Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Antigen Purification
2.3. Murine Peritoneal Exudate Cell (PEC) and Porcine PBMC Isolation
2.4. Cell Viability Assay
2.5. Enzyme-Linked Immune Absorbent Spot (ELISpot) Assay
2.6. Early Host Defense in Mice Administered with Bestatin Alone
2.7. FMDV Challenge after Immunization with the FMD Vaccine Containing Bestatin in Mice
2.8. Early, Intermediate-, and Long-Term Immune Responses in Mice after Immunization with the FMD Vaccine Containing Bestatin
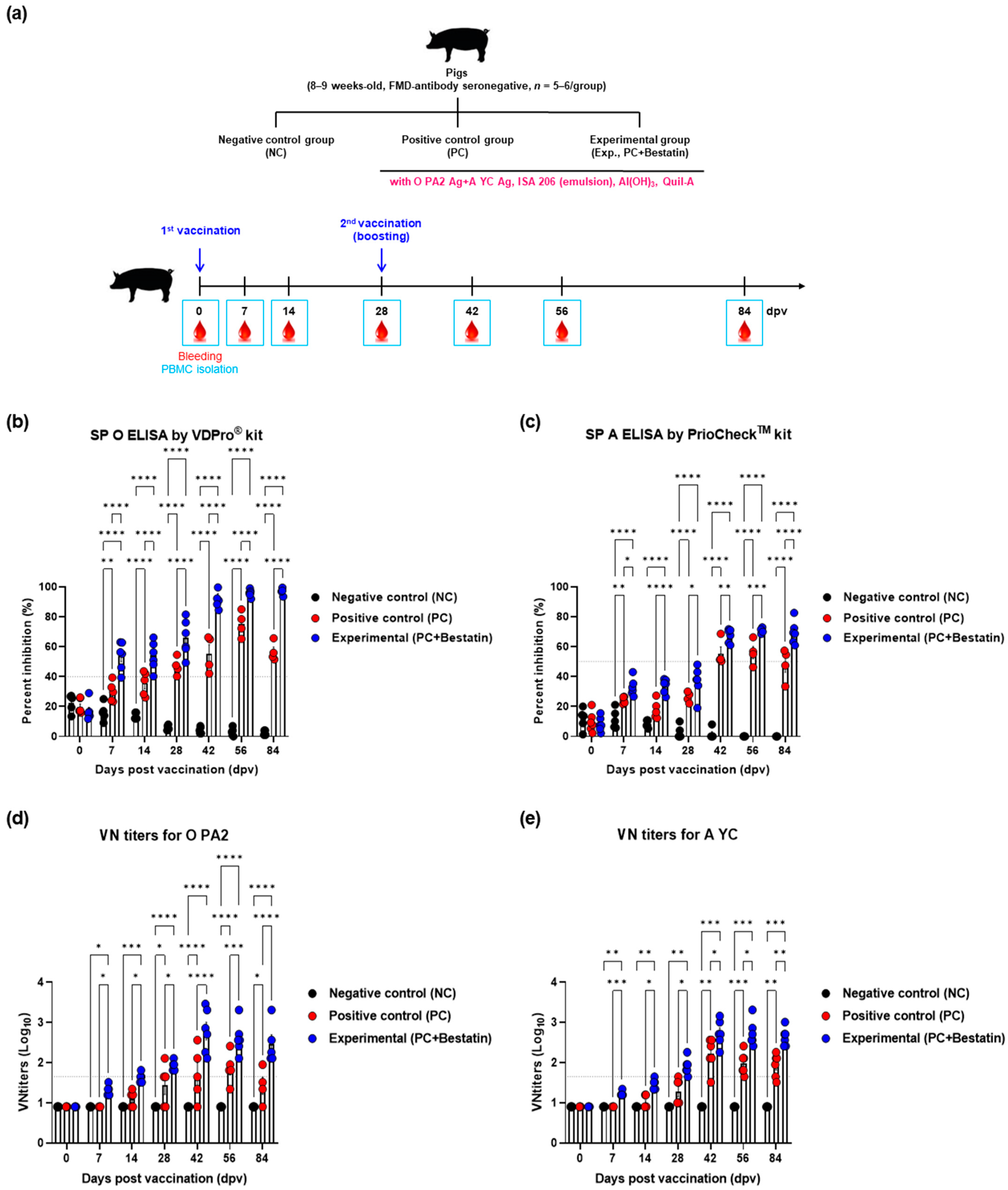
2.9. Early, Intermediate-, and Long-Term Immune Responses in Pigs after Immunization with the FMD Vaccine Containing Bestatin
2.10. SP ELISA
2.11. VN Test
2.12. ELISA for the Detection of Isotype-Specific Antibodies (IgG, IgA, and IgM)
2.13. RNA Extraction, Complementary DNA (cDNA) Synthesis, and RT-qPCR
2.14. Statistical Analysis
3. Results
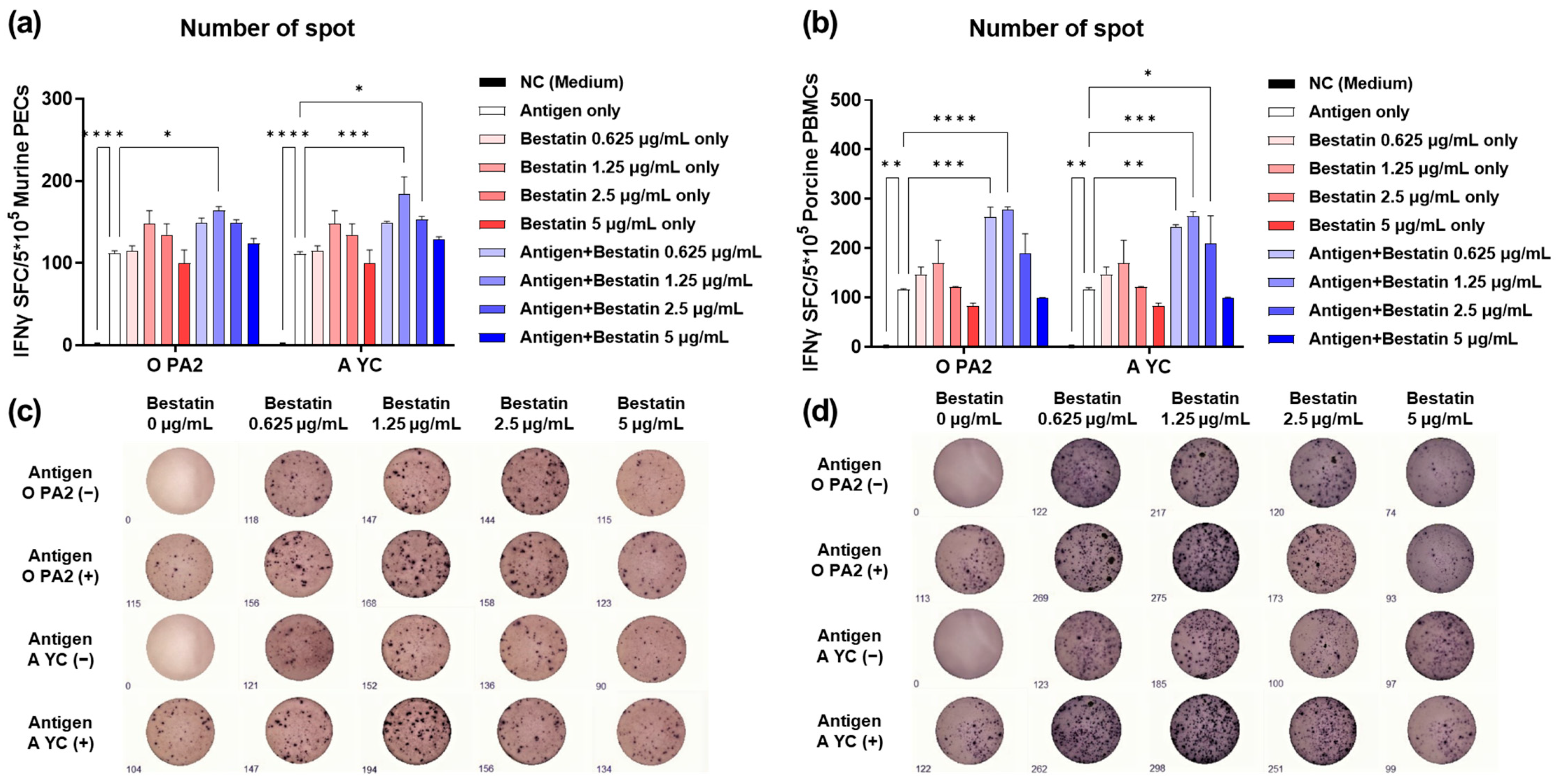
3.1. Bestatin Induces Robust IFNγ Secretion in Murine PECs and Porcine PBMCs In Vitro
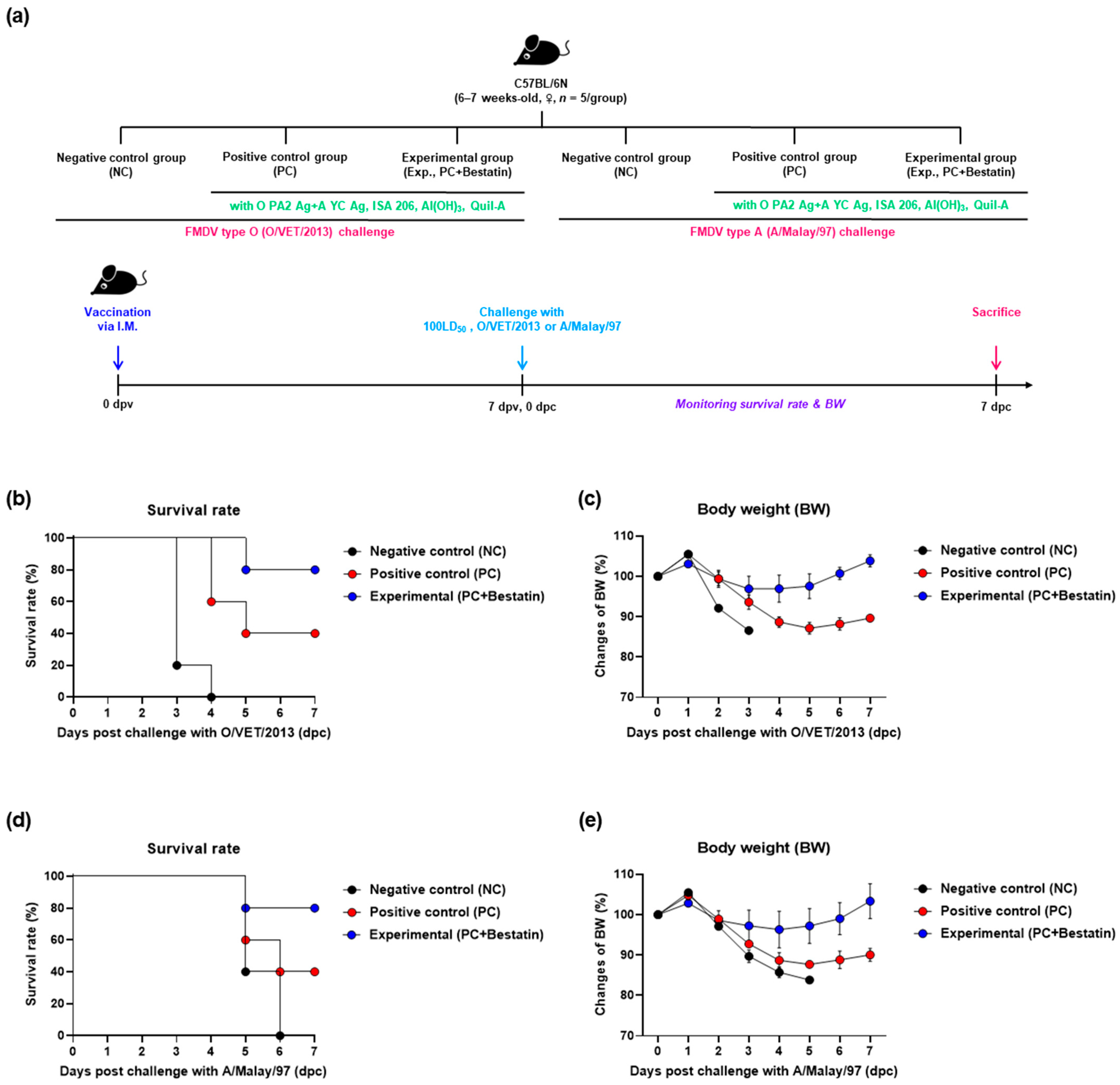
3.2. Inactivated FMD Vaccine Containing Bestatin Elicits Potent Protective Effects in the Early Stages of FMDV Infection in Mice
3.3. Bestatin Induces Early, Intermediate-, and Long-Term Immunity in Mice and Pigs
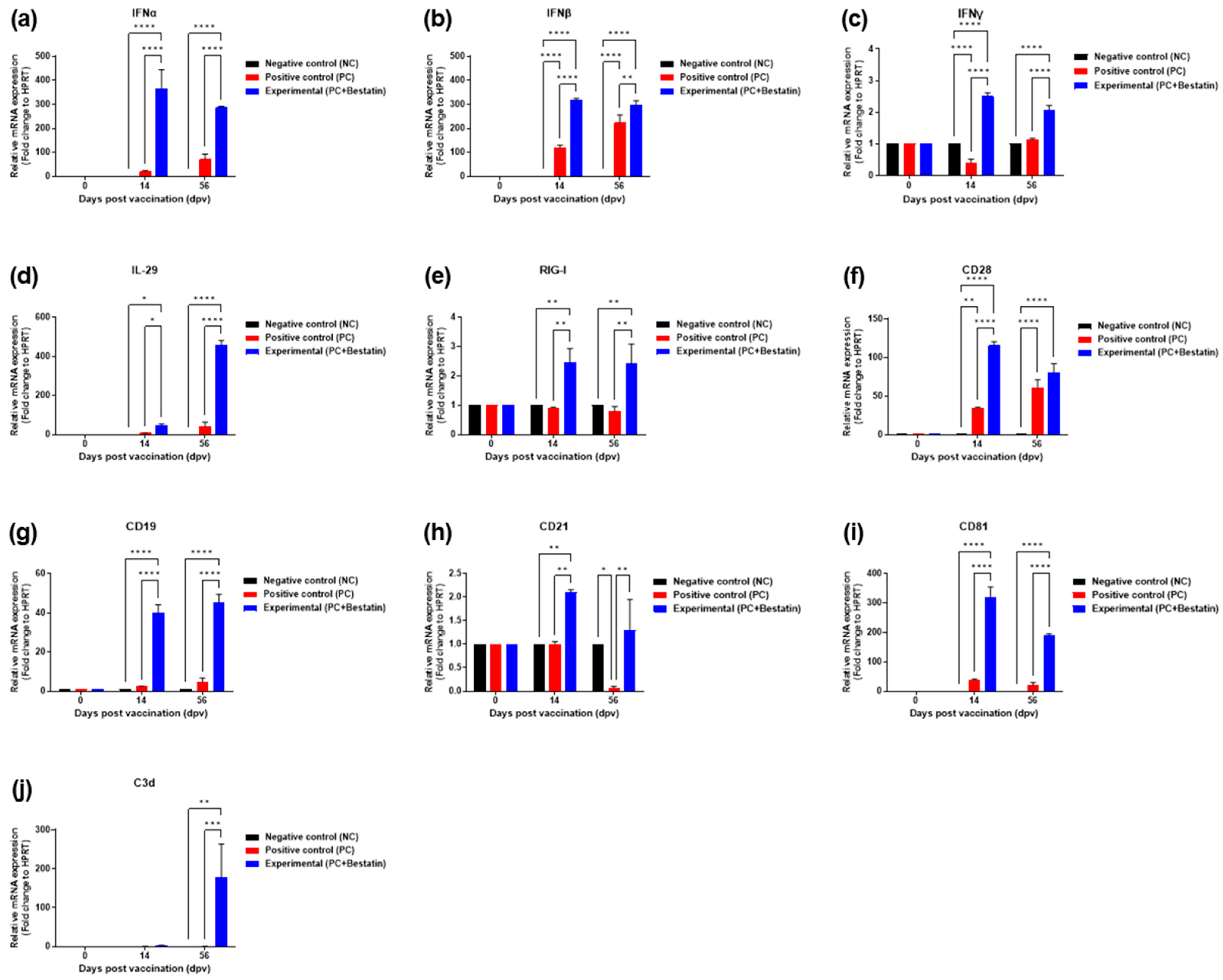
3.4. Bestatin Induces Robust and Long-Lasting Immune Responses by Enhancing the Expression of Immunomodulatory Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Wang, C.; Yang, F.; Cao, W.; Zhu, Z.; Zheng, H. Virus-host interactions in foot-and-mouth disease virus infection. Front. Immunol. 2021, 12, 571509. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lee, G.; Kim, A.; Park, J.H.; Lee, M.J.; Kim, B.; Kim, S.M. A vaccine strain of the A/ASIA/Sea-97 lineage of foot-and-mouth disease virus with a single amino acid substitution in the P1 region that is adapted to suspension culture provides high immunogenicity. Vaccines 2021, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Kim, B.Y.; Park, S.H.; Kim, H.M.; Shin, S.H.; Hwang, S.Y.; Kim, S.M.; Kim, B.; Park, J.H.; Lee, M.J. The HSP70-fused foot-and-mouth disease epitope elicits cellular and humoral immunity and drives broad-spectrum protective efficacy. npj Vaccines 2021, 6, 42. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, H.M.; Shin, S.; Jo, H.; Park, S.H.; Kim, S.M.; Park, J.H. The C3d-fused foot-and-mouth disease vaccine platform overcomes maternally derived antibody interference by inducing a potent adaptive immunity. npj Vaccines 2022, 7, 70. [Google Scholar] [CrossRef]
- Lee, M.J.; Jo, H.; Shin, S.H.; Kim, S.M.; Kim, B.; Shim, H.S.; Park, J.H. Mincle and STING-stimulating adjuvants elicit robust cellular immunity and drive long-lasting memory responses in a foot-and-mouth disease vaccine. Front. Immunol. 2019, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Knight-Jones, T.J.; Charleston, B.; Rodriguez, L.L.; Gay, C.G.; Sumption, K.J.; Vosloo, W. Global foot-and-mouth disease research update and gap analysis: 3-Vaccines. Transbound. Emerg. Dis. 2016, 63 (Suppl. 1), 30–41. [Google Scholar] [CrossRef]
- Park, J.H. Requirements for improved vaccines against foot-and-mouth disease epidemics. Clin. Exp. Vaccine Res. 2013, 2, 8–18. [Google Scholar] [CrossRef]
- Çokçalışkan, C.; Türkoğlu, T.; Sareyyüpoğlu, B.; Uzunlu, E.; Babak, A.; Özbilge, B.B.; Gülyaz, V. QS-21 enhances the early antibody response to oil adjuvant foot-and-mouth disease vaccine in cattle. Clin. Exp. Vaccine Res. 2016, 5, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, M.; Lalun, N.; Bobichon, H.; Plé, K.; Voutquenne-Nazabadioko, L. Structure-activity relationships of some hederagenin diglycosides: Haemolysis, cytotoxicity and apoptosis induction. Biochim. Biophys. Acta 2006, 1760, 1418–1427. [Google Scholar] [CrossRef]
- Cheong, Y.; Kim, M.; Ahn, J.; Oh, H.; Lim, J.; Chae, W.; Yang, S.W.; Kim, M.S.; Yu, J.E.; Byun, S.; et al. Epigallocatechin-3-gallate as a novel vaccine adjuvant. Front. Immunol. 2021, 12, 769088. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Natural and synthetic saponins as vaccine adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Ngo, H.X.; Garneau-Tsodikova, S. What are the drugs of the future? MedChemComm 2018, 9, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Ziadlou, R.; Lang, G.; Pfannkuche, J.; Cui, S.; Li, Z.; Richards, R.G.; Alini, M.; Grad, S. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics 2021, 11, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.V.; Gurevich, V.V. Therapeutic potential of small molecules and engineered proteins. Handb. Exp. Pharmacol. 2014, 219, 1–12. [Google Scholar] [CrossRef] [PubMed]
- John, S.P.; Singh, A.; Sun, J.; Pierre, M.J.; Alsalih, L.; Lipsey, C.; Traore, Z.; Balcom-Luker, S.; Bradfield, C.J.; Song, J.; et al. Small-molecule screening identifies Syk kinase inhibition and rutaecarpine as modulators of macrophage training and SARS-CoV-2 infection. Cell. Rep. 2022, 41, 111441. [Google Scholar] [CrossRef]
- Lkhagvaa, B.; Tani, K.; Sato, K.; Toyoda, Y.; Suzuka, C.; Sone, S. Bestatin, an inhibitor for aminopeptidases, modulates the production of cytokines and chemokines by activated monocytes and macrophages. Cytokine 2008, 44, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Handa, J.; Irie, Y.; Hagiwara, T.; Sakai, Y.; Hayashi, M.; Sakakibara, T.; Suzuki, M.; Irie, Y.; Horiguchi, M. Toxicological studies on bestatin. III. Chronic toxicity test and recovery study in beagle dogs. Jpn. J. Antibiot. 1983, 36, 3053–3193. [Google Scholar]
- Liu, S.; Xie, F.; Wang, H.; Liu, Z.; Liu, X.; Sun, L.; Niu, Z. Ubenimex inhibits cell proliferation, migration and invasion in renal cell carcinoma: The effect is autophagy-associated. Oncol. Rep. 2015, 33, 1372–1380. [Google Scholar] [CrossRef]
- Nakamura, K.; Fujiwara, H.; Higuchi, T.; Honda, T.; Yamada, S.; Nakayama, T.; Fujita, J.; Maeda, M.; Tachibana, T.; Suginami, H.; et al. Bestatin, an aminopeptidase inhibitor, promotes follicular growth and ovulation suppressed by stress in mice. Endocr. J. 1998, 45, 547–553. [Google Scholar] [CrossRef]
- Bauvois, B.; Dauzonne, D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects. Med. Res. Rev. 2006, 26, 88–130. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Fukushima, J.; Hamajima, K.; Ishii, N.; Tsuji, T.; Xin, K.Q.; Mohri, H.; Okuda, K. Adjuvant effect of ubenimex on a DNA vaccine for HIV-1. Clin. Exp. Immunol. 1998, 111, 30–35. [Google Scholar] [CrossRef]
- Tsunogake, S.; Furusawa, S.; Nagashima, S.; Nakamura, Y.; Enokihara, H.; Shishido, H. Effect of aminopeptidase inhibitors on the production of various cytokines by peripheral blood mononuclear cells and stromal cells and on stem cell factor gene expression in stromal cells: Comparison of ubenimex with its stereoisomers. Int. J. Immunother. 1994, 10, 41–47. [Google Scholar]
- Yamazaki, T.; Sugiyama, K.; Ichihara, K. Effect of ubenimex on the immune system of patients with hematological malignancies. Biomed. Pharmacother. 1991, 45, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Lenz, B.F.; Pennington, R.; Long, C.; Phillips, H.; Schneider, M.; Tribble, H. Immunomodulatory and therapeutic properties of bestatin in mice. Cancer Res. 1986, 46, 4505–4510. [Google Scholar]
- Florentin, I.; Kiger, N.; Bruley-Rosset, M.; Schults, J.; Mathe, G. Effect of seven immunomodulators on different types of immune responses in mice. In Hum Lymphocyte Differentiation; North Holland Biomedical Press: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Knoblich, A.; Müller, W.E.; Harle-Grupp, V.; Falke, D. Enhancement of antibody formation against herpes simplex virus in mice by the T-cell mitogen bestatin. J. Gen. Virol. 1984, 65, 1675–1686. [Google Scholar] [CrossRef]
- Jia, M.R.; Wei, T.; Xu, W.F. The analgesic activity of bestatin as a potent APN inhibitor. Front. Neurosci. 2010, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Mathé, G. Bestatin, an aminopeptidase inhibitor with a multi-pharmacological function. Biomed. Pharmacother. 1991, 45, 49–54. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, K.; Li, D.; Liu, K.; Jin, G.; Yan, M.; Wu, Q.; Chen, H.; Shin, S.H.; Bai, R.; et al. Inhibition of LTA4H by bestatin in human and mouse colorectal cancer. eBioMedicine 2019, 44, 361–374. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Q.; Jing, F.; Zhou, C.; Xiu, T.; Shi, Y.; Jing, F. Ubenimex suppresses the ability of migration and invasion in gastric cancer cells by alleviating the activity of the CD13/NAB1/MAPK pathway. Cancer Manag. Res. 2021, 13, 4483–4495. [Google Scholar] [CrossRef]
- Lee, M.J.; Jo, H.; Park, S.H.; Ko, M.K.; Kim, S.M.; Kim, B.; Park, J.H. Advanced foot-and-mouth disease vaccine platform for stimulation of simultaneous cellular and humoral immune responses. Vaccines 2020, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Ko, M.K.; Park, S.H.; Hwang, S.Y.; Kim, D.H.; Park, S.Y.; Ko, Y.J.; Kim, S.M.; Park, J.H.; Lee, M.J. Dectin-1 signaling coordinates innate and adaptive immunity for potent host defense against viral infection. Front. Immunol. 2023, 14, 1194502. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, H.G. Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch. Virol. 1975, 47, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.L.; Knowles, N.J.; Paton, D.J.; Barnett, P.V. Marker vaccine potential of a foot-and-mouth disease virus with a partial VP1 G-H loop deletion. Vaccine 2010, 28, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; El-Sayed, A.; Castañeda Vazquez, H. Foot-and-mouth disease vaccines: Recent updates and future perspectives. Arch. Virol. 2019, 164, 1501–1513. [Google Scholar] [CrossRef]
- Lim, Y.T. Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease. Clin. Exp. Vaccine Res. 2015, 4, 54–58. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Lamprecht, A. TLR4-based immunotherapeutics in cancer: A review of the achievements and shortcomings. Mol. Pharm. 2018, 15, 4777–4800. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, R.; Chhabra, R. Interleukin-2 potentiates foot-and-mouth disease vaccinal immune responses in mice. Vaccine 2005, 23, 3005–3009. [Google Scholar] [CrossRef]
- Liu, W.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Characterization of co-stimulatory ligand CD80/86 and its effect as a molecular adjuvant on DNA vaccine against Vibrio anguillarum in flounder (Paralichthys olivaceus). Front. Immunol. 2022, 13, 881753. [Google Scholar] [CrossRef]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Leggatt, G.; Waterhouse, N.; Frazer, I.H. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017, 8, e2836. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef]
- Baxter, V.K.; Griffin, D.E. Interferon-gamma modulation of the local T cell response to Alphavirus encephalomyelitis. Viruses 2020, 12, 113. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Raulf-Heimsoth, M. T cell—primary culture from peripheral blood. Methods Mol. Med. 2008, 138, 17–30. [Google Scholar] [CrossRef]
- Black, L.; Francis, M.J.; Rweyemamu, M.M.; Umebara, O.; Boge, A. The relationship between serum antibody titres and protection from foot and mouth disease in pigs after oil emulsion vaccination. J. Biol. Stand. 1984, 12, 379–389. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Chathuranga, W.A.G.; Shim, Y.J.; Haluwana, D.K.; Kim, E.H.; Yoon, I.J.; Lim, Y.T.; Shin, S.H.; Jo, H.; Hwang, S.Y.; et al. The potential adjuvanticity of CAvant®SOE for foot-and-mouth disease vaccine. Vaccines 2021, 9, 1091. [Google Scholar] [CrossRef]
- Hertzog, P.J.; Williams, B.R. Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 2013, 24, 217–225. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef]
- Kell, A.M.; Gale, M., Jr. RIG-I in RNA virus recognition. Virology 2015, 479–480, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Vilcek, J. Novel interferons. Nat. Immunol. 2003, 4, 8–9. [Google Scholar] [CrossRef]
- Kelm, N.E.; Zhu, Z.; Ding, V.A.; Xiao, H.; Wakefield, M.R.; Bai, Q.; Fang, Y. The role of IL-29 in immunity and cancer. Crit. Rev. Oncol. Hematol. 2016, 106, 91–98. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Durbin, J.E. Contribution of type III interferons to antiviral immunity: Location, location, location. J. Biol. Chem. 2017, 292, 7295–7303. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Woo, S.R.; Burnett, B.; Fu, Y.X.; Gajewski, T.F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013, 34, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Schnepf, D.; Staeheli, P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 2019, 19, 614–625. [Google Scholar] [CrossRef]
- Kelly, A.; Robinson, M.W.; Roche, G.; Biron, C.A.; O’Farrelly, C.; Ryan, E.J. Immune cell profiling of IFN-λ response shows pDCs express highest level of IFN-λR1 and are directly responsive via the JAK-STAT pathway. J. Interferon Cytokine Res. 2016, 36, 671–680. [Google Scholar] [CrossRef]
- Megjugorac, N.J.; Gallagher, G.E.; Gallagher, G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29). J. Leukoc. Biol. 2009, 86, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Huang, A.F.; Xu, W.D.; Su, L.C. Insights into IL-29: Emerging role in inflammatory autoimmune diseases. J. Cell. Mol. Med. 2019, 23, 7926–7932. [Google Scholar] [CrossRef]
- Ye, L.; Schnepf, D.; Becker, J.; Ebert, K.; Tanriver, Y.; Bernasconi, V.; Gad, H.H.; Hartmann, R.; Lycke, N.; Staeheli, P. Interferon-λ enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat. Immunol. 2019, 20, 593–601. [Google Scholar] [CrossRef]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Gauld, S.B.; Dal Porto, J.M.; Cambier, J.C. B cell antigen receptor signaling: Roles in cell development and disease. Science 2002, 296, 1641–1642. [Google Scholar] [CrossRef]
- Masilamani, M.; Kassahn, D.; Mikkat, S.; Glocker, M.O.; Illges, H. B cell activation leads to shedding of complement receptor type II (CR2/CD21). Eur. J. Immunol. 2003, 33, 2391–2397. [Google Scholar] [CrossRef]
- Bradbury, L.E.; Kansas, G.S.; Levy, S.; Evans, R.L.; Tedder, T.F. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J. Immunol. 1992, 149, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Feest, C.; Depoil, D.; Treanor, B.; Montaner, B.; Otipoby, K.L.; Carter, R.; Justement, L.B.; Bruckbauer, A.; Batista, F.D. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity 2013, 38, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Susa, K.J.; Seegar, T.C.; Blacklow, S.C.; Kruse, A.C. A dynamic interaction between CD19 and the tetraspanin CD81 controls B cell co-receptor trafficking. eLife 2020, 9, e52337. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004, 5, 981–986. [Google Scholar] [CrossRef]
- Fearon, D.T. The complement system and adaptive immunity. Semin. Immunol. 1998, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.W.; Allison, M.E.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef]
- De Groot, A.S.; Ross, T.M.; Levitz, L.; Messitt, T.J.; Tassone, R.; Boyle, C.M.; Vincelli, A.J.; Moise, L.; Martin, W.; Knopf, P.M. C3d adjuvant effects are mediated through the activation of C3d-specific autoreactive T cells. Immunol. Cell Biol. 2015, 93, 189–197. [Google Scholar] [CrossRef]
- Kovács, K.G.; Mácsik-Valent, B.; Matkó, J.; Bajtay, Z.; Erdei, A. Revisiting the coreceptor function of complement receptor type 2 (Cr2, Cd21); Coengagement with the B-cell receptor inhibits the activation, proliferation, and antibody production of human B cells. Front. Immunol. 2021, 12, 620427. [Google Scholar] [CrossRef] [PubMed]
- Hainline, K.M.; Shores, L.S.; Votaw, N.L.; Bernstein, Z.J.; Kelly, S.H.; Fries, C.N.; Madhira, M.S.; Gilroy, C.A.; Chilkoti, A.; Collier, J.H. Modular complement assemblies for mitigating inflammatory conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2018627118. [Google Scholar] [CrossRef] [PubMed]
- Gorham, R.D.; Nuñez, V.; Lin, J.H.; Rooijakkers, S.H.; Vullev, V.I.; Morikis, D. Discovery of small molecules for fluorescent detection of complement activation product C3d. J. Med. Chem. 2015, 58, 9535–9545. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.W.; Ko, M.-K.; Park, S.H.; Shin, S.; Kim, S.-M.; Park, J.-H.; Lee, M.J. Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines. Vaccines 2023, 11, 1690. https://doi.org/10.3390/vaccines11111690
Kim HW, Ko M-K, Park SH, Shin S, Kim S-M, Park J-H, Lee MJ. Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines. Vaccines. 2023; 11(11):1690. https://doi.org/10.3390/vaccines11111690
Chicago/Turabian StyleKim, Hyeong Won, Mi-Kyeong Ko, So Hui Park, Seokwon Shin, Su-Mi Kim, Jong-Hyeon Park, and Min Ja Lee. 2023. "Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines" Vaccines 11, no. 11: 1690. https://doi.org/10.3390/vaccines11111690
APA StyleKim, H. W., Ko, M.-K., Park, S. H., Shin, S., Kim, S.-M., Park, J.-H., & Lee, M. J. (2023). Bestatin, A Pluripotent Immunomodulatory Small Molecule, Drives Robust and Long-Lasting Immune Responses as an Adjuvant in Viral Vaccines. Vaccines, 11(11), 1690. https://doi.org/10.3390/vaccines11111690





