An Evaluation of Urease A Subunit Nanocapsules as a Vaccine in a Mouse Model of Helicobacter pylori Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silica Templates
2.2. Preparation of Recombinant UreA
2.3. Nanocapsule Synthesis and Characterisation Protocols
2.4. Animal Trial
2.5. H. pylori Challenge
2.6. Serum, Blood and Tissue Collection
2.7. Statistical Analysis
2.8. Immunoassay Protocols
2.9. Measurement of Humoral Response by Serum Antibody Detection—Indirect ELISA
2.10. Immune Cell Population Quantification by FACS
2.11. H. pylori Stomach DNA Analysis
2.12. H. pylori Burdens Analysis by qPCR
3. Results
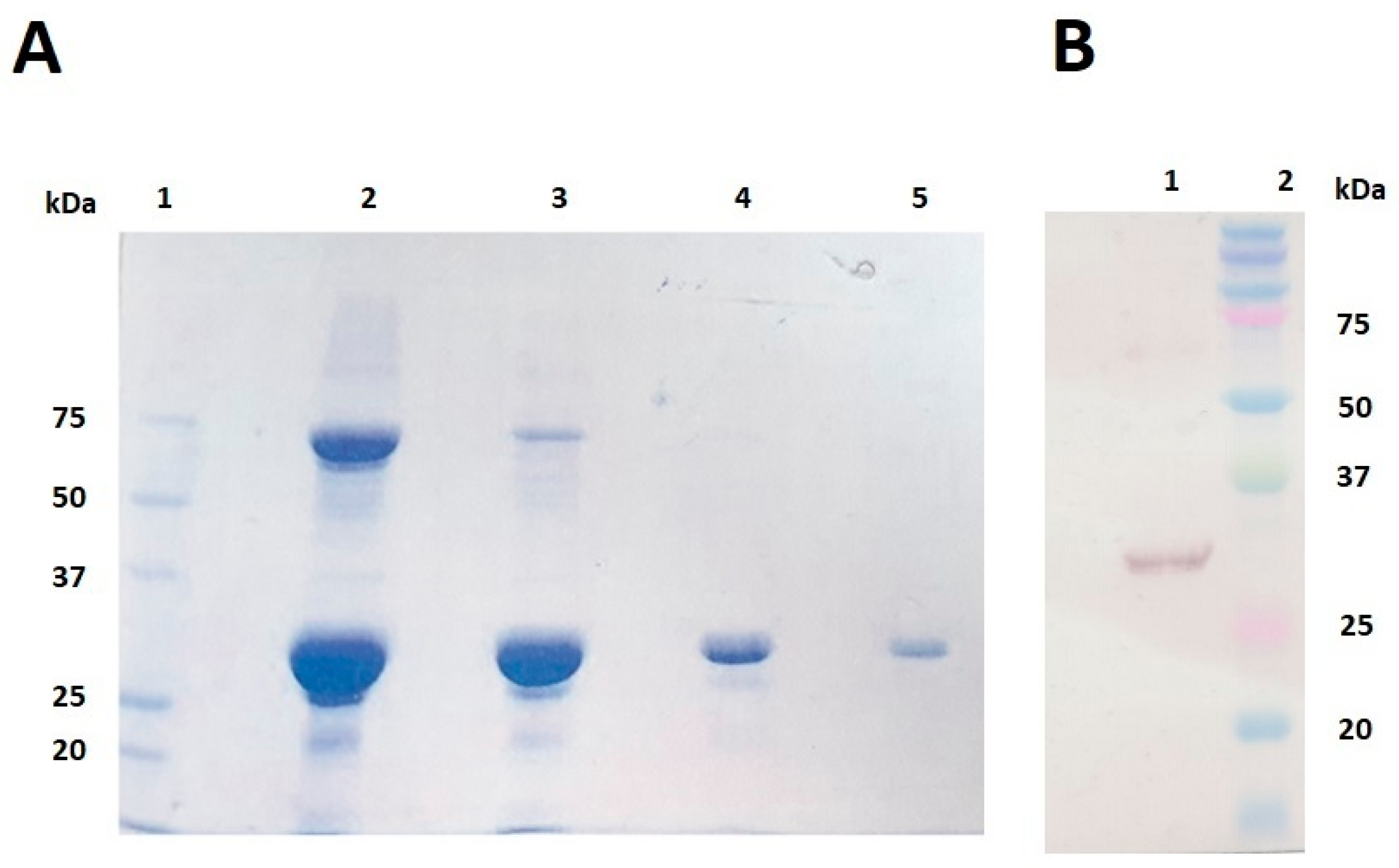
3.1. Protein Expression and Characterisation
3.2. Protein Loading of Silica Templates
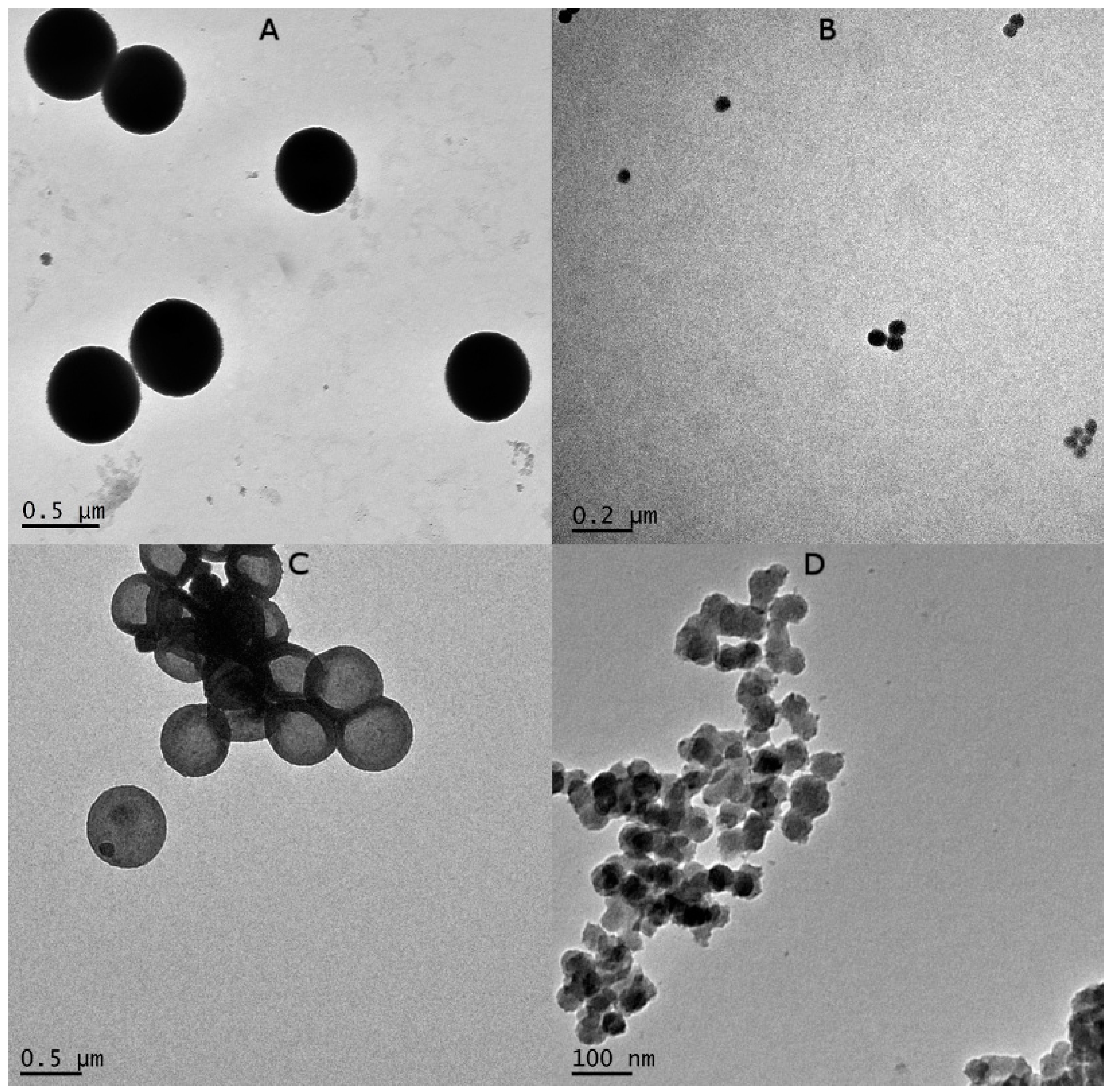
3.3. Characterisation of Silica Templates and UreA Nanocapsules by Transmission Electron Microscopy (TEM)
3.4. Size and Zeta Potential Characterisation by Dynamic Light Scattering (DLS)
3.5. Levels of Antigen-Specific IgG, IgG1 and IgG2c Antibodies
3.6. Determination of H. pylori Burdens
3.7. Analysis of Immune Cell Populations from Mouse Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiotani, A.; Cen, P.; Graham, D.Y. Eradication of gastric cancer is now both possible and practical. Semin. Cancer Biol. 2013, 23, 492–501. [Google Scholar] [CrossRef]
- Emerenini, F.C.; Nwolisa, E.C.; Iregbu, F.U.; Eke, C.B.; Ikefuna, A.N. Prevalence and risk factors for helicobacter pylori infection among children in Owerri, Nigeria. Niger. J. Clin. Pract. 2021, 24, 1188–1193. [Google Scholar] [CrossRef]
- Salih, B.A. Helicobacter pylori infection in developing countries: The burden for how long? Saudi J. Gastroenterol. 2009, 15, 201–207. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Ye, J.; Ning, L.; Luo, J.; Zhang, L.; Jiang, Y.; Xi, Y.; Ning, Y. Antibody Production and Th1-biased Response Induced by an Epitope Vaccine Composed of Cholera Toxin B Unit and Helicobacter pylori Lpp20 Epitopes. Helicobacter 2016, 21, 234–248. [Google Scholar] [CrossRef]
- Moss, S.F.; Moise, L.; Lee, D.S.; Kim, W.; Zhang, S.; Lee, J.; Rogers, A.B.; Martin, W.; De Groot, A.S. HelicoVax: Epitope-based therapeutic Helicobacter pylori vaccination in a mouse model. Vaccine 2011, 29, 2085–2091. [Google Scholar] [CrossRef]
- Tobias, J.; Lebens, M.; Wai, S.N.; Holmgren, J.; Svennerholm, A.M. Surface expression of Helicobacter pylori HpaA adhesion antigen on Vibrio cholerae, enhanced by co-expressed enterotoxigenic Escherichia coli fimbrial antigens. Microb. Pathog. 2017, 105, 177–184. [Google Scholar] [CrossRef]
- Yang, J.; Dai, L.X.; Pan, X.; Wang, H.; Li, B.; Zhu, J.; Li, M.Y.; Shi, X.L.; Wang, B.N. Protection against Helicobacter pylori infection in BALB/c mice by oral administration of multi-epitope vaccine of CTB-UreI-UreB. Pathog. Dis. 2015, 73, ftv026. [Google Scholar] [CrossRef]
- Dos Santos Viana, I.; Cordeiro Santos, M.L.; Santos Marques, H.; Lima de Souza Goncalves, V.; Bittencourt de Brito, B.; Franca da Silva, F.A.; Oliveira, E.S.N.; Dantas Pinheiro, F.; Fernandes Teixeira, A.; Tanajura Costa, D.; et al. Vaccine development against Helicobacter pylori: From ideal antigens to the current landscape. Expert. Rev. Vaccines 2021, 20, 989–999. [Google Scholar] [CrossRef]
- Keikha, M.; Eslami, M.; Yousefi, B.; Ghasemian, A.; Karbalaei, M. Potential antigen candidates for subunit vaccine development against Helicobacter pylori infection. J. Cell Physiol. 2019, 234, 21460–21470. [Google Scholar] [CrossRef]
- Michetti, P.; Kreiss, C.; Kotloff, K.L.; Porta, N.; Blanco, J.L.; Bachmann, D.; Herranz, M.; Saldinger, P.F.; Corthesy-Theulaz, I.; Losonsky, G.; et al. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 1999, 116, 804–812. [Google Scholar] [CrossRef]
- Xiao, S.; Shang, K.; Li, W.; Wang, X. An efficient biosensor based on the synergistic catalysis of Helicobacter pylori urease b subunit and nanoplatinum for urease inhibitors screening and antagonistic mechanism analyzing. Sens. Actuators B Chem. 2022, 355, 131284. [Google Scholar] [CrossRef]
- Huang, J.Y.; Sweeney, E.G.; Sigal, M.; Zhang, H.C.; Remington, S.J.; Cantrell, M.A.; Kuo, C.J.; Guillemin, K.; Amieva, M.R. Chemodetection and Destruction of Host Urea Allows Helicobacter pylori to Locate the Epithelium. Cell Host Microbe 2015, 18, 147–156. [Google Scholar] [CrossRef]
- Debowski, A.W.; Walton, S.M.; Chua, E.G.; Tay, A.C.; Liao, T.; Lamichhane, B.; Himbeck, R.; Stubbs, K.A.; Marshall, B.J.; Fulurija, A.; et al. Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection. PLoS Pathog. 2017, 13, e1006464. [Google Scholar] [CrossRef]
- de Jesus Souza, M.; de Moraes, J.A.; Da Silva, V.N.; Helal-Neto, E.; Uberti, A.F.; Scopel-Guerra, A.; Olivera-Severo, D.; Carlini, C.R.; Barja-Fidalgo, C. Helicobacter pylori urease induces pro-inflammatory effects and differentiation of human endothelial cells: Cellular and molecular mechanism. Helicobacter 2019, 24, e12573. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Benoit, S.L.; Misra, S.K.; Sharp, J.S.; Maier, R.J. Noncatalytic Antioxidant Role for Helicobacter pylori Urease. J. Bacteriol. 2018, 200, e00124-18. [Google Scholar] [CrossRef]
- Zhang, H.X.; Qiu, Y.Y.; Zhao, Y.H.; Liu, X.T.; Liu, M.; Yu, A.L. Immunogenicity of oral vaccination with Lactococcus lactis derived vaccine candidate antigen (UreB) of Helicobacter pylori fused with the human interleukin 2 as adjuvant. Mol. Cell Probes 2014, 28, 25–30. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Y.; Xu, Z.; Ma, R.; Ding, Y.; Bai, X.; Rong, Q.; Zhang, Y.; Li, B.; Ji, X. Mechanistic Insight Into the Interaction Between Helicobacter pylori Urease Subunit alpha and Its Molecular Chaperone Hsp60. Front. Microbiol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Lucas, B.; Bumann, D.; Walduck, A.; Koesling, J.; Develioglu, L.; Meyer, T.F.; Aebischer, T. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 2001, 69, 1714–1721. [Google Scholar] [CrossRef]
- Katsande, P.M.; Nguyen, V.D.; Nguyen, T.L.P.; Nguyen, T.K.C.; Mills, G.; Bailey, D.M.D.; Christie, G.; Hong, H.A.; Cutting, S.M. Prophylactic immunization to Helicobacter pylori infection using spore vectored vaccines. Helicobacter 2023, 28, e12997. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Taki, A.; Smooker, P. Small Wonders-The Use of Nanoparticles for Delivering Antigen. Vaccines 2015, 3, 638–661. [Google Scholar] [CrossRef] [PubMed]
- Taki, A.C.; Francis, J.E.; Skakic, I.; Dekiwadia, C.; McLean, T.R.; Bansal, V.; Smooker, P.M. Protein-only nanocapsules induce cross-presentation in dendritic cells, demonstrating potential as an antigen delivery system. Nanomedicine 2020, 28, 102234. [Google Scholar] [CrossRef] [PubMed]
- Skakic, I.; Francis, J.E.; Smooker, P.M. Design and Synthesis of Protein-Based Nanocapsule Vaccines. Methods Mol. Biol. 2022, 2412, 339–354. [Google Scholar] [CrossRef]
- Büchel, G.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. A Novel Pathway for Synthesis of Submicrometer-Size Solid Core/Mesoporous Shell Silica Spheres. Adv. Mater. 1998, 10, 1036–1038. [Google Scholar] [CrossRef]
- Möller, K.; Kobler, J.; Bein, T. Colloidal Suspensions of Nanometer-Sized Mesoporous Silica. Adv. Funct. Mater. 2007, 17, 605–612. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Carrion-Recio, D.; Seijo, B.; Remunan-Lopez, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control Release 2012, 157, 383–390. [Google Scholar] [CrossRef]
- Gomez-Duarte, O.G.; Lucas, B.; Yan, Z.X.; Panthel, K.; Haas, R.; Meyer, T.F. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 1998, 16, 460–471. [Google Scholar] [CrossRef]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef]
- Becher, D.; Deutscher, M.E.; Simpfendorfer, K.R.; Wijburg, O.L.; Pederson, J.S.; Lew, A.M.; Strugnell, R.A.; Walduck, A.K. Local recall responses in the stomach involving reduced regulation and expanded help mediate vaccine-induced protection against Helicobacter pylori in mice. Eur. J. Immunol. 2010, 40, 2778–2790. [Google Scholar] [CrossRef]
- Ng, G.Z.; Sutton, P. An optimised perfusion technique for extracting murine gastric leukocytes. J. Immunol. Methods 2015, 427, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Zangala, T. Isolation of genomic DNA from mouse tails. J. Vis. Exp. 2007, 6, 246. [Google Scholar] [CrossRef]
- Tan, M.P.; Kaparakis, M.; Galic, M.; Pedersen, J.; Pearse, M.; Wijburg, O.L.C.; Janssen, P.H.; Strugnell, R.A. Chronic Helicobacter pylori infection does not significantly alter the microbiota of the murine stomach. Appl. Environ. Microbiol. 2007, 73, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, T.; Bumann, D.; Epple, H.J.; Metzger, W.; Schneider, T.; Cherepnev, G.; Walduck, A.K.; Kunkel, D.; Moos, V.; Loddenkemper, C.; et al. Correlation of T cell response and bacterial clearance in human volunteers challenged with Helicobacter pylori revealed by randomised controlled vaccination with Ty21a-based Salmonella vaccines. Gut 2008, 57, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Jeverstam, F.; Lundgren, A.; Magnusson, M.K.; Walduck, A.; Qadri, F.; Bhuiyan, T.R.; Raghavan, S. The frequency of circulating integrin alpha4beta7(+) cells correlates with protection against Helicobacter pylori infection in immunized mice. Helicobacter 2019, 24, e12658. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3—Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Firacative, C.; Gressler, A.E.; Schubert, K.; Schulze, B.; Muller, U.; Brombacher, F.; von Bergen, M.; Alber, G. Identification of T helper (Th)1- and Th2-associated antigens of Cryptococcus neoformans in a murine model of pulmonary infection. Sci. Rep. 2018, 8, 2681. [Google Scholar] [CrossRef]
- Martin, R.M.; Brady, J.L.; Lew, A.M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 1998, 212, 187–192. [Google Scholar] [CrossRef]
- Sayi, A.; Kohler, E.; Hitzler, I.; Arnold, I.; Schwendener, R.; Rehrauer, H.; Muller, A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J. Immunol. 2009, 182, 7085–7101. [Google Scholar] [CrossRef]
- Akhiani, A.A.; Schon, K.; Franzen, L.E.; Pappo, J.; Lycke, N. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 2004, 172, 5024–5033. [Google Scholar] [CrossRef]
- Loffeld, R.J.; Werdmuller, B.F.; Kusters, J.G.; Kuipers, E.J. IgG antibody titer against Helicobacter pylori correlates with presence of cytotoxin associated gene A-positive H. pylori strains. FEMS Immunol. Med. Microbiol. 2000, 28, 139–141. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Wolf, N.; Ward, R.W.; Hearnden, C.H.; Sharp, F.A.; Geoghegan, J.; O’Grady, K.; McEntee, C.P.; Shanahan, K.A.; Guy, C.; Bowie, A.G.; et al. Non-canonical inflammasome activation mediates the adjuvanticity of nanoparticles. Cell Rep. Med. 2023, 4, 100899. [Google Scholar] [CrossRef]
- Bagheri, N.; Razavi, A.; Pourgheysari, B.; Azadegan-Dehkordi, F.; Rahimian, G.; Pirayesh, A.; Shafigh, M.; Rafieian-Kopaei, M.; Fereidani, R.; Tahmasbi, K.; et al. Up-regulated Th17 cell function is associated with increased peptic ulcer disease in Helicobacter pylori-infection. Infect. Genet. Evol. 2018, 60, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Konjar, S.; Ferreira, C.; Blankenhaus, B.; Veldhoen, M. Intestinal Barrier Interactions with Specialized CD8 T Cells. Front. Immunol. 2017, 8, 1281. [Google Scholar] [CrossRef] [PubMed]
- Bamford, K.B.; Fan, X.; Crowe, S.E.; Leary, J.F.; Gourley, W.K.; Luthra, G.K.; Brooks, E.G.; Graham, D.Y.; Reyes, V.E.; Ernst, P.B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 1998, 114, 482–492. [Google Scholar] [CrossRef]
- Nurgalieva, Z.Z.; Conner, M.E.; Opekun, A.R.; Zheng, C.Q.; Elliott, S.N.; Ernst, P.B.; Osato, M.; Estes, M.K.; Graham, D.Y. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infect. Immun. 2005, 73, 2999–3006. [Google Scholar] [CrossRef]
- Pappo, J.; Torrey, D.; Castriotta, L.; Savinainen, A.; Kabok, Z.; Ibraghimov, A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 1999, 67, 337–341. [Google Scholar] [CrossRef]
- Kaparakis, M.; Walduck, A.K.; Price, J.D.; Pedersen, J.S.; van Rooijen, N.; Pearse, M.J.; Wijburg, O.L.; Strugnell, R.A. Macrophages are mediators of gastritis in acute Helicobacter pylori infection in C57BL/6 mice. Infect. Immun. 2008, 76, 2235–2239. [Google Scholar] [CrossRef]
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef]
- Jennings, V.M. Review of Selected Adjuvants Used in Antibody Production. ILAR J. 1995, 37, 119–125. [Google Scholar] [CrossRef]
- Melssen, M.M.; Fisher, C.T.; Slingluff, C.L.; Melief, C.J.M. Peptide emulsions in incomplete Freund’s adjuvant create effective nurseries promoting egress of systemic CD4(+) and CD8(+) T cells for immunotherapy of cancer. J. Immunother. Cancer 2022, 10, e004709. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Cresswell, P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 2004, 5, 678–684. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.L.; Thiberge, J.M.; Huerre, M.; Labigne, A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect. Immun. 1998, 66, 1349–1355. [Google Scholar] [CrossRef]
- Bland, D.A.; Suarez, G.; Beswick, E.J.; Sierra, J.C.; Reyes, V.E. H pylori receptor MHC class II contributes to the dynamic gastric epithelial apoptotic response. World J. Gastroenterol. 2006, 12, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, A.; Aebischer, T.; Meyer, T.F.; Walduck, A.K. Leptin receptor signaling is required for vaccine-induced protection against Helicobacter pylori. Helicobacter 2008, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]




| Group | Vaccine | Boost | Dose per Vaccination | Challenged |
|---|---|---|---|---|
| A | Salmonella positive control | - | 107 CFU | ✓ |
| B | PBS | PBS | 100 µL PBS | ✓ |
| C | PBS | PBS | 100 µL PBS | ✗ |
| D | TiterMax® Gold Adjuvant only | TiterMax® Gold Adjuvant only | 100 µL PBS | ✓ |
| E | Soluble UreA with TiterMax® Gold adjuvant | Soluble UreA with TiterMax® Gold adjuvant | 20 µg of antigen in 100 µL PBS | ✓ |
| F | Large nanocapsules with TiterMax® Gold adjuvant | Large nanocapsules with TiterMax® Gold adjuvant | 20 µg of nanocapsule in 100 µL PBS | ✓ |
| G | Small nanocapsules with TiterMax® Gold adjuvant | Small nanocapsules with TiterMax® Gold adjuvant | 20 µg of nanocapsule in 100 µL PBS | ✓ |
| H | Combination nanocapsules with TiterMax® Gold adjuvant | Combination nanocapsules with TiterMax® Gold adjuvant | 10 µg of large nanocapsule + 10 µg of small nanocapsule in 100 µL PBS | ✓ |
| I | Soluble UreA without adjuvant | Soluble UreA without adjuvant | 20 µg of antigen in 100 µL PBS | ✓ |
| J | Large nanocapsules without adjuvant | Large nanocapsules without adjuvant | 20 µg of nanocapsule in 100 µL PBS | ✓ |
| K | Small nanocapsules without adjuvant | Small nanocapsules without adjuvant | 20 µg of nanocapsule in 100 µL PBS | ✓ |
| L | Combination nanocapsules without adjuvant | Combination nanocapsules without adjuvant | 10 µg of large nanocapsules + 10 µg of small nanocapsules in 100 µL PBS | ✓ |
| FORWARD | 5′-CTTAACCATAGAACTGCATTTGAAACTAC-3′ |
| REVERSE | 5′-GGTCGCCTTCGCAATGAGTA-3′ |
| PROBE | 5′-[FAM]TAC CTC TCC CAC ACT CT[TAMRA]-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skakic, I.; Francis, J.E.; Dekiwadia, C.; Aibinu, I.; Huq, M.; Taki, A.C.; Walduck, A.; Smooker, P.M. An Evaluation of Urease A Subunit Nanocapsules as a Vaccine in a Mouse Model of Helicobacter pylori Infection. Vaccines 2023, 11, 1652. https://doi.org/10.3390/vaccines11111652
Skakic I, Francis JE, Dekiwadia C, Aibinu I, Huq M, Taki AC, Walduck A, Smooker PM. An Evaluation of Urease A Subunit Nanocapsules as a Vaccine in a Mouse Model of Helicobacter pylori Infection. Vaccines. 2023; 11(11):1652. https://doi.org/10.3390/vaccines11111652
Chicago/Turabian StyleSkakic, Ivana, Jasmine E. Francis, Chaitali Dekiwadia, Ibukun Aibinu, Mohsina Huq, Aya C. Taki, Anna Walduck, and Peter M. Smooker. 2023. "An Evaluation of Urease A Subunit Nanocapsules as a Vaccine in a Mouse Model of Helicobacter pylori Infection" Vaccines 11, no. 11: 1652. https://doi.org/10.3390/vaccines11111652
APA StyleSkakic, I., Francis, J. E., Dekiwadia, C., Aibinu, I., Huq, M., Taki, A. C., Walduck, A., & Smooker, P. M. (2023). An Evaluation of Urease A Subunit Nanocapsules as a Vaccine in a Mouse Model of Helicobacter pylori Infection. Vaccines, 11(11), 1652. https://doi.org/10.3390/vaccines11111652









