Immunogenicity, Efficacy, and Safety of a Novel Synthetic Microparticle Pre-Erythrocytic Malaria Vaccine in Multiple Host Species
Abstract
1. Introduction
2. Materials and Methods
2.1. LbL Particle Fabrication
2.2. Animals and Immunizations
2.3. Antibody Assays
2.4. T-Cell ELISPOT
2.5. Efficacy Measurement
2.6. Statistical Analyses
3. Results
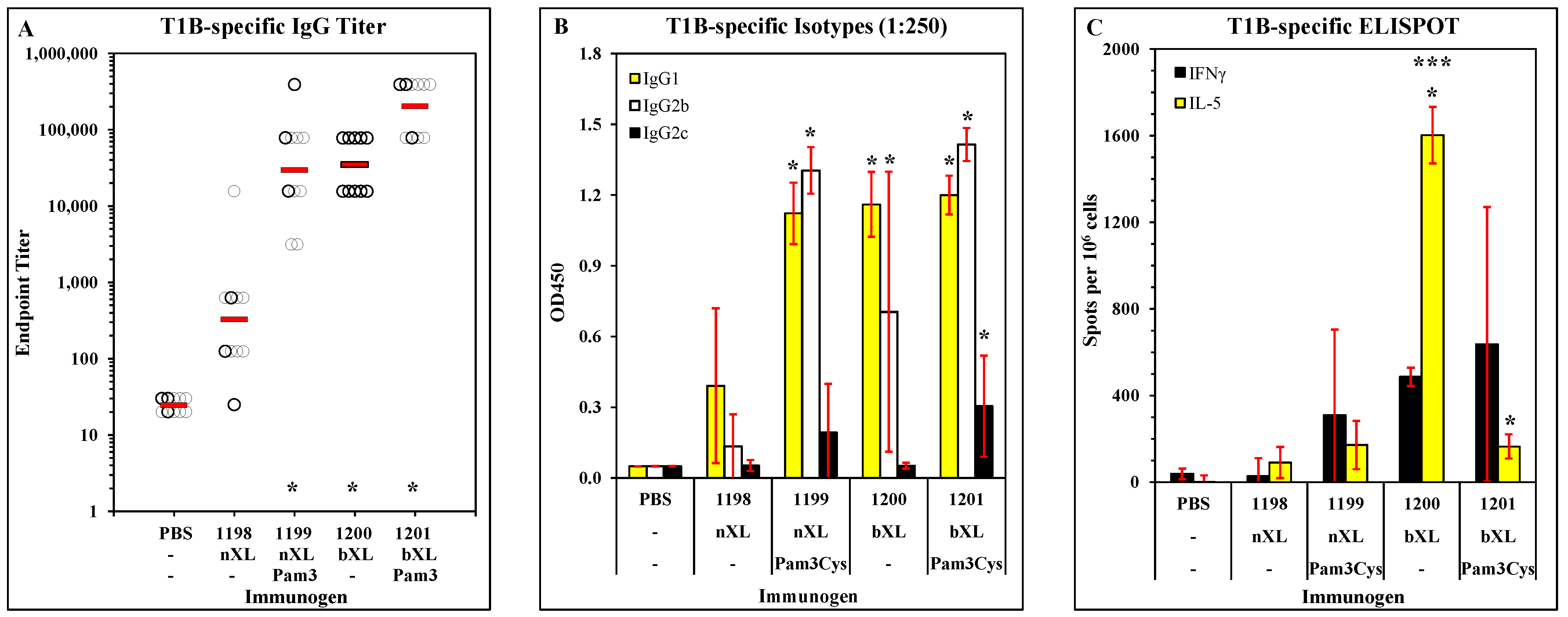
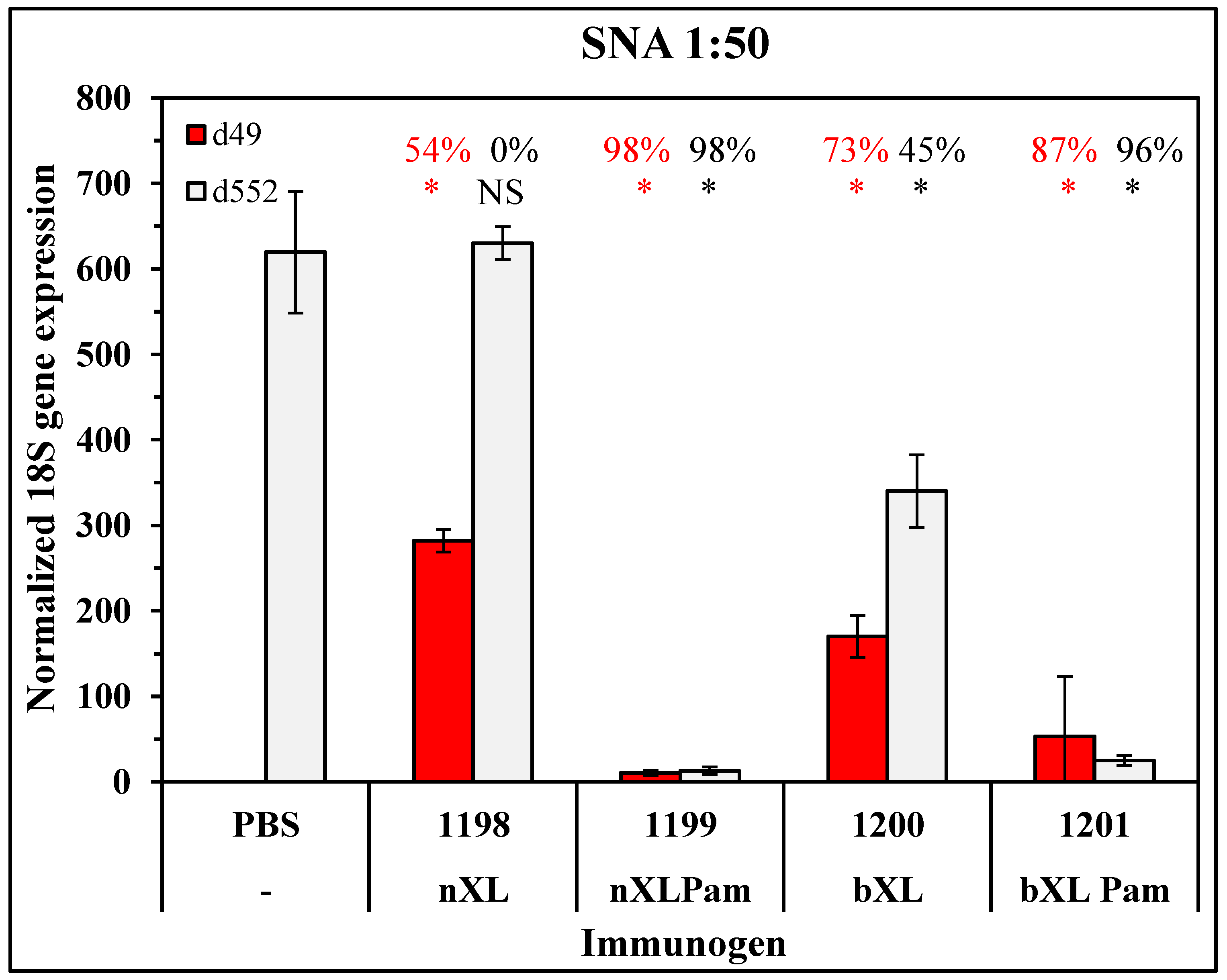
3.1. Candidate Optimization and Potency in Mice
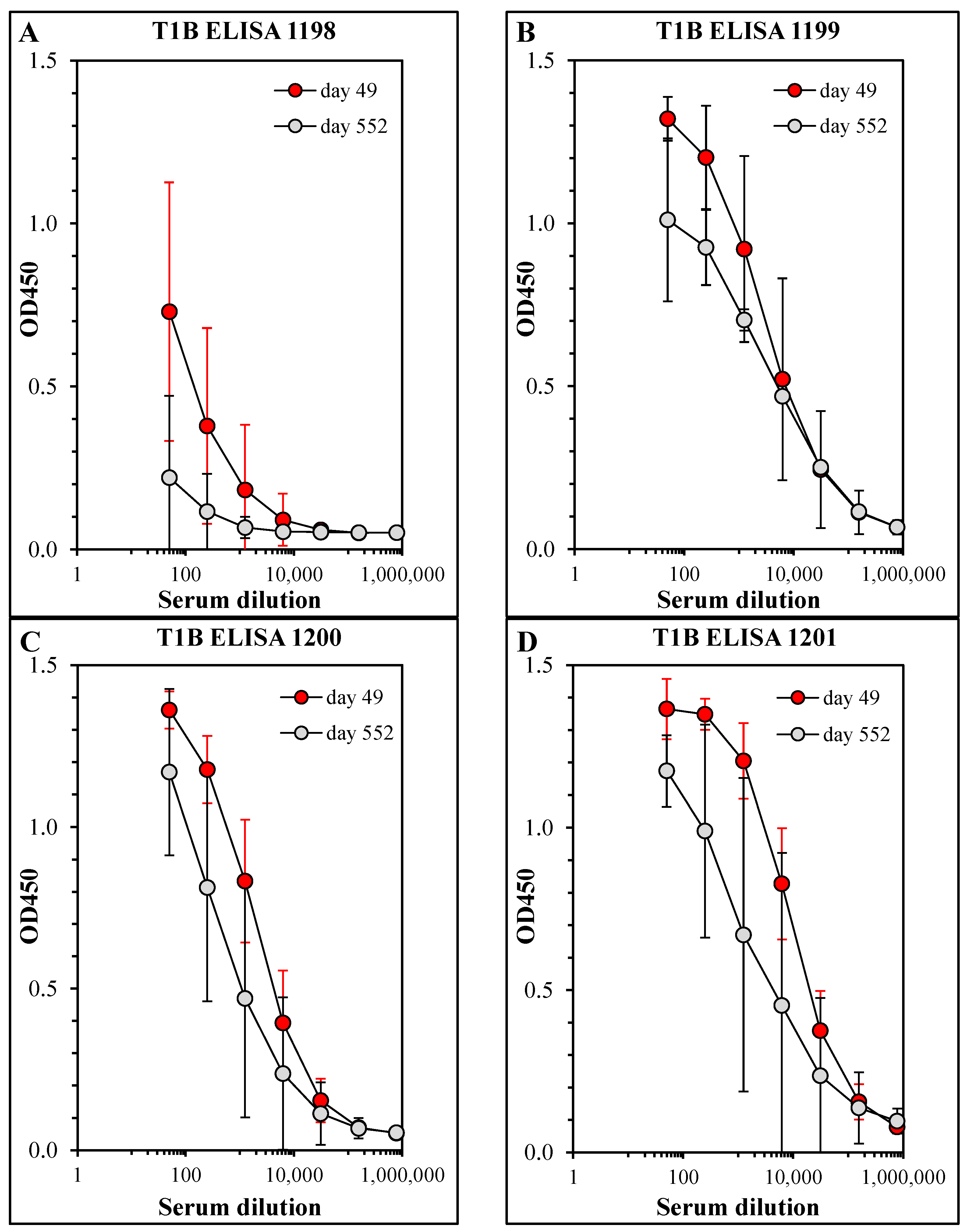
3.2. Persistence of Antibody Responses
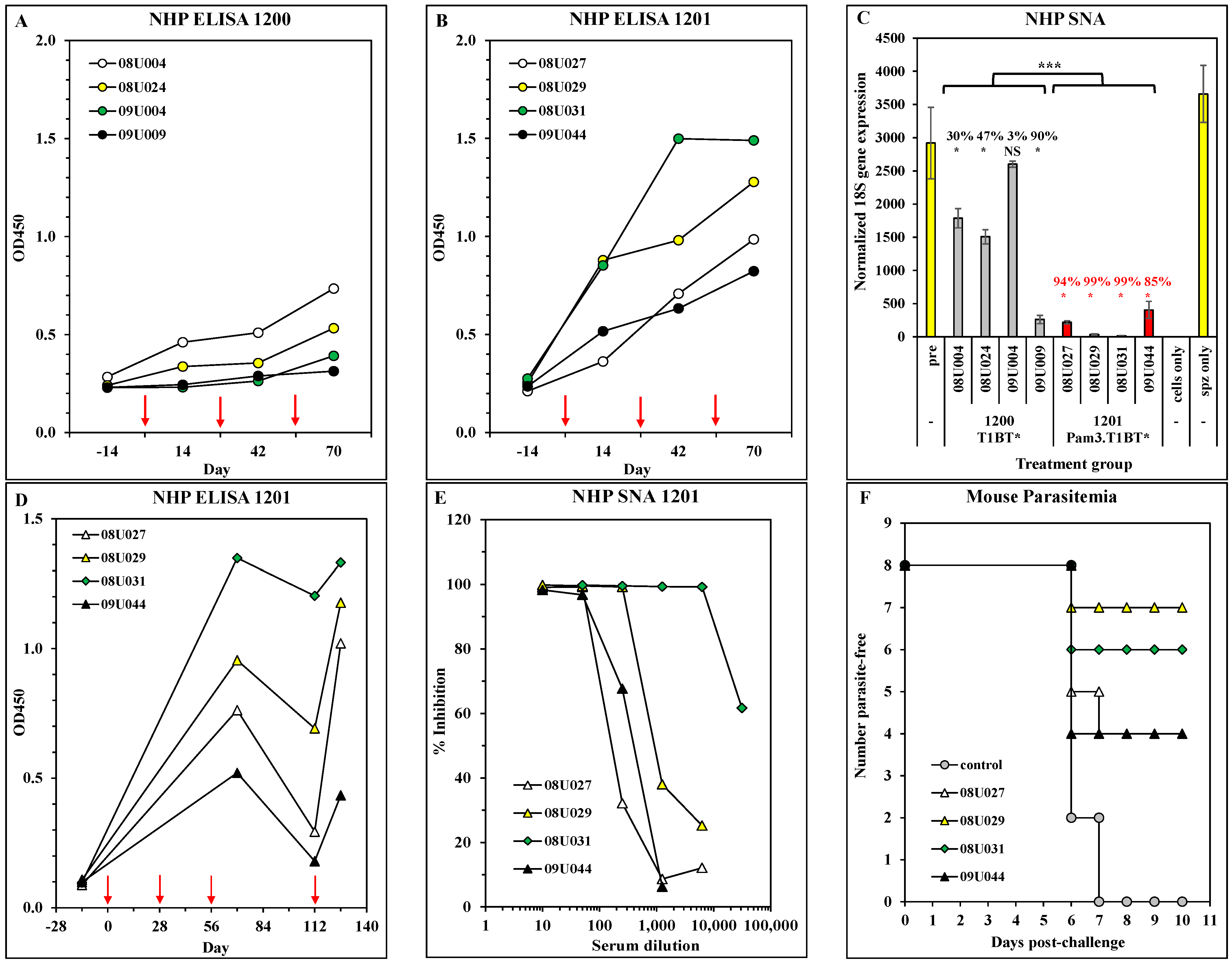
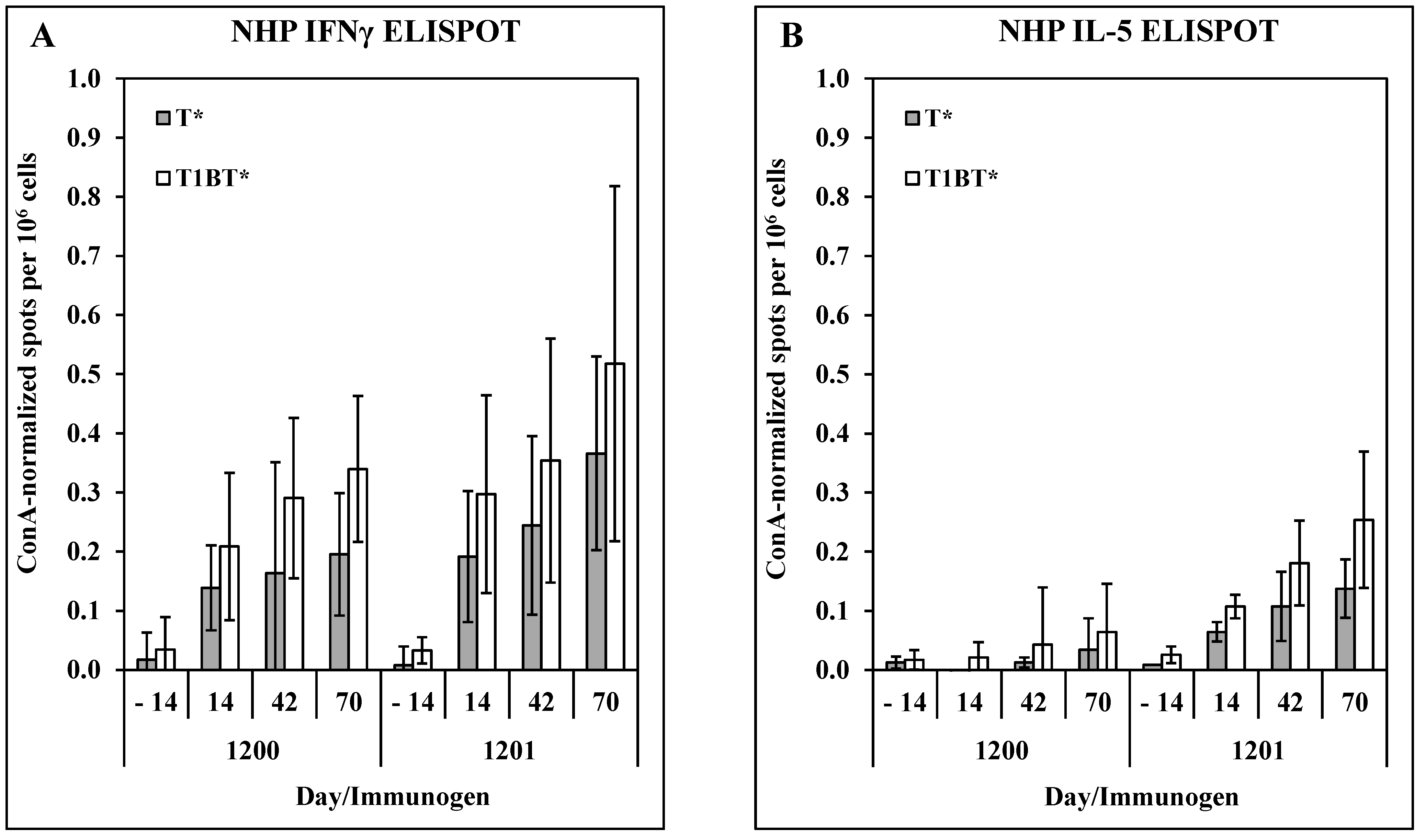
3.3. Immunogenicity and Efficacy in Non-Human Primates
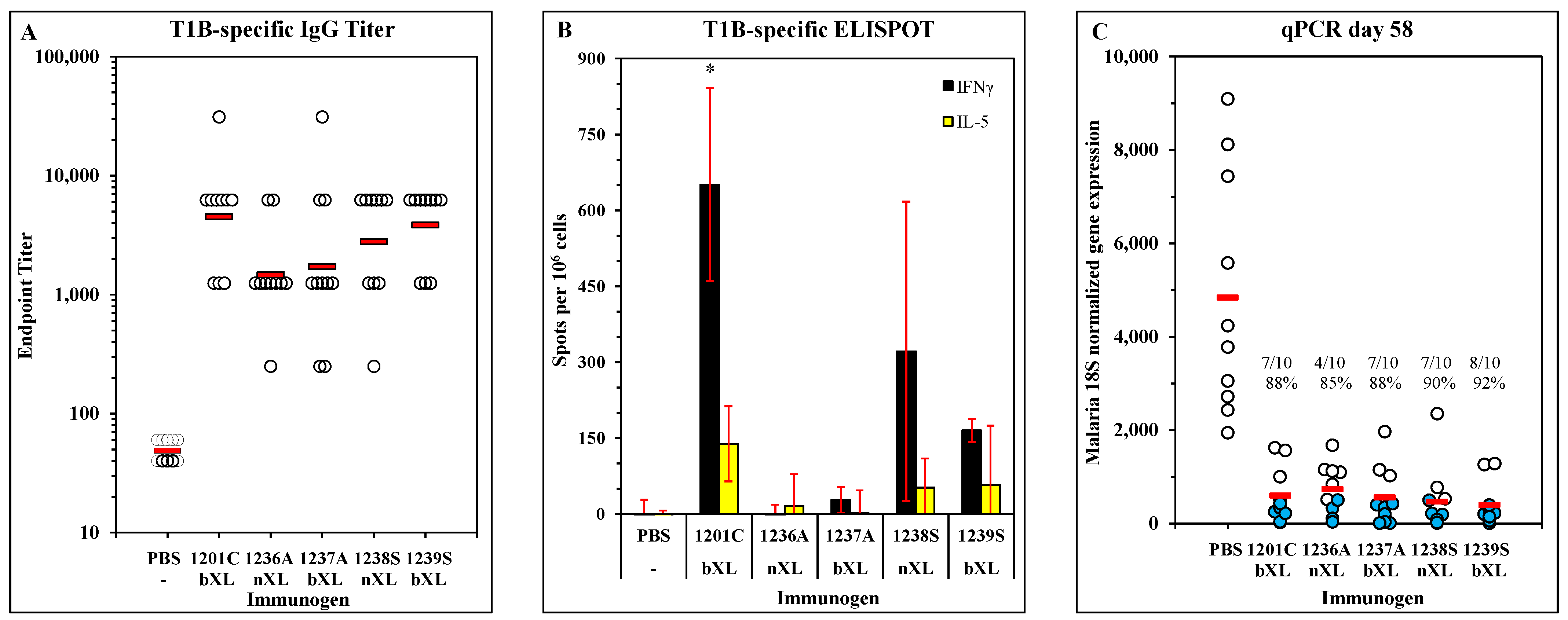
3.4. Immunogenicity and Efficacy of LbL-MP with Substitution for Unpaired Cysteine Residue
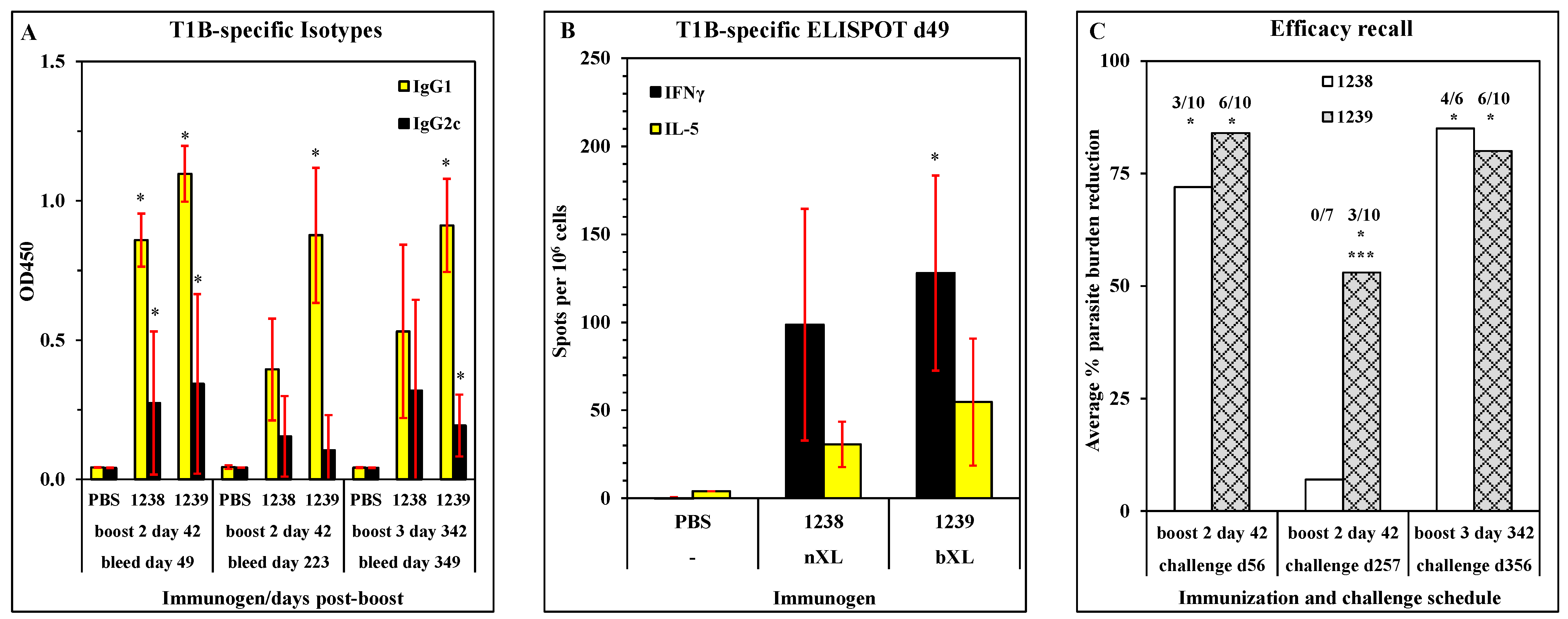
3.5. Persistence of Antibody Response in Mice Immunized with ACT-1239
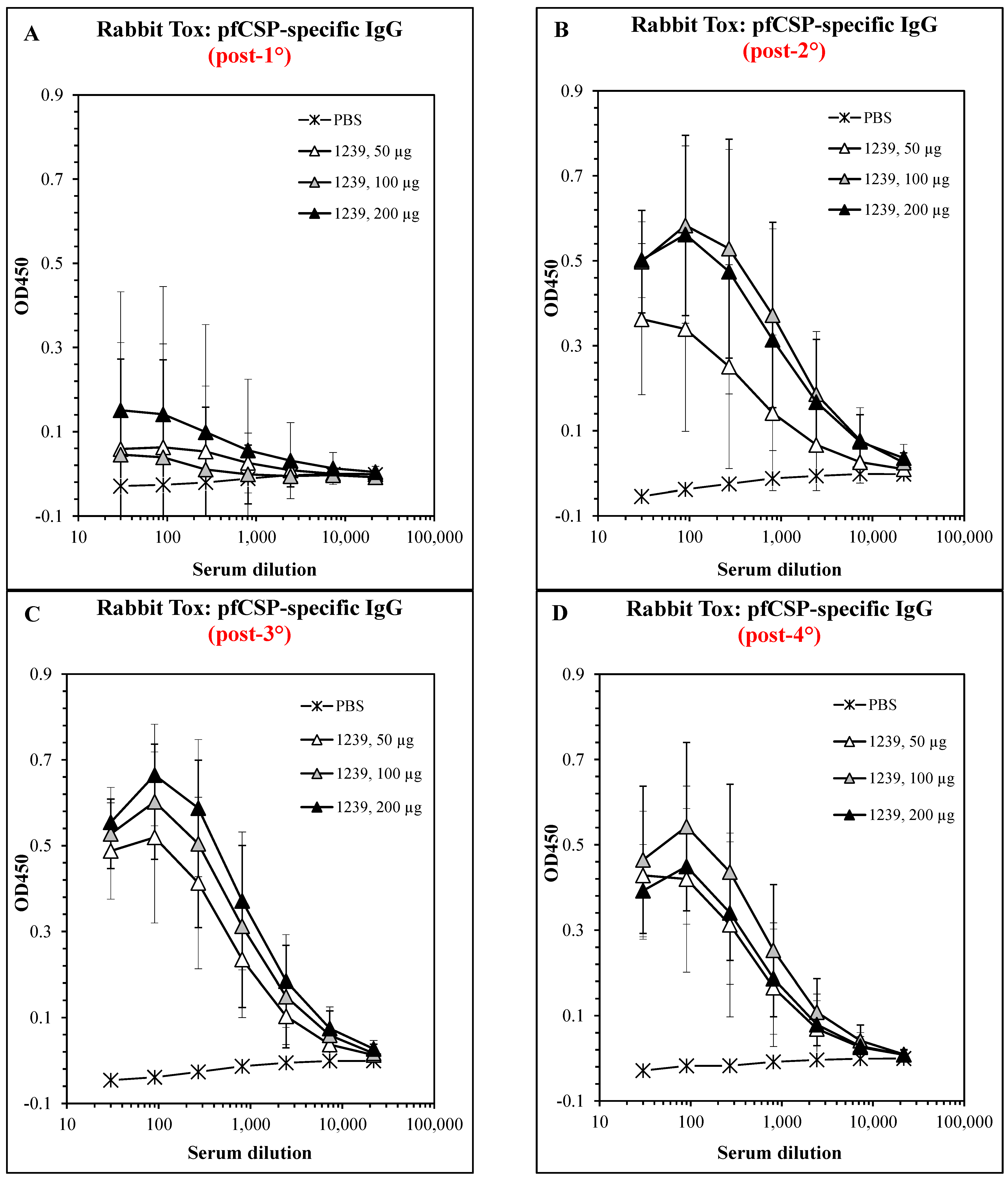
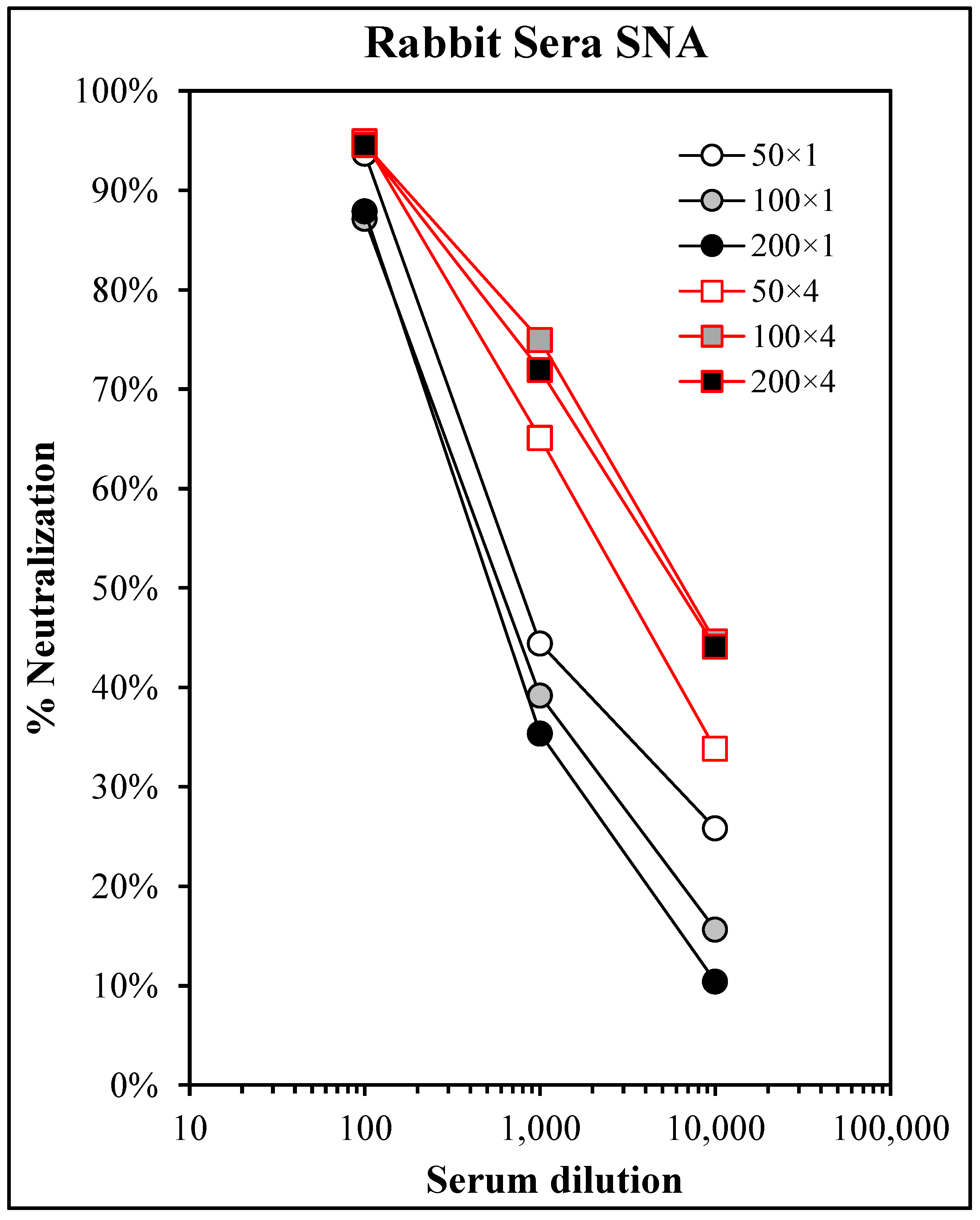
3.6. Safety and Immunogenicity in GLP-Compliant Rabbit Toxicology Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Nardin, E.H.; Nussenzweig, R.S. T cell responses to pre-erythrocytic stages of malaria: Role in protection and vaccine development against pre-erythrocytic stages. Annu. Rev. Immunol. 1993, 11, 687–727. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Nussenzweig, R.S.; Nussenzweig, V. Development of sporozoite vaccines. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1984, 307, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Luke, T.C.; Hoffman, S.L. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 2003, 206, 3803–3808. [Google Scholar] [CrossRef]
- Roestenberg, M.; McCall, M.; Hopman, J.; Wiersma, J.; Luty, A.J.; van Gemert, G.J.; van de Vegte-Bolmer, M.; van Schaijk, B.; Teelen, K.; Arens, T.; et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009, 361, 468–477. [Google Scholar] [CrossRef]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Lell, B.; Soulanoudjingar, S.S.; Fernandes, J.F.; Abossolo, B.P.; Conzelmann, C.; Methogo, B.G.; Doucka, Y.; Flamen, A.; Mordmuller, B.; et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Methogo, B.G.; Kabwende, A.L.; Adegnika, A.A.; Mordmuller, B.; Issifou, S.; Kremsner, P.G.; et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012, 367, 2284–2295. [Google Scholar] [CrossRef]
- Bejon, P.; White, M.T.; Olotu, A.; Bojang, K.; Lusingu, J.P.; Salim, N.; Otsyula, N.N.; Agnandji, S.T.; Asante, K.P.; Owusu-Agyei, S.; et al. Efficacy of RTS,S malaria vaccines: Individual-participant pooled analysis of phase 2 data. Lancet Infect. Dis. 2013, 13, 319–327. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Awuondo, K.O.; Leach, A.; Lievens, M.; Leboulleux, D.; Njuguna, P.; Peshu, N.; et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 2013, 368, 1111–1120. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef]
- Sissoko, M.S.; Healy, S.A.; Katile, A.; Omaswa, F.; Zaidi, I.; Gabriel, E.E.; Kamate, B.; Samake, Y.; Guindo, M.A.; Dolo, A.; et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect. Dis. 2017, 17, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.J.; Tang, J.; Derome, M.E.; Mitchell, R.A.; Jacobs, A.; Deng, Y.; Palath, N.; Cardenas, E.; Boyd, J.G.; Nardin, E. Plasmodium falciparum synthetic LbL microparticle vaccine elicits protective neutralizing antibody and parasite-specific cellular immune responses. Vaccine 2013, 31, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Molano, A.; Park, S.H.; Chiu, Y.H.; Nosseir, S.; Bendelac, A.; Tsuji, M. Cutting edge: The IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: Exploring the role of GPIs in NK T cell activation and antimalarial responses. J. Immunol. 2000, 164, 5005–5009. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.H.; Sano, G.; Hafalla, J.C.; Morrot, A.; Curotto de Lafaille, M.A.; Zavala, F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 2002, 8, 166–170. [Google Scholar] [CrossRef]
- Ferreira, A.; Schofield, L.; Enea, V.; Schellekens, H.; van der Meide, P.; Collins, W.E.; Nussenzweig, R.S.; Nussenzweig, V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 1986, 232, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Mellouk, S.; Hoffman, S.L.; Liu, Z.Z.; de la Vega, P.; Billiar, T.R.; Nussler, A.K. Nitric oxide-mediated antiplasmodial activity in human and murine hepatocytes induced by gamma interferon and the parasite itself: Enhancement by exogenous tetrahydrobiopterin. Infect Immun. 1994, 62, 4043–4046. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.J.; Palath, N.; Derome, M.E.; Tang, J.; Jacobs, A.; Boyd, J.G. Synthetic nanoparticle vaccines produced by layer-by-layer assembly of artificial biofilms induce potent protective T-cell and antibody responses in vivo. Vaccine 2011, 29, 558–569. [Google Scholar] [CrossRef]
- Mwakingwe-Omari, A.; Healy, S.A.; Lane, J.; Cook, D.M.; Kalhori, S.; Wyatt, C.; Kolluri, A.; Marte-Salcedo, O.; Imeru, A.; Nason, M.; et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 2021, 595, 289–294. [Google Scholar] [CrossRef]
- Kumar, K.A.; Oliveira, G.A.; Edelman, R.; Nardin, E.; Nussenzweig, V. Quantitative Plasmodium sporozoite neutralization assay (TSNA). J. Immunol. Methods 2004, 292, 157–164. [Google Scholar] [CrossRef]
- Othoro, C.; Johnston, D.; Lee, R.; Soverow, J.; Bystryn, J.C.; Nardin, E. Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod. Infect Immun. 2009, 77, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Przysiecki, C.; Lucas, B.; Mitchell, R.; Carapau, D.; Wen, Z.; Xu, H.; Wang, X.M.; Nahas, D.; Wu, C.; Hepler, R.; et al. Sporozoite neutralizing antibodies elicited in mice and rhesus macaques immunized with a Plasmodium falciparum repeat peptide conjugated to meningococcal outer membrane protein complex. Front. Cell. Infect. Microbiol. 2012, 2, 146. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Spaccapelo, R.; Bistoni, F.; Holder, A.A.; Crisanti, A. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J. Biol. Chem. 2002, 277, 47613–47618. [Google Scholar] [CrossRef]
- McCoy, M.E.; Golden, H.E.; Doll, T.A.; Yang, Y.; Kaba, S.A.; Zou, X.; Gerbasi, V.R.; Burkhard, P.; Lanar, D.E. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine. Malar J. 2013, 12, 136. [Google Scholar] [CrossRef]
- Nardin, E.H.; Calvo-Calle, J.M.; Oliveira, G.A.; Nussenzweig, R.S.; Schneider, M.; Tiercy, J.M.; Loutan, L.; Hochstrasser, D.; Rose, K. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J. Immunol. 2001, 166, 481–489. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Bejon, P.; Olotu, A.; Griffin, J.T.; Riley, E.M.; Kester, K.E.; Ockenhouse, C.F.; Ghani, A.C. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS ONE 2013, 8, e61395. [Google Scholar] [CrossRef] [PubMed]
- Stoute, J.A.; Slaoui, M.; Heppner, D.G.; Momin, P.; Kester, K.E.; Desmons, P.; Wellde, B.T.; Garcon, N.; Krzych, U.; Marchand, M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 1997, 336, 86–91. [Google Scholar] [CrossRef]
- Seder, R.A.; Chang, L.J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection Against Malaria by Intravenous Immunization with a Nonreplicating Sporozoite Vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef]
- Hoffman, S.L.; Goh, L.M.; Luke, T.C.; Schneider, I.; Le, T.P.; Doolan, D.L.; Sacci, J.; de la Vega, P.; Dowler, M.; Paul, C.; et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 2002, 185, 1155–1164. [Google Scholar] [CrossRef]
- Hoffman, S.L.; Isenbarger, D.; Long, G.W.; Sedegah, M.; Szarfman, A.; Waters, L.; Hollingdale, M.R.; van der Meide, P.H.; Finbloom, D.S.; Ballou, W.R. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science 1989, 244, 1078–1081. [Google Scholar] [CrossRef]
- Feng, G.; Kurtovic, L.; Agius, P.A.; Aitken, E.H.; Sacarlal, J.; Wines, B.D.; Hogarth, P.M.; Rogerson, S.J.; Fowkes, F.J.I.; Dobano, C.; et al. Induction, decay, and determinants of functional antibodies following vaccination with the RTS,S malaria vaccine in young children. BMC Med. 2022, 20, 289. [Google Scholar] [CrossRef]
- Casares, S.; Brumeanu, T.D.; Richie, T.L. The RTS, S malaria vaccine. Vaccine 2010, 28, 4880–4894. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Natama, M.H.; Some, A.; Traore, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Natama, H.M.; Some, A.; Bellamy, D.; Traore, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: A phase 1/2b randomised controlled trial. Lancet. Infect. Dis. 2022, 22, 1728–1736. [Google Scholar] [CrossRef]
- Syed, Y.Y. RTS,S/AS01 malaria vaccine (Mosquirix((R))): A profile of its use. Drugs. Ther. Perspect. 2022, 38, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. Mosquirix RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Bejon, P.; Lusingu, J.; Olotu, A.; Leach, A.; Lievens, M.; Vekemans, J.; Mshamu, S.; Lang, T.; Gould, J.; Dubois, M.C.; et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 2008, 359, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Eappen, A.G.; Li, T.; Marquette, M.; Chakravarty, S.; Kc, N.; Zanghi, G.; Hoffman, B.U.; Hettiarachchi, H.; Patil, A.; Abebe, Y.; et al. In vitro production of infectious Plasmodium falciparum sporozoites. Nature 2022, 612, 534–539. [Google Scholar] [CrossRef]
- Zavala, F.; Cochrane, A.H.; Nardin, E.H.; Nussenzweig, R.S.; Nussenzweig, V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J. Exp. Med. 1983, 157, 1947–1957. [Google Scholar] [CrossRef]
- Nardin, E.H.; Nussenzweig, V.; Nussenzweig, R.S.; Collins, W.E.; Harinasuta, K.T.; Tapchaisri, P.; Chomcharn, Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 1982, 156, 20–30. [Google Scholar] [CrossRef]
- Enea, V.; Ellis, J.; Zavala, F.; Arnot, D.E.; Asavanich, A.; Masuda, A.; Quakyi, I.; Nussenzweig, R.S. DNA cloning of Plasmodium falciparum circumsporozoite gene: Amino acid sequence of repetitive epitope. Science 1984, 225, 628–630. [Google Scholar] [CrossRef]
- Zavala, F.; Masuda, A.; Graves, P.M.; Nussenzweig, V.; Nussenzweig, R.S. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J. Immunol. 1985, 135, 2790–2793. [Google Scholar] [CrossRef]
- Vanderberg, J.P.; Frevert, U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 2004, 34, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Sidjanski, S.; Vanderberg, J.P. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am. J. Trop. Med. Hyg. 1997, 57, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Amino, R.; Thiberge, S.; Blazquez, S.; Baldacci, P.; Renaud, O.; Shorte, S.; Menard, R. Imaging malaria sporozoites in the dermis of the mammalian host. Nat. Protoc. 2007, 2, 1705–1712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamauchi, L.M.; Coppi, A.; Snounou, G.; Sinnis, P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007, 9, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Vanderberg, J.; Nussenzweig, R.; Most, H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil Med. 1969, 134, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.H.; Aikawa, M.; Jeng, M.; Nussenzweig, R.S. Antibody-induced ultrastructural changes of malarial sporozoites. J. Immunol. 1976, 116, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Hollingdale, M.R.; Nardin, E.H.; Tharavanij, S.; Schwartz, A.L.; Nussenzweig, R.S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J. Immunol. 1984, 132, 909–913. [Google Scholar] [CrossRef]
- Nardin, E.H.; Herrington, D.A.; Davis, J.; Levine, M.; Stuber, D.; Takacs, B.; Caspers, P.; Barr, P.; Altszuler, R.; Clavijo, P.; et al. Conserved repetitive epitope recognized by CD4+ clones from a malaria-immunized volunteer. Science 1989, 246, 1603–1606. [Google Scholar] [CrossRef]
- Moreno, A.; Clavijo, P.; Edelman, R.; Davis, J.; Sztein, M.; Herrington, D.; Nardin, E. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int. Immunol. 1991, 3, 997–1003. [Google Scholar] [CrossRef]
- Moreno, A.; Clavijo, P.; Edelman, R.; Davis, J.; Sztein, M.; Sinigaglia, F.; Nardin, E. CD4+ T cell clones obtained from Plasmodium falciparum sporozoite-immunized volunteers recognize polymorphic sequences of the circumsporozoite protein. J. Immunol. 1993, 151, 489–499. [Google Scholar] [CrossRef]
- Calvo-Calle, J.M.; Oliveira, G.A.; Nardin, E.H. Human CD4+ T cells induced by synthetic peptide malaria vaccine are comparable to cells elicited by attenuated Plasmodium falciparum sporozoites. J. Immunol. 2005, 175, 7575–7585. [Google Scholar] [CrossRef]
- Shafique, M.; Meijerhof, T.; Wilschut, J.; de Haan, A. Evaluation of an intranasal virosomal vaccine against respiratory syncytial virus in mice: Effect of TLR2 and NOD2 ligands on induction of systemic and mucosal immune responses. PLoS ONE 2013, 8, e61287. [Google Scholar] [CrossRef]
- Powell, T.J.; Jacobs, A.; Tang, J.; Cardenas, E.; Palath, N.; Daniels, J.; Boyd, J.G.; Bergeron, H.C.; Jorquera, P.A.; Tripp, R.A. Microparticle RSV Vaccines Presenting the G Protein CX3C Chemokine Motif in the Context of TLR Signaling Induce Protective Th1 Immune Responses and Prevent Pulmonary Eosinophilia Post-Challenge. Vaccines 2022, 10, 2078. [Google Scholar] [CrossRef]
- Shears, M.J.; Watson, F.N.; Stone, B.C.; Cruz Talavera, I.; Parthiban, C.; Matsubara, J.; Kc, N.; Sim, B.K.L.; Hoffman, S.L.; Murphy, S.C. Preliminary studies on the immunogenicity of a prime-and-trap malaria vaccine in nonhuman primates. Vaccine 2023, 41, 5494–5498. [Google Scholar] [CrossRef]
- Rainho-Tomko, J.N.; Pavot, V.; Kishko, M.; Swanson, K.; Edwards, D.; Yoon, H.; Lanza, L.; Alamares-Sapuay, J.; Osei-Bonsu, R.; Mundle, S.T.; et al. Immunogenicity and protective efficacy of RSV G central conserved domain vaccine with a prefusion nanoparticle. NPJ Vaccines 2022, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bao, L.; Deng, W.; Xu, L.; Li, F.; Lv, Q.; Yu, P.; Chen, T.; Xu, Y.; Zhu, H.; et al. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J. Infect. Dis. 2014, 209, 236–242. [Google Scholar] [CrossRef]
- Overstreet, M.G.; Cockburn, I.A.; Chen, Y.C.; Zavala, F. Protective CD8 T cells against Plasmodium liver stages: Immunobiology of an ’unnatural’ immune response. Immunol. Rev. 2008, 225, 272–283. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Kumar, K.A.; Calvo-Calle, J.M.; Othoro, C.; Altszuler, D.; Nussenzweig, V.; Nardin, E.H. Class II-restricted protective immunity induced by malaria sporozoites. Infect. Immun. 2008, 76, 1200–1206. [Google Scholar] [CrossRef]
- Chakravarty, S.; Cockburn, I.A.; Kuk, S.; Overstreet, M.G.; Sacci, J.B.; Zavala, F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 2007, 13, 1035–1041. [Google Scholar] [CrossRef]
- Kumar, K.A.; Sano, G.; Boscardin, S.; Nussenzweig, R.S.; Nussenzweig, M.C.; Zavala, F.; Nussenzweig, V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 2006, 444, 937–940. [Google Scholar] [CrossRef]
- Vigdorovich, V.; Patel, H.; Watson, A.; Raappana, A.; Reynolds, L.; Selman, W.; Beeman, S.; Edlefsen, P.T.; Kappe, S.H.I.; Sather, D.N. Coimmunization with preerythrocytic antigens alongside circumsporozoite protein can enhance sterile protection against Plasmodium sporozoite infection. Microbiol. Spectr. 2023, 11, e03791-22. [Google Scholar] [CrossRef]
- Watson, F.; Shears, M.; Matsubara, J.; Kalata, A.; Seilie, A.; Talavera, I.C.; Olsen, T.; Tsuji, M.; Chakravarty, S.; Sim, B.K.L.; et al. Cryopreserved Sporozoites with and without the Glycolipid Adjuvant 7DW8-5 Protect in Prime-and-Trap Malaria Vaccination. Am. J. Trop. Med. Hyg. 2022, 106, 1227–1236. [Google Scholar] [CrossRef]
- MacMillen, Z.; Hatzakis, K.; Simpson, A.; Shears, M.J.; Watson, F.; Erasmus, J.H.; Khandhar, A.P.; Wilder, B.; Murphy, S.C.; Reed, S.G.; et al. Accelerated prime-and-trap vaccine regimen in mice using repRNA-based CSP malaria vaccine. bioRxiv 2023. [Google Scholar] [CrossRef]
| Construct | Crosslinking | DP Shorthand |
|---|---|---|
| ACT-1198 | nXL | T1BT* |
| ACT-1199 | nXL | Pam3Cys.T1BT* |
| ACT-1200 | bXL | T1BT* |
| ACT-1201 | bXL | Pam3Cys.T1BT* |
| ACT-1236 | nXL | Pam3Cys.T1BT*(A) |
| ACT-1237 | bXL | Pam3Cys.T1BT*(A) |
| ACT-1238 | nXL | Pam3Cys.T1BT*(S) |
| ACT-1239 | bXL | Pam3Cys.T1BT*(S) |
| Day of Study | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 0 | 21 | 42 | 49 | 56 | 58 | 223 | 257 | 259 | 342 | 349 | 356 | 358 |
| 3 | 1° | 2° | 3° | ELISPOT | - | - | - | - | - | - | - | - | - |
| 10 | 1° | 2° | 3° | ELISA | PfPb | liver | - | - | - | - | - | - | - |
| 10 | 1° | 2° | 3° | ELISA | ELISA | PfPb | liver | - | - | - | - | ||
| 10 | 1° | 2° | 3° | ELISA | 4° | ELISA | PfPb | liver | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, T.J.; Tang, J.; Mitchell, R.; DeRome, M.E.; Jacobs, A.; Palath, N.; Cardenas, E.; Yorke, M.; Boyd, J.G.; Kaba, S.A.; et al. Immunogenicity, Efficacy, and Safety of a Novel Synthetic Microparticle Pre-Erythrocytic Malaria Vaccine in Multiple Host Species. Vaccines 2023, 11, 1789. https://doi.org/10.3390/vaccines11121789
Powell TJ, Tang J, Mitchell R, DeRome ME, Jacobs A, Palath N, Cardenas E, Yorke M, Boyd JG, Kaba SA, et al. Immunogenicity, Efficacy, and Safety of a Novel Synthetic Microparticle Pre-Erythrocytic Malaria Vaccine in Multiple Host Species. Vaccines. 2023; 11(12):1789. https://doi.org/10.3390/vaccines11121789
Chicago/Turabian StylePowell, Thomas J., Jie Tang, Robert Mitchell, Mary E. DeRome, Andrea Jacobs, Naveen Palath, Edwin Cardenas, Michelle Yorke, James G. Boyd, Stephen A. Kaba, and et al. 2023. "Immunogenicity, Efficacy, and Safety of a Novel Synthetic Microparticle Pre-Erythrocytic Malaria Vaccine in Multiple Host Species" Vaccines 11, no. 12: 1789. https://doi.org/10.3390/vaccines11121789
APA StylePowell, T. J., Tang, J., Mitchell, R., DeRome, M. E., Jacobs, A., Palath, N., Cardenas, E., Yorke, M., Boyd, J. G., Kaba, S. A., & Nardin, E. (2023). Immunogenicity, Efficacy, and Safety of a Novel Synthetic Microparticle Pre-Erythrocytic Malaria Vaccine in Multiple Host Species. Vaccines, 11(12), 1789. https://doi.org/10.3390/vaccines11121789





