Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A
Abstract
:1. Introduction
2. Materials and Methods
3. Results
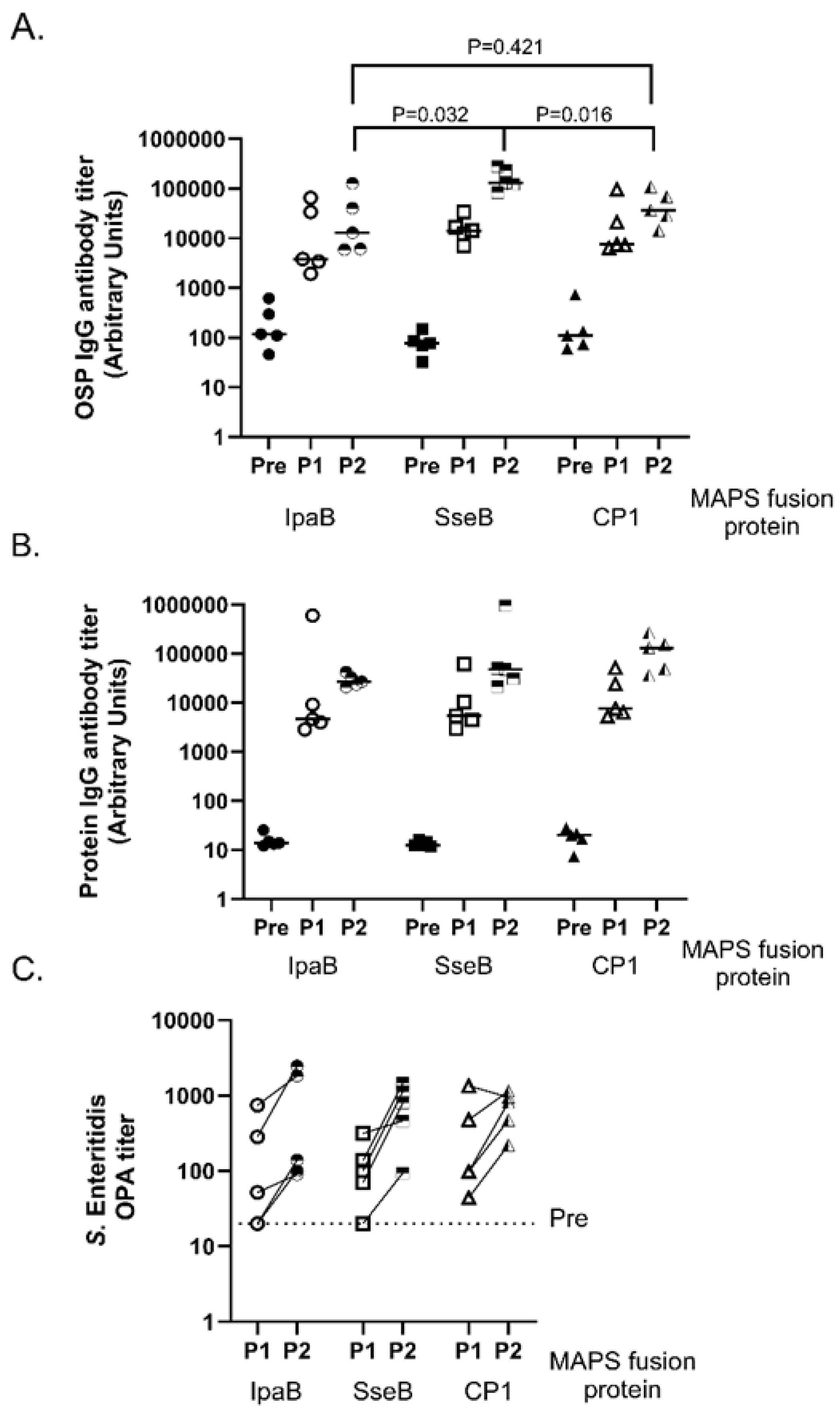
3.1. Comparison of Different Fusion Proteins in S. Enteritidis MAPS Complex
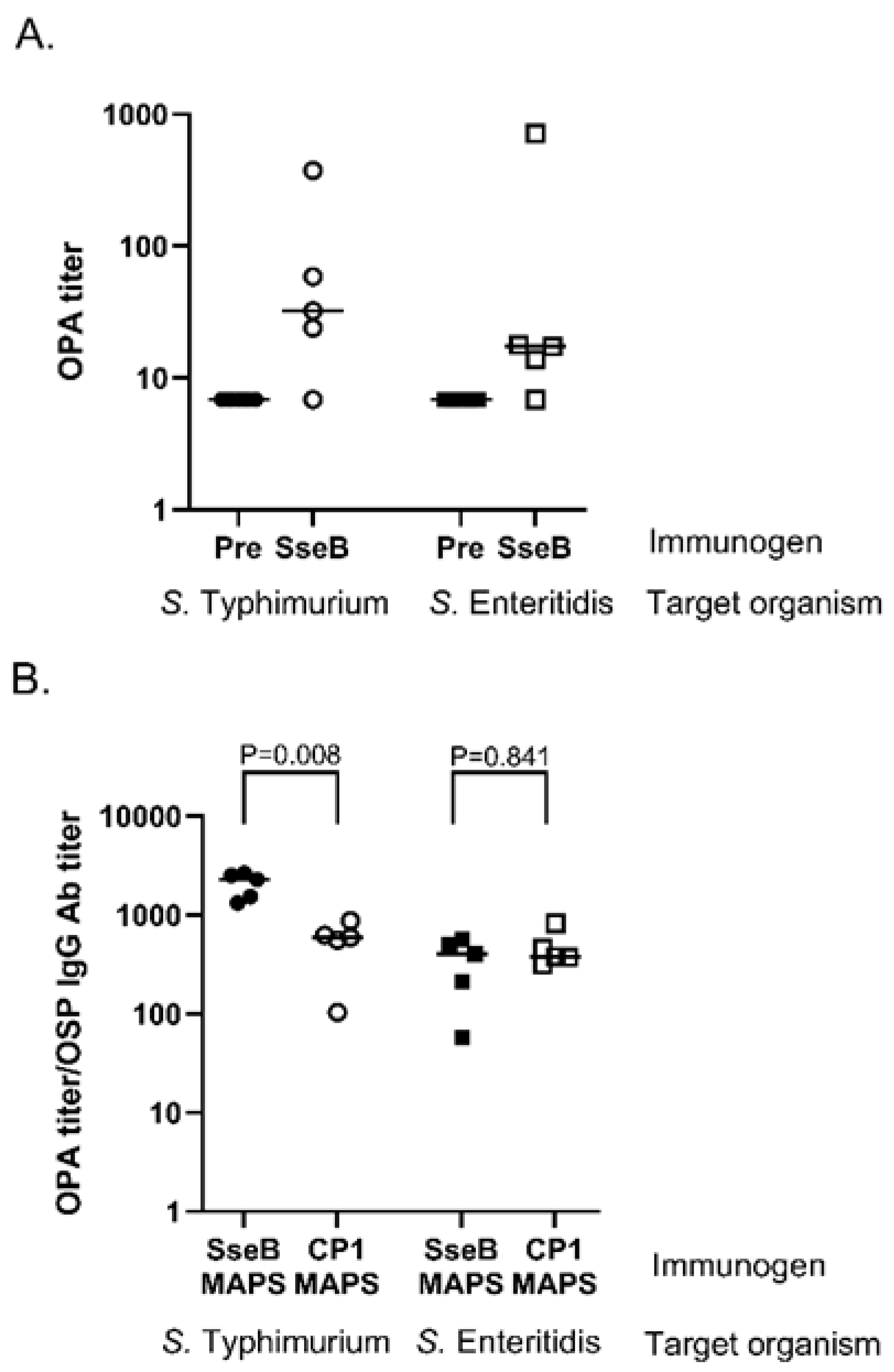
3.2. Role of Fusion Protein Rhavi-SseB
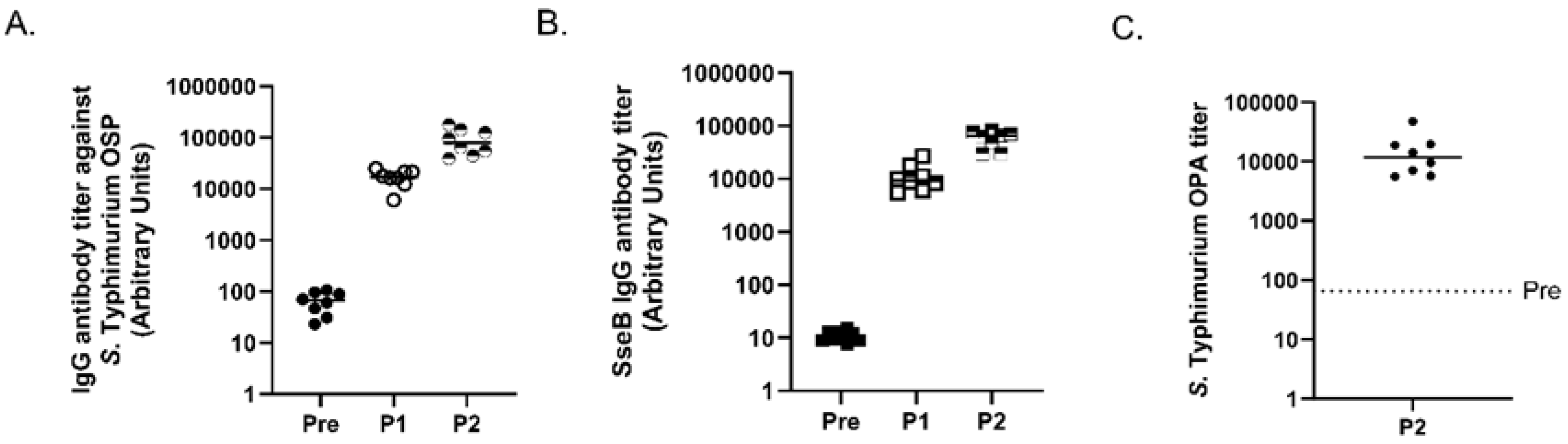
3.3. Analysis of S. Typhimurium MAPS
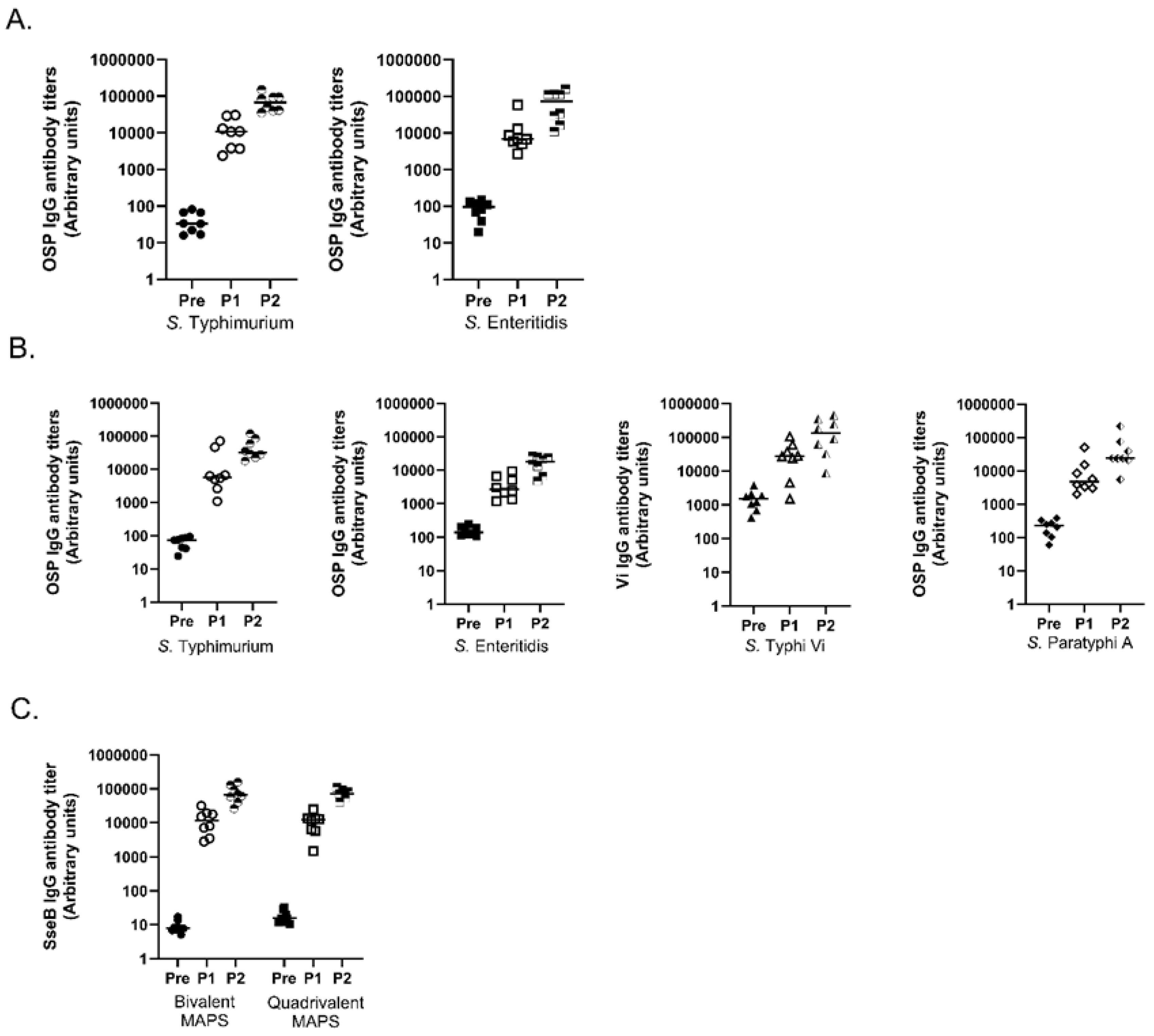
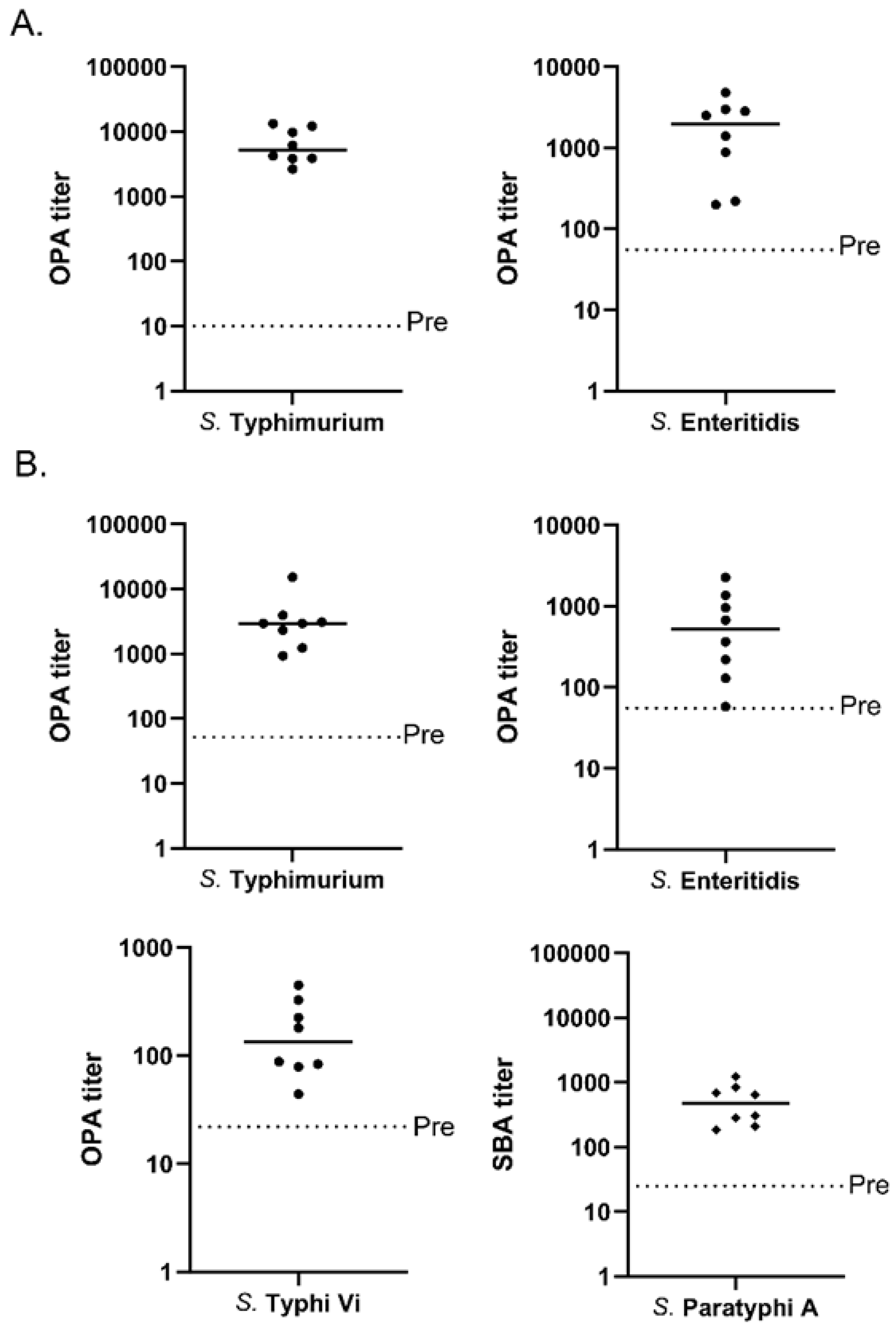
3.4. Multivalent MAPS against Two and Four Salmonella Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Boerth, E.M.; Gong, J.; Ma, N.; Lucas, K.; Ledue, O.; Malley, R.; Lu, Y.-J. A Bivalent MAPS Vaccine Induces Protective Antibody Responses against Salmonella Typhi and Paratyphi A. Vaccines 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Haselbeck, A.H.; Panzner, U.; Im, J.; Baker, S.; Meyer, C.G.; Marks, F. Current perspectives on invasive nontyphoidal Salmonella disease. Curr. Opin. Infect. Dis. 2017, 30, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; MacLennan, C.A.; Simon, R.; Martin, L.B.; Khan, M.I. Nontyphoidal salmonella disease: Current status of vaccine research and development. Vaccine 2016, 34, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, N.N.; Murthy, S.; Birkhold, M.; Marchello, C.S.; Crump, J.A. Prevalence and distribution of non-typhoidal Salmonella enterica serogroups and serovars isolated from normally sterile sites: A global systematic review. medRxiv 2023. [Google Scholar] [CrossRef]
- Baliban, S.M.; Lu, Y.J.; Malley, R. Overview of the Nontyphoidal and Paratyphoidal Salmonella Vaccine Pipeline: Current Status and Future Prospects. Clin. Infect. Dis. 2020, 71 (Suppl. S2), S151–S154. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Stanaway, J.; Grow, S.; Vannice, K.; Steele, A.D. Salmonella Combination Vaccines: Moving Beyond Typhoid. Open Forum. Infect. Dis. 2023, 10 (Suppl. S1), S58–S66. [Google Scholar] [CrossRef]
- Micoli, F.; Ravenscroft, N.; Cescutti, P.; Stefanetti, G.; Londero, S.; Rondini, S.; MacLennan, C.A. Structural analysis of O-polysaccharide chains extracted from different Salmonella Typhimurium strains. Carbohydr. Res. 2014, 385, 1–8. [Google Scholar] [CrossRef]
- Baliban, S.M.; Curtis, B.; Toema, D.; Tennant, S.M.; Levine, M.M.; Pasetti, M.F.; Simon, R. Immunogenicity and efficacy following sequential parenterally-administered doses of Salmonella Enteritidis COPS:FliC glycoconjugates in infant and adult mice. PLoS Negl. Trop. Dis. 2018, 12, e0006522. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef]
- Schuster, O.; Sears, K.T.; Ramachandran, G.; Fuche, F.J.; Curtis, B.; Tennant, S.M.; Simon, R. Immunogenicity and protective efficacy against Salmonella C(2)-C(3) infection in mice immunized with a glycoconjugate of S. Newport Core-O polysaccharide linked to the homologous serovar FliC protein. Hum. Vaccin. Immunother. 2019, 15, 1436–1444. [Google Scholar] [CrossRef]
- Goh, Y.S.; Clare, S.; Micoli, F.; Saul, A.; Mastroeni, P.; MacLennan, C.A. Monoclonal Antibodies of a Diverse Isotype Induced by an O-Antigen Glycoconjugate Vaccine Mediate In Vitro and In Vivo Killing of African Invasive Nontyphoidal Salmonella. Infect. Immun. 2015, 83, 3722–3731. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Albert, J.; Mundy, R.; Yu, X.J.; Beuzón, C.R.; Holden, D.W. SseA is a chaperone for the SseB and SseD translocon components of the Salmonella pathogenicity-island-2-encoded type III secretion system. Microbiology 2003, 149 (Pt. 5), 1103–1111. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Jones, C.; Blohmke, C.J.; Darton, T.C.; Goudet, A.; Sergeant, R.; Maillere, B.; Pollard, A.J.; Altmann, D.M.; Boyton, R.J. The serodominant secreted effector protein of Salmonella, SseB, is a strong CD4 antigen containing an immunodominant epitope presented by diverse HLA class II alleles. Immunology 2014, 143, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Liang, L.; Juarez, S.; Nanton, M.R.; Gondwe, E.N.; Msefula, C.L.; Kayala, M.A.; Necchi, F.; Heath, J.N.; Hart, P.; et al. Identification of a common immune signature in murine and human systemic Salmonellosis. Proc. Natl. Acad. Sci. USA 2012, 109, 4998–5003. [Google Scholar] [CrossRef] [PubMed]
- Barat, S.; Willer, Y.; Rizos, K.; Claudi, B.; Mazé, A.; Schemmer, A.K.; Kirchhoff, D.; Schmidt, A.; Burton, N.; Bumann, D. Immunity to intracellular Salmonella depends on surface-associated antigens. PLoS Pathog. 2012, 8, e1002966. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Huang, T.; Shen, H.; Meng, C.; Jiao, X.; Pan, Z. Salmonella Enteritidis Subunit Vaccine Candidate Based on SseB Protein Co-Delivered with Simvastatin as Adjuvant. Pathogens 2022, 11, 443. [Google Scholar] [CrossRef]
- Desin, T.S.; Köster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Expert. Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.J.; Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 13564–13569. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Szu, S.C.; Li, X.R.; Schneerson, R.; Vickers, J.H.; Bryla, D.; Robbins, J.B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect. Immun. 1989, 57, 3823–3827. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Gavini, M.; Pisoni, I.; Lanzilao, L.; Colucci, A.; Giannelli, C.; Pippi, F.; Sollai, L.; Pinto, V.; et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal. Biochem. 2013, 434, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.H. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955, 212, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.; Shiloach, J.; Bryla, D.A.; Robbins, J.B.; Szu, S.C. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid with emphasis on the role of O acetyls. Infect. Immun. 1996, 64, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Zhang, F.; Sayeed, S.; Thompson, C.M.; Szu, S.; Anderson, P.W.; Malley, R. A bivalent vaccine to protect against Streptococcus pneumoniae and Salmonella typhi. Vaccine 2012, 30, 3405–3412. [Google Scholar] [CrossRef]
- Hale, C.; Bowe, F.; Pickard, D.; Clare, S.; Haeuw, J.-F.; Powers, U.; Menager, N.; Mastroeni, P.; Dougan, G. Evaluation of a novel Vi conjugate vaccine in a murine model of salmonellosis. Vaccine 2006, 24, 4312–4320. [Google Scholar] [CrossRef]
- Boyd, M.A.; Tennant, S.M.; Saague, V.A.; Simon, R.; Muhsen, K.; Ramachandran, G.; Cross, A.S.; Galen, J.E.; Pasetti, M.F.; Levine, M.M. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin. Vaccine Immunol. 2014, 21, 712–721. [Google Scholar] [CrossRef]
- Gondwe, E.N.; Molyneux, M.E.; Goodall, M.; Graham, S.M.; Mastroeni, P.; Drayson, M.T.; MacLennan, C.A. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc. Natl. Acad. Sci. USA 2010, 107, 3070–3075. [Google Scholar] [CrossRef]
- Gilchrist, J.J.; MacLennan, C.A. Invasive Nontyphoidal Salmonella Disease in Africa. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Hindle, Z.; Chatfield, S.N.; Phillimore, J.; Bentley, M.; Johnson, J.; Cosgrove, C.A.; Ghaem-Maghami, M.; Sexton, A.; Khan, M.; Brennan, F.R.; et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 2002, 70, 3457–3467. [Google Scholar] [CrossRef]
- Sears, K.T.; Galen, J.E.; Tennant, S.M. Advances in the development of Salmonella-based vaccine strategies for protection against Salmonellosis in humans. J. Appl. Microbiol. 2021, 131, 2640–2658. [Google Scholar] [CrossRef]
- Allam, U.S.; Krishna, M.G.; Lahiri, A.; Joy, O.; Chakravortty, D. Salmonella enterica serovar Typhimurium lacking hfq gene confers protective immunity against murine typhoid. PLoS ONE 2011, 6, e16667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Xiong, K.; Chen, Z.; Zheng, C.; Tan, Y.; Cong, Y. Elimination of persistent vaccine bacteria of Salmonella enterica serovar Typhimurium in the guts of immunized mice by inducible expression of truncated YncE. PLoS ONE 2017, 12, e0179649. [Google Scholar] [CrossRef] [PubMed]
- El Ghany, M.A.; Jansen, A.; Clare, S.; Hall, L.; Pickard, D.; Kingsley, R.A.; Dougan, G. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect. Immun. 2007, 75, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef]
- Launay, O.; Lewis, D.J.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results from Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Obiero, C.W.; Ndiaye, A.G.W.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Baliban, S.M.; Allen, J.C.; Curtis, B.; Amin, M.N.; Lees, A.; Rao, R.N.; Naidu, G.; Venkatesan, R.; Rao, D.Y.; Mohan, V.K.; et al. Immunogenicity and Induction of Functional Antibodies in Rabbits Immunized with a Trivalent Typhoid-Invasive Nontyphoidal Salmonella Glycoconjugate Formulation. Molecules 2018, 23, 1749. [Google Scholar] [CrossRef]
- Li, P.; Liu, Q.; Luo, H.; Liang, K.; Yi, J.; Luo, Y.; Hu, Y.; Han, Y.; Kong, Q. O-Serotype Conversion in Salmonella Typhimurium Induces Protective Immune Responses against Invasive Non-Typhoidal Salmonella Infections. Front. Immunol. 2017, 8, 1647. [Google Scholar] [CrossRef]
- Liu, Q.; Li, P.; Luo, H.; Curtiss, R., 3rd; Kong, Q. Attenuated Salmonella Typhimurium expressing Salmonella Paratyphoid A O-antigen induces protective immune responses against two Salmonella strains. Virulence 2019, 10, 82–96. [Google Scholar] [CrossRef]
- Watson, D.C.; Robbins, J.B.; Szu, S.C. Protection of mice against Salmonella typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect. Immun. 1992, 60, 4679–4686. [Google Scholar] [CrossRef] [PubMed]
- Jörbeck, H.J.; Svenson, S.B.; Lindberg, A.A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit opsonizing antibodies that enhance phagocytosis. Infect. Immun. 1981, 32, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Rondini, S.; Micoli, F.; Lanzilao, L.; Gavini, M.; Alfini, R.; Brandt, C.; Clare, S.; Mastroeni, P.; Saul, A.; MacLennan, C.A. Design of glycoconjugate vaccines against invasive African Salmonella enterica serovar Typhimurium. Infect. Immun. 2015, 83, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, C.A.; Tennant, S.M. Comparing the roles of antibodies to nontyphoidal Salmonella enterica in high- and low-income countries and implications for vaccine development. Clin. Vaccine Immunol. 2013, 20, 1487–1490. [Google Scholar] [CrossRef]
- Trebicka, E.; Jacob, S.; Pirzai, W.; Hurley, B.P.; Cherayil, B.J. Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States. Clin. Vaccine Immunol. 2013, 20, 1491–1498. [Google Scholar] [CrossRef]
- Goh, Y.S.; Necchi, F.; O’shaughnessy, C.M.; Micoli, F.; Gavini, M.; Young, S.P.; Msefula, C.; Gondwe, E.N.; Mandala, W.L.; Gordon, M.; et al. Bactericidal Immunity to Salmonella in Africans and Mechanisms Causing Its Failure in HIV Infection. PLoS Neglected Trop. Dis. 2016, 10, e0004604. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Martin, L.B.; Micoli, F. Vaccines against invasive Salmonella disease: Current status and future directions. Hum. Vaccin. Immunother. 2014, 10, 1478–1493. [Google Scholar] [CrossRef]
- Simon, R.; Tennant, S.M.; Wang, J.Y.; Schmidlein, P.J.; Lees, A.; Ernst, R.K.; Pasetti, M.F.; Galen, J.E.; Levine, M.M. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect. Immun. 2011, 79, 4240–4249. [Google Scholar] [CrossRef]
- Ahmed, A.; Akhade, A.S.; Qadri, A. Accessibility of O Antigens Shared between Salmonella Serovars Determines Antibody-Mediated Cross-Protection. J. Immunol. 2020, 205, 438–446. [Google Scholar] [CrossRef]
- Lee, S.-J.; Benoun, J.; Sheridan, B.S.; Fogassy, Z.; Pham, O.; Pham, Q.-M.; Puddington, L.; McSorley, S.J. Dual Immunization with SseB/Flagellin Provides Enhanced Protection against Salmonella Infection Mediated by Circulating Memory Cells. J. Immunol. 2017, 199, 1353–1361. [Google Scholar] [CrossRef]
- Cunningham, A.F.; Gaspal, F.; Serre, K.; Mohr, E.; Henderson, I.R.; Scott-Tucker, A.; Kenny, S.M.; Khan, M.; Toellner, K.M.; Lane, P.J.; et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 2007, 178, 6200–6207. [Google Scholar] [CrossRef]
- Gil-Cruz, C.; Bobat, S.; Marshall, J.L.; Kingsley, R.A.; Ross, E.A.; Henderson, I.R.; Leyton, D.L.; Coughlan, R.E.; Khan, M.; Jensen, K.T.; et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. USA 2009, 106, 9803–9808. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Becerra, F.J.; Kumar, P.; Vishwakarma, V.; Kim, J.H.; Arizmendi, O.; Middaugh, C.R.; Picking, W.D.; Picking, W.L. Characterization and Protective Efficacy of Type III Secretion Proteins as a Broadly Protective Subunit Vaccine against Salmonella enterica Serotypes. Infect. Immun. 2018, 86, e00473-17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yi, J.; Liang, K.; Liu, T.; Roland, K.L.; Jiang, Y.; Kong, Q. Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model. Int. J. Med. Microbiol. 2016, 306, 697–706. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boerth, E.M.; Gong, J.; Roffler, B.; Thompson, C.M.; Song, B.; Malley, S.F.; Hirsch, A.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines 2023, 11, 1671. https://doi.org/10.3390/vaccines11111671
Boerth EM, Gong J, Roffler B, Thompson CM, Song B, Malley SF, Hirsch A, MacLennan CA, Zhang F, Malley R, et al. Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines. 2023; 11(11):1671. https://doi.org/10.3390/vaccines11111671
Chicago/Turabian StyleBoerth, Emily M., Joyce Gong, Becky Roffler, Claudette M. Thompson, Boni Song, Sasha F. Malley, Angelika Hirsch, Calman A. MacLennan, Fan Zhang, Richard Malley, and et al. 2023. "Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A" Vaccines 11, no. 11: 1671. https://doi.org/10.3390/vaccines11111671
APA StyleBoerth, E. M., Gong, J., Roffler, B., Thompson, C. M., Song, B., Malley, S. F., Hirsch, A., MacLennan, C. A., Zhang, F., Malley, R., & Lu, Y.-J. (2023). Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines, 11(11), 1671. https://doi.org/10.3390/vaccines11111671





