Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Study Procedures
2.3. Anti-SARS-CoV-2 Antibody Testing
2.4. IFN-γ-Specific T-Cell Response against SARS-CoV-2
2.5. Statistical Analysis
3. Results
3.1. Patients
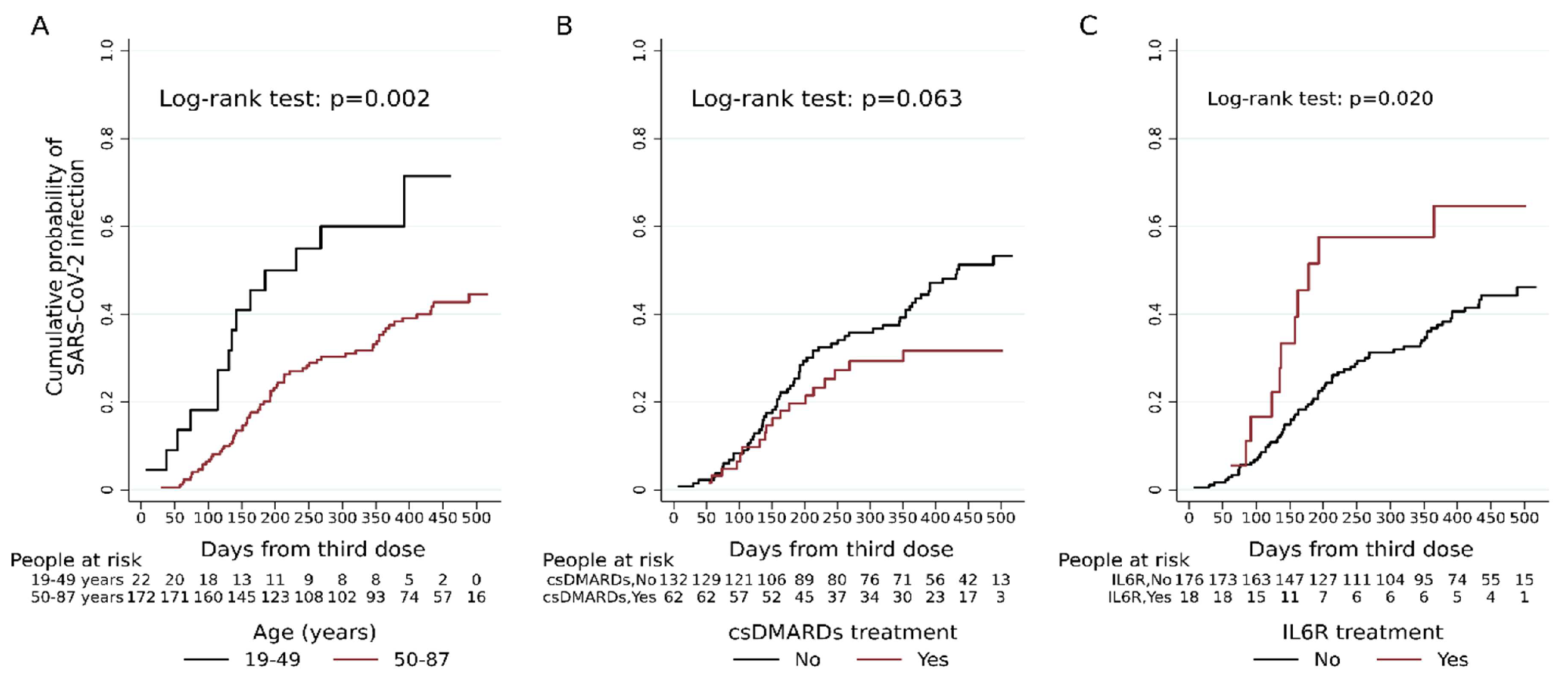
3.2. Risk of Breakthrough Infections and Clinical Parameters
3.3. Risk of Breakthrough Infection and B and T Cell Immune-Specific Responses
3.4. Severity of Breakthrough Infection
3.5. Comparison between the Risk of Breakthrough Infection in RA and HCWs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 5 September 2023).
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 Vaccination Induces Immunological T Cell Memory Able to Cross-Recognize Variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.-D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Trentini, F.; Petrone, D.; Mammone, A.; Ambrosio, L.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Zardini, A.; et al. Tracking the Progressive Spread of the SARS-CoV-2 Omicron Variant in Italy, December 2021 to January 2022. Eurosurveillance 2022, 27, 2200125. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Dayam, R.M.; Law, J.C.; Goetgebuer, R.L.; Chao, G.Y.; Abe, K.T.; Sutton, M.; Finkelstein, N.; Stempak, J.M.; Pereira, D.; Croitoru, D.; et al. Accelerated Waning of Immunity to SARS-CoV-2 mRNA Vaccines in Patients with Immune-Mediated Inflammatory Diseases. JCI Insight 2022, 7, e159721. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised (accessed on 5 September 2023).
- Pablos, J.L.; Galindo, M.; Carmona, L.; Lledó, A.; Retuerto, M.; Blanco, R.; Gonzalez-Gay, M.A.; Martinez-Lopez, D.; Castrejón, I.; Alvaro-Gracia, J.M.; et al. Clinical Outcomes of Hospitalised Patients with COVID-19 and Chronic Inflammatory and Autoimmune Rheumatic Diseases: A Multicentric Matched Cohort Study. Ann. Rheum. Dis. 2020, 79, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Grainger, R.; Kim, A.H.J.; Conway, R.; Yazdany, J.; Robinson, P.C. COVID-19 in People with Rheumatic Diseases: Risks, Outcomes, Treatment Considerations. Nat. Rev. Rheumatol. 2022, 18, 191–204. [Google Scholar] [CrossRef]
- Landewé, R.B.M.; Kroon, F.P.B.; Alunno, A.; Najm, A.; Bijlsma, J.W.; Burmester, G.-R.R.; Caporali, R.; Combe, B.; Conway, R.; Curtis, J.R.; et al. EULAR Recommendations for the Management and Vaccination of People with Rheumatic and Musculoskeletal Diseases in the Context of SARS-CoV-2: The November 2021 Update. Ann. Rheum. Dis. 2022, 81, 1628–1639. [Google Scholar] [CrossRef]
- Tang, K.-T.; Hsu, B.-C.; Chen, D.-Y. Immunogenicity, Effectiveness, and Safety of COVID-19 Vaccines in Rheumatic Patients: An Updated Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 834. [Google Scholar] [CrossRef]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar-M, P.; Sharma, A.; Patel, Y.I.; Kennedy, N.A.; Kim, A.H.J.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 Vaccination in Immune Mediated Inflammatory Diseases: Systematic Review and Meta-Analysis. Autoimmun. Rev. 2022, 21, 102927. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Aiello, A.; Laganà, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Farroni, C.; Lapa, D.; Najafi Fard, S.; Cuzzi, G.; et al. ImmunosuppressiveTherapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 mRNA Vaccine in Rheumatoid Arthritis Patients. Front. Immunol. 2021, 12, 740249. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Bohnert, A.S.B.; O’Hare, A.M.; Boyko, E.J.; Maciejewski, M.L.; Smith, V.A.; Bowling, C.B.; Viglianti, E.; Iwashyna, T.J.; Hynes, D.M.; et al. Effectiveness of mRNA COVID-19 Vaccine Boosters Against Infection, Hospitalization, and Death: A Target Trial Emulation in the Omicron (B.1.1.529) Variant Era. Ann. Intern. Med. 2022, 175, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Perry, J.; Osman, S.; Wright, J.; Richard-Greenblatt, M.; Buchan, S.A.; Sadarangani, M.; Bolotin, S. Does a Humoral Correlate of Protection Exist for SARS-CoV-2? A Systematic Review. PLoS ONE 2022, 17, e0266852. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Meschi, S.; Matusali, G.; Colavita, F.; Lapa, D.; Bordi, L.; Puro, V.; Leoni, B.D.; Galli, C.; Capobianchi, M.R.; Castilletti, C.; et al. Predicting the Protective Humoral Response to a SARS-CoV-2 mRNA Vaccine. Clin. Chem. Lab. Med. 2021, 59, 2010–2018. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Santoro, A.; Capri, A.; Petrone, D.; Colavita, F.; Meschi, S.; Matusali, G.; Mizzoni, K.; Notari, S.; Agrati, C.; Goletti, D.; et al. SARS-CoV-2 Breakthrough Infections According to the Immune Response Elicited after mRNA Third Dose Vaccination in COVID-19-Naïve Hospital Personnel. Biomedicines 2023, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Picchianti Diamanti, A.; Navarra, A.; Cuzzi, G.; Aiello, A.; Salemi, S.; Di Rosa, R.; De Lorenzo, C.; Vio, D.; Sebastiani, G.; Ferraioli, M.; et al. The Third Dose of BNT162b2 COVID-19 Vaccine Does Not “Boost” Disease Flares and Adverse Events in Patients with Rheumatoid Arthritis. Biomedicines 2023, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Najafi Fard, S.; Petruccioli, E.; Petrone, L.; Vanini, V.; Farroni, C.; Cuzzi, G.; Navarra, A.; Gualano, G.; Mosti, S.; et al. Spike Is the Most Recognized Antigen in the Whole-Blood Platform in Both Acute and Convalescent COVID-19 Patients. Int. J. Infect. Dis. 2021, 106, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Colavita, F.; Lapa, D.; Meschi, S.; Bordi, L.; Piselli, P.; Gagliardini, R.; Corpolongo, A.; Nicastri, E.; Antinori, A.; et al. SARS-CoV-2 Serum Neutralization Assay: A Traditional Tool for a Brand-New Virus. Viruses 2021, 13, 655. [Google Scholar] [CrossRef]
- Dunkler, D.; Ploner, M.; Schemper, M.; Heinze, G. Weighted Cox Regression Using the R Package Coxphw. J. Stat. Softw. 2018, 84, 1–26. [Google Scholar] [CrossRef]
- CDC. Interim Guidelines for COVID-19 Antibody Testing in Clinical and Public Health Settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html (accessed on 5 September 2023).
- Machado, P.M.; Lawson-Tovey, S.; Strangfeld, A.; Mateus, E.F.; Hyrich, K.L.; Gossec, L.; Carmona, L.; Rodrigues, A.; Raffeiner, B.; Duarte, C.; et al. Safety of Vaccination against SARS-CoV-2 in People with Rheumatic and Musculoskeletal Diseases: Results from the EULAR Coronavirus Vaccine (COVAX) Physician-Reported Registry. Ann. Rheum. Dis. 2022, 81, 695–709. [Google Scholar] [CrossRef]
- Zaccardelli, A.; Wallace, Z.S.; Sparks, J.A. Acute and Postacute COVID-19 Outcomes for Patients with Rheumatoid Arthritis: Lessons Learned and Emerging Directions 3 Years into the Pandemic. Curr. Opin. Rheumatol. 2023, 35, 175–184. [Google Scholar] [CrossRef]
- Li, H.; Wallace, Z.S.; Sparks, J.A.; Lu, N.; Wei, J.; Xie, D.; Wang, Y.; Zeng, C.; Lei, G.; Zhang, Y. Risk of COVID-19 Among Unvaccinated and Vaccinated Patients With Rheumatoid Arthritis: A General Population Study. Arthritis Care Res. 2023, 75, 956–966. [Google Scholar] [CrossRef]
- Cordtz, R.; Kristensen, S.; Westermann, R.; Duch, K.; Pearce, F.; Lindhardsen, J.; Torp-Pedersen, C.; Andersen, M.P.; Dreyer, L. COVID-19 Infection and Hospitalisation Risk According to Vaccination Status and DMARD Treatment in Patients with Rheumatoid Arthritis. Rheumatology 2022, 62, 77–88. [Google Scholar] [CrossRef]
- Kawano, Y.; Patel, N.J.; Wang, X.; Cook, C.E.; Vanni, K.M.; Kowalski, E.N.; Banasiak, E.P.; Qian, G.; DiIorio, M.; Hsu, T.Y.-T.; et al. Temporal Trends in COVID-19 Outcomes among Patients with Systemic Autoimmune Rheumatic Diseases: From the First Wave through the Initial Omicron Wave. Ann. Rheum. Dis. 2022, 81, 1742–1749. [Google Scholar] [CrossRef]
- CDC. Estimated COVID-19 Burden. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html (accessed on 5 September 2023).
- Md Yusof, M.Y.; Arnold, J.; Saleem, B.; Vandevelde, C.; Dass, S.; Savic, S.; Vital, E.M.; Emery, P. Breakthrough SARS-CoV-2 Infections and Prediction of Moderate-to-Severe Outcomes during Rituximab Therapy in Patients with Rheumatic and Musculoskeletal Diseases in the UK: A Single-Centre Cohort Study. Lancet Rheumatol. 2023, 5, e88–e98. [Google Scholar] [CrossRef]
- Avouac, J.; Drumez, E.; Hachulla, E.; Seror, R.; Georgin-Lavialle, S.; El Mahou, S.; Pertuiset, E.; Pham, T.; Marotte, H.; Servettaz, A.; et al. COVID-19 Outcomes in Patients with Inflammatory Rheumatic and Musculoskeletal Diseases Treated with Rituximab: A Cohort Study. Lancet Rheumatol. 2021, 3, e419–e426. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.; Mrdenovic, N.; Scheicht, D.; Pons-Kühnemann, J.; Scheibelhut, C.; Strunk, J. Humoral Immunogenicity of COVID-19 Vaccines in Patients with Inflammatory Rheumatic Diseases under Treatment with Rituximab: A Case-Control Study (COVID-19VacRTX). Rheumatology 2022, 61, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.; Montgomery, A.; Dalbeth, N.; Sapsford, M.; Ngan Kee, R.; Cooper, A.; Quincey, V.; Bhana, S.; Gore-Massy, M.; Hausmann, J.; et al. Omicron Variant Infection in Inflammatory Rheumatological Conditions—Outcomes from a COVID-19 Naive Population in Aotearoa New Zealand. Lancet Reg. Health West Pac. 2023, 38, 100843. [Google Scholar] [CrossRef] [PubMed]
- Doran, M.F.; Crowson, C.S.; Pond, G.R.; O’Fallon, W.M.; Gabriel, S.E. Frequency of Infection in Patients with Rheumatoid Arthritis Compared with Controls: A Population-Based Study. Arthritis Rheum. 2002, 46, 2287–2293. [Google Scholar] [CrossRef]
- Mehta, B.; Pedro, S.; Ozen, G.; Kalil, A.; Wolfe, F.; Mikuls, T.; Michaud, K. Serious Infection Risk in Rheumatoid Arthritis Compared with Non-Inflammatory Rheumatic and Musculoskeletal Diseases: A US National Cohort Study. RMD Open 2019, 5, e000935. [Google Scholar] [CrossRef] [PubMed]
- Picchianti Diamanti, A.; Rosado, M.M.; Nicastri, E.; Sesti, G.; Pioli, C.; Laganà, B. Severe Acute Respiratory Syndrome Coronavirus-2 Infection and Autoimmunity 1 Year Later: The Era of Vaccines. Front. Immunol. 2021, 12, 708848. [Google Scholar] [CrossRef] [PubMed]
- Rondaan, C.; Furer, V.; Heijstek, M.W.; Agmon-Levin, N.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; van Laar, J.M.; et al. Efficacy, Immunogenicity and Safety of Vaccination in Adult Patients with Autoimmune Inflammatory Rheumatic Diseases: A Systematic Literature Review for the 2019 Update of EULAR Recommendations. RMD Open 2019, 5, e001035. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, Y.J.; Shin, K.; Ha, Y.-J.; Lee, E.Y.; Song, Y.W.; Choi, Y.; Winthrop, K.L.; Lee, E.B. Impact of Temporary Methotrexate Discontinuation for 2 Weeks on Immunogenicity of Seasonal Influenza Vaccination in Patients with Rheumatoid Arthritis: A Randomised Clinical Trial. Ann. Rheum. Dis. 2018, 77, 898–904. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, M.A.; Lee, E.Y.; Song, Y.W.; Choi, Y.; Winthrop, K.L.; Lee, E.B. Effect of Methotrexate Discontinuation on Efficacy of Seasonal Influenza Vaccination in Patients with Rheumatoid Arthritis: A Randomised Clinical Trial. Ann. Rheum. Dis. 2017, 76, 1559–1565. [Google Scholar] [CrossRef]
- Abhishek, A.; Boyton, R.; McKnight, Á.; Coates, L.; Bluett, J.; Barber, V.S.; Cureton, L.; Francis, A.; Appelbe, D.; Eldridge, L.; et al. Effects of Temporarily Suspending Low-Dose Methotrexate Treatment for 2 Weeks after SARS-CoV-2 Vaccine Booster on Vaccine Response in Immunosuppressed Adults with Inflammatory Conditions: Protocol for a Multicentre Randomised Controlled Trial and Nested Mechanistic Substudy (Vaccine Response On/Off Methotrexate (VROOM) Study). BMJ Open 2022, 12, e062599. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Boyton, R.J.; Peckham, N.; McKnight, Á.; Coates, L.C.; Bluett, J.; Barber, V.; Cureton, L.; Francis, A.; Appelbe, D.; et al. Effect of a 2-Week Interruption in Methotrexate Treatment versus Continued Treatment on COVID-19 Booster Vaccine Immunity in Adults with Inflammatory Conditions (VROOM Study): A Randomised, Open Label, Superiority Trial. Lancet Respir. Med. 2022, 10, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Al-Haideri, M.T.; Mannani, R.; Kaboli, R.; Gharebakhshi, F.; Darvishzadehdeldari, S.; Tahmasebi, S.; Faramarzi, F.; Cotrina-Aliaga, J.C.; Khorasani, S.; Alimohammadi, M.; et al. The Effects of Methotrexate on the Immune Responses to the COVID-19 Vaccines in the Patients with Immune-Mediated Inflammatory Disease: A Systematic Review of Clinical Evidence. Transpl. Immunol. 2023, 79, 101858. [Google Scholar] [CrossRef] [PubMed]
- Bitoun, S.; Nocturne, G.; Ly, B.; Krzysiek, R.; Roques, P.; Pruvost, A.; Paoletti, A.; Pascaud, J.; Dönnes, P.; Florence, K.; et al. Methotrexate and BAFF Interaction Prevents Immunization against TNF Inhibitors. Ann. Rheum. Dis. 2018, 77, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and Safety of the BNT162b2 mRNA COVID-19 Vaccine in Adult Patients with Autoimmune Inflammatory Rheumatic Diseases and in the General Population: A Multicentre Study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The Origins, Function, and Regulation of T Follicular Helper Cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef]
- Kim, W.-J.; Choi, S.-H.; Park, J.Y.; Song, J.S.; Chung, J.-W.; Choi, S.T. SARS-CoV-2 Omicron Escapes mRNA Vaccine Booster-Induced Antibody Neutralisation in Patients with Autoimmune Rheumatic Diseases: An Observational Cohort Study. Ann. Rheum. Dis. 2022, 81, 1585–1593. [Google Scholar] [CrossRef]

| Patients’ Characteristics | RA Patients |
|---|---|
| Total | 194 (100) |
| Age, median (IQR) years | 63 (54–71) |
| Age class, n (%) | |
| 19–49 | 22 (11.3) |
| 50–59 | 49 (25.3) |
| 60–69 | 66 (34.0) |
| 70–87 | 57 (29.4) |
| Gender: Female, n (%) | 137 (70.6) |
| Presence of comorbidities, n (%) | 130 (67.0) |
| No | 64 (33.0) |
| Yes, one | 78 (40.2) |
| Yes, two or more | 52 (26.8) |
| BMI, median (IQR) | 26.9 (23.3–28.4) |
| Smoking habits, n (%) | 39 (20.1) |
| Flu vaccination (2021/2022), n (%) | 112 (57.7) |
| Vaccination booster type, n (%) | |
| Comirnaty | 190 (97.9) |
| Spikevax/other | 4 (2.1) |
| Rheumatoid arthritis disease | |
| Disease duration, median (IQR) months | 120 (72–186) |
| Immunosuppressive drugs; regimens containing: | |
| Conventional DMARDs, n (%) | 62 (32.0) |
| TNFα-i, n (%) | 39 (20.1) |
| IL6R-i, n (%) | 18 (9.3) |
| CTLA4-Ig, n (%) | 38 (19.6) |
| JAK-i, n (%) | 49 (25.3) |
| CD20-i, n (%) | 11 (5.7) |
| Corticosteroids, n (%) | 33 (17.1) |
| Immunogenicity data, Samples available, n (%) | 41 (21.1) |
| Days from booster dose to sample, median (IQR) | 28 (28–73) |
| Among 82 RA patients with breakthrough infection | |
| Days from 3rd dose to infection, median (IQR) | 176 (118–268) |
| Early therapy with antivirals or monoclonal antibodies, n (%) | |
| Yes | 22 (26.8) |
| No | 60 (73.2) |
| Severity of COVID-19 disease, n (%) | |
| Asymptomatic | 8 (9.8) |
| Pauci-symptomatic | 67 (81.7) |
| Hospitalised * | 7 (8.5) |
| Characteristics | Uninfected | Breakthrough Infection | Cox Regression | |||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||||
| HR | 95% CI | p | aHR | 95% CI | p | |||
| Overall | 112 (57.7) | 82 (42.3) | ||||||
| Age in years, median (IQR) | 66 (57–72) | 62 (53–71) | 0.74 * | 0.61–0.90 | 0.003 | |||
| Age class under/over 50 years, n (%) | ||||||||
| 19–49 | 7 (31.8) | 15 (68.2) | 1 | 1 | ||||
| 50–87 | 105 (61.0) | 67 (39.0) | 0.41 | 0.24–0.73 | 0.002 | 0.38 | 0.20–0.74 | 0.004 |
| Gender, n (%) | ||||||||
| Male | 30 (52.6) | 27 (47.4) | 1 | |||||
| Female | 82 (59.9) | 55 (40.1) | 1.14 | 0.72–1.81 | 0.568 | |||
| Presence of comorbidities, n (%) | ||||||||
| No | 30 (46.9) | 34 (53.1) | 1 | |||||
| Yes | 82 (63.1) | 48 (36.9) | 0.59 | 0.38–0.91 | 0.018 | 0.68 | 0.43–1.10 | 0.113 |
| BMI | 27 (23.5–28.6) | 26 (22.7–28) | 0.94 | 0.89–1.00 | 0.050 | 0.96 | 0.90–1.02 | 0.218 |
| Smoking habits, n (%) | ||||||||
| No | 94 (60.7) | 61 (39.4) | 1 | 1 | ||||
| Yes | 18 (46.2) | 21 (53.8) | 1.68 | 1.02–2.76 | 0.040 | 1.61 | 0.97–2.68 | 0.066 |
| Flu vaccination (season 2021/2022), n(%) | ||||||||
| No | 46 (56.1) | 36 (43.9) | 1 | |||||
| Yes | 66 (58.9) | 46 (41.1) | 0.92 | 0.59–1.42 | 0.703 | |||
| Third-dose vaccine, n (%) | ||||||||
| Comirnaty | 110 (57.9) | 80 (42.1) | 1 | |||||
| Spikevax/other | 2 (50.0) | 2 (50.0) | 1.03 | 0.25–4.19 | 0.967 | |||
| Rheumatoid arthritis disease | ||||||||
| Disease duration in months, median (IQR) | 126 (72–198) | 120 (65–180) | 1 | 1.00–1.00 | 0.956 | |||
| Immunosuppressive drugs: regimens containing | ||||||||
| Conventional DMARDs, n (%) | ||||||||
| No | 68 (51.5) | 64 (48.5) | 1 | 1 | ||||
| Yes | 44 (71.0) | 18 (29.0) | 0.61 | 0.36–1.03 | 0.065 | 0.52 | 0.30–0.90 | 0.021 |
| TNFα-i, n (%) | ||||||||
| No | 88 (56.8) | 67 (43.2) | 1 | |||||
| Yes | 24 (61.5) | 15 (38.5) | 0.89 | 0.51–1.56 | 0.688 | |||
| IL6-i, n (%) | ||||||||
| No | 105 (59.7) | 71 (40.3) | 1 | 1 | ||||
| Yes | 7 (38.9) | 11 (61.1) | 2.09 | 1.11–3.95 | 0.023 | 2.01 | 1.03–3.89 | 0.039 |
| CTLA4-Ig, n (%) | ||||||||
| No | 88 (56.4) | 68 (43.6) | 1 | |||||
| Yes | 24 (63.2) | 14 (36.8) | 0.68 | 0.38–1.22 | 0.200 | |||
| JAK-i, n (%) | ||||||||
| No | 83 (57.2) | 62 (42.8) | 1 | |||||
| Yes | 29 (59.2) | 20 (40.8) | 0.89 | 0.54–1.48 | 0.657 | |||
| CD20-i, n (%) | ||||||||
| No | 108 (59.0) | 75 (41.0) | 1 | 1 | ||||
| Yes | 4 (36.4) | 7 (63.6) | 1.74 | 0.80–3.78 | 0.162 | 2.88 | 1.27–6.51 | 0.011 |
| Corticosteroids, n (%) | ||||||||
| No | 90 (55.9) | 71 (44.1) | 1 | |||||
| Yes | 22 (66.7) | 11 (33.3) | 0.86 | 0.45–1.68 | 0.666 | |||
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | SARS-CoV-2 BI | ||||||||
| No | Yes | p | HR | 95% CI | p | aHR | 95% CI | p | |
| Total, n (%) | 25 (61%) | 16 (39%) | |||||||
| Immune responses | |||||||||
| anti-S/RBD (BAU/mL), n (%) | |||||||||
| <809 | 7 (43.8) | 9 (56.3) | 1 | ||||||
| ≥809 | 18 (72.0) | 7 (28.0) | 0.43 | 0.16–1.15 | 0.092 | 0.60 | 0.21–1.74 | 0.346 | |
| Neutralising antibodies, n (%) * | |||||||||
| <20 | 2 (25.0) | 6 (75.0) | 1 | 1 | |||||
| ≥20 | 22 (71.0) | 9 (29.0) | 0.27 | 0.10–0.74 | 0.011 | 0.36 | 0.12–1.07 | 0.067 | |
| T cell-specific response detected by IFN-γ, n (%) | |||||||||
| <16 | 10 (52.6) | 9 (47.4) | 1 | 1 | |||||
| ≥16 | 15 (68.2) | 7 (31.8) | 0.352 | 0.69 | 0.26–1.85 | 0.457 | 1.07 | 0.35–3.28 | 0.906 |
| Hospitalisation | ||||
|---|---|---|---|---|
| No | Yes | Total | p | |
| Overall | 75 (91.5) | 7 (8.5) | 82 (100) | |
| Age, median (IQR) | 61 (53–71) | 67 (52–80) | 62 (53–71) | 0.778 |
| Age class, over 50, n (%) | 1.000 | |||
| 19–49 | 14 (93.3) | 1 (6.7) | 15 (100) | |
| 50–87 | 61 (91.0) | 6 (8.9) | 67 (100) | |
| Age class, over 65, n (%) | 0.694 | |||
| 19–64 | 43 (93.5) | 3 (6.5) | 46 (100) | |
| 65–87 | 32 (88.9) | 4 (11.1) | 36 (100) | |
| Gender, n (%) | ||||
| Female | 51 (92.7) | 4 (7.3) | 55 (100) | 0.679 |
| Male | 24 (88.9) | 3 (11.1) | 27 (100) | |
| Comorbidities, n (%) | 0.127 | |||
| None | 33 (97.1) | 1 (2.9) | 34 (100) | |
| At least one | 42 (87.5) | 6 (12.5) | 48 (100) | |
| BMI, median (IQR) | 26 (23–28) | 27 (20–27) | 26 (22.7–28) | 0.601 |
| Smoking habits,n (%) | 0.274 | |||
| No | 57 (93.4) | 4 (6.6) | 61 (100) | |
| Yes | 18 (85.7) | 3 (14.3) | 21 (100) | |
| Flu vaccination (2021/2022), n (%) | 0.382 | |||
| No | 34 (94.4) | 2 (5.6) | 36 (100) | |
| Yes | 41 (89.1) | 5 (10.9) | 46 (100) | |
| Disease duration (months), median (IQR) | 120 (74–181) | 60 (60–96) | 120 (65–180) | 0.045 |
| Regimens containing: | ||||
| Conventional DMARDs, n (%) | 0.645 | |||
| No | 59 (92.2) | 5 (7.8) | 64 (100) | |
| Yes | 16 (88.9) | 2 (11.1) | 18 (100) | |
| TNFα-i, n (%) | 1.000 | |||
| No | 61 (91.0) | 6 (8.9) | 67 (100) | |
| Yes | 14 (93.3) | 1 (6.7) | 15 (100) | |
| IL6R-i, n (%) | 0.586 | |||
| No | 64 (90.1) | 7 (9.9) | 71 (100) | |
| Yes | 11 (100) | 0 (0) | 11 (100) | |
| CTLA4-Ig, n (%) | 0.597 | |||
| No | 61 (89.7) | 7 (10.3) | 68 (100) | |
| Yes | 14 (100) | 0 (0) | 14 (100) | |
| JAK-i, n (%) | 1.000 | |||
| No | 56 (90.3) | 6 (9.7) | 62 (100) | |
| Yes | 19 (95.0) | 1 (5.0) | 20 (100) | |
| CD20-i, n (%) | 0.001 | |||
| No | 72 (96.0) | 3 (4.0) | 75 (100) | |
| Yes | 3 (42.9) | 4 (57.1) | 7 (100) | |
| Corticosteroids, n (%) | 1.000 | |||
| No | 65 (91.5) | 6 (8.5) | 71 (100) | |
| Yes | 10 (90.9) | 1 (9.1) | 11 (100) | |
| Characteristics | RA | HCW | Total | p |
|---|---|---|---|---|
| Total, n (%) | 194 (16.2) | 1002 (83.8) | 1.196 (100) | |
| Age | 64 (54–71) | 45 (34–54) | 48 (36–57) | <0.001 |
| Gender, n (%) | 0.435 | |||
| Female | 137 (70.6) | 679 (67.8) | 816 (68.2) | |
| Male | 57 (29.4) | 323 (32.2) | 380 (31.8) | |
| Flu vaccination, n (%) | <0.001 | |||
| No | 82 (42.3) | 787 (78.5) | 869 (72.7) | |
| Yes | 112 (57.7) | 215 (21.5) | 327 (27.3) | |
| Third dose vaccine, n (%) | <0.001 | |||
| Comirnaty | 190 (97.9) | 567 (56.6) | 757 (63.3) | |
| Spikevax/other | 4 (2.1) | 435 (43.4) | 439 (36.7) | |
| Breakthrough infection, n (%) | ||||
| Yes | 82 (42.3) | 530 (52.9) | 612 (51.2) | 0.007 |
| No | 112 (57.7) | 472 (47.1) | 584 (48.8) |
| Characteristics | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | aHR | 95% CI | p | |
| RA vs. HCW | 0.74 | 0.59–0.93 | 0.012 | 0.92 | 0.70–1.20 | 0.524 |
| Age (10 years increment) | 0.88 | 0.83–0.93 | 0.000 | 0.89 | 0.83–0.95 | 0.001 |
| Male vs. Female | 0.77 | 0.64–0.91 | 0.003 | 0.78 | 0.65–0.93 | 0.007 |
| Flu vaccination, yes vs. no | 0.96 | 0.80–1.15 | 0.636 | |||
| Spikevax/others vs. Comirnaty | 1.09 | 0.93–1.28 | 0.314 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picchianti-Diamanti, A.; Navarra, A.; Aiello, A.; Laganà, B.; Cuzzi, G.; Salmi, A.; Vanini, V.; Maggi, F.; Meschi, S.; Matusali, G.; et al. Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine. Vaccines 2023, 11, 1684. https://doi.org/10.3390/vaccines11111684
Picchianti-Diamanti A, Navarra A, Aiello A, Laganà B, Cuzzi G, Salmi A, Vanini V, Maggi F, Meschi S, Matusali G, et al. Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine. Vaccines. 2023; 11(11):1684. https://doi.org/10.3390/vaccines11111684
Chicago/Turabian StylePicchianti-Diamanti, Andrea, Assunta Navarra, Alessandra Aiello, Bruno Laganà, Gilda Cuzzi, Andrea Salmi, Valentina Vanini, Fabrizio Maggi, Silvia Meschi, Giulia Matusali, and et al. 2023. "Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine" Vaccines 11, no. 11: 1684. https://doi.org/10.3390/vaccines11111684
APA StylePicchianti-Diamanti, A., Navarra, A., Aiello, A., Laganà, B., Cuzzi, G., Salmi, A., Vanini, V., Maggi, F., Meschi, S., Matusali, G., Notari, S., Agrati, C., Salemi, S., Di Rosa, R., Passarini, D., Di Gioia, V., Sesti, G., Conti, F., Spinelli, F. R., ... Goletti, D. (2023). Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine. Vaccines, 11(11), 1684. https://doi.org/10.3390/vaccines11111684








