockdown of Virus Antigen Expression Increases Therapeutic Vaccine Efficacy in High-Titer Hepatitis B Virus Carrier Mice. Gastroenterology 2020, 158, 1762–1775.e1769. [Google Scholar] [CrossRef] [PubMed]
- Bunse, T.; Kosinska, A.D.; Michler, T.; Protzer, U. PD-L1 Silencing in Liver Using siRNAs Enhances Efficacy of Therapeutic Vaccination for Chronic Hepatitis B. Biomolecules 2022, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, A.D.; Moeed, A.; Kallin, N.; Festag, J.; Su, J.; Steiger, K.; Michel, M.L.; Protzer, U.; Knolle, P.A. Synergy of therapeutic heterologous prime-boost hepatitis B vaccination with CpG-application to improve immune control of persistent HBV infection. Sci. Rep. 2019, 9, 10808. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, R.Q.; Lens, S.M.; Lammers, K.; Kuiper, H.; Beckmann, M.P.; van Lier, R.A. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J. Immunol. 1995, 154, 2612–2623. [Google Scholar] [CrossRef]
- Arens, R.; Tesselaar, K.; Baars, P.A.; van Schijndel, G.M.; Hendriks, J.; Pals, S.T.; Krimpenfort, P.; Borst, J.; van Oers, M.H.; van Lier, R.A. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity 2001, 15, 801–812. [Google Scholar] [CrossRef]
- Hendriks, J.; Gravestein, L.A.; Tesselaar, K.; van Lier, R.A.; Schumacher, T.N.; Borst, J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000, 1, 433–440. [Google Scholar] [CrossRef]
- Hendriks, J.; Xiao, Y.; Borst, J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 2003, 198, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Salama, A.D.; Sho, M.; Najafian, N.; Ito, T.; Forman, J.P.; Kewalramani, R.; Sandner, S.; Harada, H.; Clarkson, M.R.; et al. CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo. J. Immunol. 2005, 174, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Boursalian, T.E.; McEarchern, J.A.; Law, C.L.; Grewal, I.S. Targeting CD70 for human therapeutic use. Adv. Exp. Med. Biol. 2009, 647, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Franklin, N.A.; Kingeter, L.M.; Yagita, H.; Tutt, A.L.; Glennie, M.J.; Bullock, T.N. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J. Immunother. 2010, 33, 769–779. [Google Scholar] [CrossRef]
- Soares, H.; Waechter, H.; Glaichenhaus, N.; Mougneau, E.; Yagita, H.; Mizenina, O.; Dudziak, D.; Nussenzweig, M.C.; Steinman, R.M. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007, 204, 1095–1106. [Google Scholar] [CrossRef]
- Denoeud, J.; Moser, M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J. Leukoc. Biol. 2011, 89, 195–203. [Google Scholar] [CrossRef]
- Glouchkova, L.; Ackermann, B.; Zibert, A.; Meisel, R.; Siepermann, M.; Janka-Schaub, G.E.; Goebel, U.; Troeger, A.; Dilloo, D. The CD70/CD27 pathway is critical for stimulation of an effective cytotoxic T cell response against B cell precursor acute lymphoblastic leukemia. J. Immunol. 2009, 182, 718–725. [Google Scholar] [CrossRef]
- Schildknecht, A.; Miescher, I.; Yagita, H.; van den Broek, M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur. J. Immunol. 2007, 37, 716–728. [Google Scholar] [CrossRef]
- Xiao, Y.; Peperzak, V.; Keller, A.M.; Borst, J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J. Immunol. 2008, 181, 1071–1082. [Google Scholar] [CrossRef]
- Jacobs, J.; Deschoolmeester, V.; Zwaenepoel, K.; Rolfo, C.; Silence, K.; Rottey, S.; Lardon, F.; Smits, E.; Pauwels, P. CD70: An emerging target in cancer immunotherapy. Pharmacol. Ther. 2015, 155, 1–10. [Google Scholar] [CrossRef]
- Arroyo Hornero, R.; Georgiadis, C.; Hua, P.; Trzupek, D.; He, L.Z.; Qasim, W.; Todd, J.A.; Ferreira, R.C.; Wood, K.J.; Issa, F.; et al. CD70 expression determines the therapeutic efficacy of expanded human regulatory T cells. Commun. Biol. 2020, 3, 375. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, K.; Borst, J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: Rationale and potential. Immunotherapy 2015, 7, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, A.D.; Festag, J.; Mück-Häusl, M.; Festag, M.M.; Asen, T.; Protzer, U. Immunogenicity and Antiviral Response of Therapeutic Hepatitis B Vaccination in a Mouse Model of HBeAg-Negative, Persistent HBV Infection. Vaccines 2021, 9, 841. [Google Scholar] [CrossRef]
- Wyatt, L.S.; Earl, P.L.; Xiao, W.; Americo, J.L.; Cotter, C.A.; Vogt, J.; Moss, B. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. J. Virol. 2009, 83, 7176–7184. [Google Scholar] [CrossRef]
- Sino Biologocal Inc. Human CD70/CD27L/TNFSF7 Protein (Fc Tag): Datasheet, Catalog Number 10780-H01H. Human CD70 Datasheet. Available online: https://cdn1.sinobiological.com/reagent/10780-H01H.pdf (accessed on 10 September 2022).
- Stross, L.; Günther, J.; Gasteiger, G.; Asen, T.; Graf, S.; Aichler, M.; Esposito, I.; Busch, D.H.; Knolle, P.; Sparwasser, T.; et al. Foxp3+ regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice. Hepatology 2012, 56, 873–883. [Google Scholar] [CrossRef]
- Luke, G.A.; de Felipe, P.; Lukashev, A.; Kallioinen, S.E.; Bruno, E.A.; Ryan, M.D. Occurrence, function and evolutionary origins of ‘2A-like’ sequences in virus genomes. J. Gen. Virol. 2008, 89, 1036–1042. [Google Scholar] [CrossRef]
- Wang, Z.; Martinez, J.; Zhou, W.; La Rosa, C.; Srivastava, T.; Dasgupta, A.; Rawal, R.; Li, Z.; Britt, W.J.; Diamond, D. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 2010, 28, 1547–1557. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Matzke, B.; Schaller, H.; Chisari, F.V. High-level hepatitis B virus replication in transgenic mice. J. Virol. 1995, 69, 6158–6169. [Google Scholar] [CrossRef]
- Tesselaar, K.; Arens, R.; van Schijndel, G.M.; Baars, P.A.; van der Valk, M.A.; Borst, J.; van Oers, M.H.; van Lier, R.A. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 2003, 4, 49–54. [Google Scholar] [CrossRef]
- Chahroudi, A.; Garber, D.A.; Reeves, P.; Liu, L.; Kalman, D.; Feinberg, M.B. Differences and similarities in viral life cycle progression and host cell physiology after infection of human dendritic cells with modified vaccinia virus Ankara and vaccinia virus. J. Virol. 2006, 80, 8469–8481. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, A.F.; van de Sandt, C.E.; Li, B.W.S.; MacLoughlin, R.J.; Fouchier, R.A.M.; van Amerongen, G.; Volz, A.; Hendriks, R.W.; de Swart, R.L.; Sutter, G.; et al. Modified Vaccinia Virus Ankara Preferentially Targets Antigen Presenting Cells In Vitro, Ex Vivo and In Vivo. Sci. Rep. 2017, 7, 8580. [Google Scholar] [CrossRef] [PubMed]
- Engelmayer, J.; Larsson, M.; Subklewe, M.; Chahroudi, A.; Cox, W.I.; Steinman, R.M.; Bhardwaj, N. Vaccinia virus inhibits the maturation of human dendritic cells: A novel mechanism of immune evasion. J. Immunol. 1999, 163, 6762–6768. [Google Scholar] [CrossRef] [PubMed]
- Bullock, T.N.; Yagita, H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J. Immunol. 2005, 174, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.M.; Xiao, Y.; Peperzak, V.; Naik, S.H.; Borst, J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood 2009, 113, 5167–5175. [Google Scholar] [CrossRef]
- Bathke, B.; Pätzold, J.; Kassub, R.; Giessel, R.; Lämmermann, K.; Hinterberger, M.; Brinkmann, K.; Chaplin, P.; Suter, M.; Hochrein, H.; et al. CD70 encoded by modified vaccinia virus Ankara enhances CD8 T-cell-dependent protective immunity in MHC class II-deficient mice. Immunology 2018, 154, 285–297. [Google Scholar] [CrossRef]
- Kastenmuller, W.; Gasteiger, G.; Gronau, J.H.; Baier, R.; Ljapoci, R.; Busch, D.H.; Drexler, I. Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination. J. Exp. Med. 2007, 204, 2187–2198. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephan, A.-S.; Kosinska, A.D.; Mück-Häusl, M.; Muschaweckh, A.; Jäger, C.; Röder, N.; Heikenwälder, M.; Dembek, C.; Protzer, U. Evaluation of the Effect of CD70 Co-Expression on CD8 T Cell Response in Protein-Prime MVA-Boost Vaccination in Mice. Vaccines 2023, 11, 245. https://doi.org/10.3390/vaccines11020245
Stephan A-S, Kosinska AD, Mück-Häusl M, Muschaweckh A, Jäger C, Röder N, Heikenwälder M, Dembek C, Protzer U. Evaluation of the Effect of CD70 Co-Expression on CD8 T Cell Response in Protein-Prime MVA-Boost Vaccination in Mice. Vaccines. 2023; 11(2):245. https://doi.org/10.3390/vaccines11020245
Chicago/Turabian StyleStephan, Ann-Sophie, Anna D. Kosinska, Martin Mück-Häusl, Andreas Muschaweckh, Clemens Jäger, Natalie Röder, Mathias Heikenwälder, Claudia Dembek, and Ulrike Protzer. 2023. "Evaluation of the Effect of CD70 Co-Expression on CD8 T Cell Response in Protein-Prime MVA-Boost Vaccination in Mice" Vaccines 11, no. 2: 245. https://doi.org/10.3390/vaccines11020245
APA StyleStephan, A.-S., Kosinska, A. D., Mück-Häusl, M., Muschaweckh, A., Jäger, C., Röder, N., Heikenwälder, M., Dembek, C., & Protzer, U. (2023). Evaluation of the Effect of CD70 Co-Expression on CD8 T Cell Response in Protein-Prime MVA-Boost Vaccination in Mice. Vaccines, 11(2), 245. https://doi.org/10.3390/vaccines11020245





