Vaccination and Antiviral Treatment against Avian Influenza H5Nx Viruses: A Harbinger of Virus Control or Evolution
Abstract
1. Introduction
2. Introductions and Evolutionary Events of AIVs in Egypt
3. Imported and Locally Produced Avian Influenza Vaccines in Egypt
| AIV Subtype | Vaccine Name/ Company/Country | Vaccine Type | Donor Strain | Clade/Lineage | Efficacy * |
|---|---|---|---|---|---|
| H5N9 | Optimune AIV/Ceva/Mexico | Inactivated | Influenza A/turkey/Wisconsin/1968(H5N9) | Classical H5N1 | 88% |
| H5N1/H5N8 | ME-Flu VAC/MEVAC/Egypt | Inactivated | Influenza A/chicken/Egypt/RG-173CAL/2017(H5N1); Influenza A/chicken/ME-2018(H5N8) | H5N1 clade 2.2.1.2 and 2.3.4.4b | 87–96% |
| H5N1 | SERA-VAC/VSVRI/Egypt | Inactivated | Influenza A/chicken/ Egypt/M2583D/2010(H5N1) | H5N1 clade 2.2.1.1 | 91–≥99% |
| H5N1/H5N8 | Vallyvac AI H5N1/Valley vaccine/Egypt | Inactivated | Influenza A/chicken/D10552B/2015(H5N1); Influenza A/green-winged tail/Egypt/877/2016(H5N8) | H5N1 clade 2.2.1.2 and 2.3.4.4b | 87–≥99% |
| H5N1 | Egy flu/Harbin Veterinary Research Institute/China | Inactivated | Influenza A/chicken/Egypt/18-H/2009(H5N1) | H5N1 clade 2.2.1.1 | 89–94% |
| H5N1 | Avian Influenza H5 Re6 + Re8 Vaccine/Harbin/China | Inactivated | Influenza A/duck/Guangdong/S1322/2006(H5N1); Influenza A/chicken/Guizhou/4/13(H5N1) | H5N1 clade 2.3.2. and 2.3.4.4b | 95–97% |
| H5N2 | Nobilis Influenza H5N2/Intervet/US | Inactivated | Influenza A/duck/Potsdam/1402/86(H5N2) | H5N2 Eurasian | 87–92% |
| H5N2 | CEVac Flukem/Ceva/Mexico | Inactivated | Influenza A/Chicken /Mexico/232/94/CPA(H5N2) | H5N2 North American | 75–90% |
| H5N3 | Zoetis H5N3/Zoetis/US | Inactivated | Influenza A/chicken/Vietnam/C58/2004(H5N3) | H5N1 clade 1 | 90–97% |
| H5N1 | Volvac® B.E.S.T. AI + ND)/Boehringer Ingelheim/Mexico | Inactivated | Influenza A/duck/ China/E319-2/2003(H5N1) | H5N1 clade 2.3.2 | 93–98% |
| H5N1 | Reassortant AIV (Subtype H5N1) Vaccine (strain Re-1)/Zhaoqing DaHuaNong Biology Medicine, Sihui, China | Inactivated | Influenza A/goose/Guangdong/1996(H5N1) (Re-1) | H5N1 clade 0 | 94% |
| H5N1 | Reassortant AIV (strain Re-5) Re-5/Merial (USA) and QYH (China) | Inactivated | Influenza A/duck/Anhui/1/2006(H5N1) (Re-5) | H5N1 clade 2.3.4 | 93–98% |
| H5N1 | Egymune/Yebio Bioengineering company/China | Inactivated | Influenza A/duck/Anhui/1/2006(H5N1) (Re-5) | H5N1 clade 2.4.4. | 95% |
| H5N1 | Reassortant Re-8/Merial (USA) and QYH (China) | inactivated | Influenza A/chicken/Guizhou/4/2013(H5N1) (Re8) | H5N1 clade 2.3.4.4 | 95–97% |
4. Improper Antiviral Drug Prescription to Control AIVs in Avian Species and Its Impact on the Evolution of Drug-Resistant Variants
5. Adaptive Mammalian Mutation Markers in Egyptian AIVs
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.A.; Chen, L.-M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Rademan, R.; Geldenhuys, M.; Markotter, W. Detection and Characterization of an H9N2 Influenza A Virus in the Egyptian Rousette Bat in Limpopo, South Africa. Viruses 2023, 15, 498. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.; Starick, E.; Karamendin, K.; Genova, C.D.; Scott, S.D.; Khan, Y.; Harder, T.; Kydyrmanov, A. Genetic characterization of a new candidate hemagglutinin subtype of influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2225645. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Alexander, D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000, 74, 3–13. [Google Scholar] [CrossRef]
- Schmier, S.; Mostafa, A.; Haarmann, T.; Bannert, N.; Ziebuhr, J.; Veljkovic, V.; Dietrich, U.; Pleschka, S. In Silico Prediction and Experimental Confirmation of HA Residues Conferring Enhanced Human Receptor Specificity of H5N1 Influenza A Viruses. Sci. Rep. 2015, 5, 11434. [Google Scholar] [CrossRef]
- Schat, K.A.; Bingham, J.; Butler, J.M.; Chen, L.M.; Lowther, S.; Crowley, T.M.; Moore, R.J.; Donis, R.O.; Lowenthal, J.W. Role of position 627 of PB2 and the multibasic cleavage site of the hemagglutinin in the virulence of H5N1 avian influenza virus in chickens and ducks. PLoS ONE 2012, 7, e30960. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Suzuki, K.; Sakurai, Y.; Kubo, M.; Okada, H.; Itoh, T.; Tsukamoto, K. NP body domain and PB2 contribute to increased virulence of H5N1 highly pathogenic avian influenza viruses in chickens. J. Virol. 2011, 85, 1834–1846. [Google Scholar] [CrossRef] [PubMed]
- Wasilenko, J.L.; Lee, C.W.; Sarmento, L.; Spackman, E.; Kapczynski, D.R.; Suarez, D.L.; Pantin-Jackwood, M.J. NP, PB1, and PB2 viral genes contribute to altered replication of H5N1 avian influenza viruses in chickens. J. Virol. 2008, 82, 4544–4553. [Google Scholar] [CrossRef]
- Wasilenko, J.L.; Sarmento, L.; Pantin-Jackwood, M.J. A single substitution in amino acid 184 of the NP protein alters the replication and pathogenicity of H5N1 avian influenza viruses in chickens. Arch. Virol. 2009, 154, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Veits, J.; Weber, S.; Stech, O.; Breithaupt, A.; Graber, M.; Gohrbandt, S.; Bogs, J.; Hundt, J.; Teifke, J.P.; Mettenleiter, T.C.; et al. Avian influenza virus hemagglutinins H2, H4, H8, and H14 support a highly pathogenic phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 2579–2584. [Google Scholar] [CrossRef]
- Tscherne, D.M.; Garcia-Sastre, A. Virulence determinants of pandemic influenza viruses. J. Clin. Investig. 2011, 121, 6–13. [Google Scholar] [CrossRef]
- Harris, E. Human Flu Cases in Cambodia Not Due to Bird Flu Outbreak Viruses. JAMA 2023, 329, 1053. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cumulative Number of Confirmed HUMAN cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2023. Available online: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2022-5-jan-2023/ (accessed on 9 October 2023).
- WHO. Human Infection with Avian Influenza A(H5) Viruses: Avian Influenza Weekly Update Number 914. Available online: https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20230922.pdf (accessed on 9 October 2023).
- El-Shesheny, R.; Kandeil, A.; Mostafa, A.; Ali, M.A.; Webby, R.J. H5 Influenza Viruses in Egypt. Cold Spring Harb. Perspect. Med. 2021, 11, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Kayed, A.; Moatasim, Y.; Webby, R.J.; McKenzie, P.P.; Kayali, G.; Ali, M.A. Genetic characterization of highly pathogenic avian influenza A H5N8 viruses isolated from wild birds in Egypt. J. Gen. Virol. 2017, 98, 1573–1586. [Google Scholar] [CrossRef]
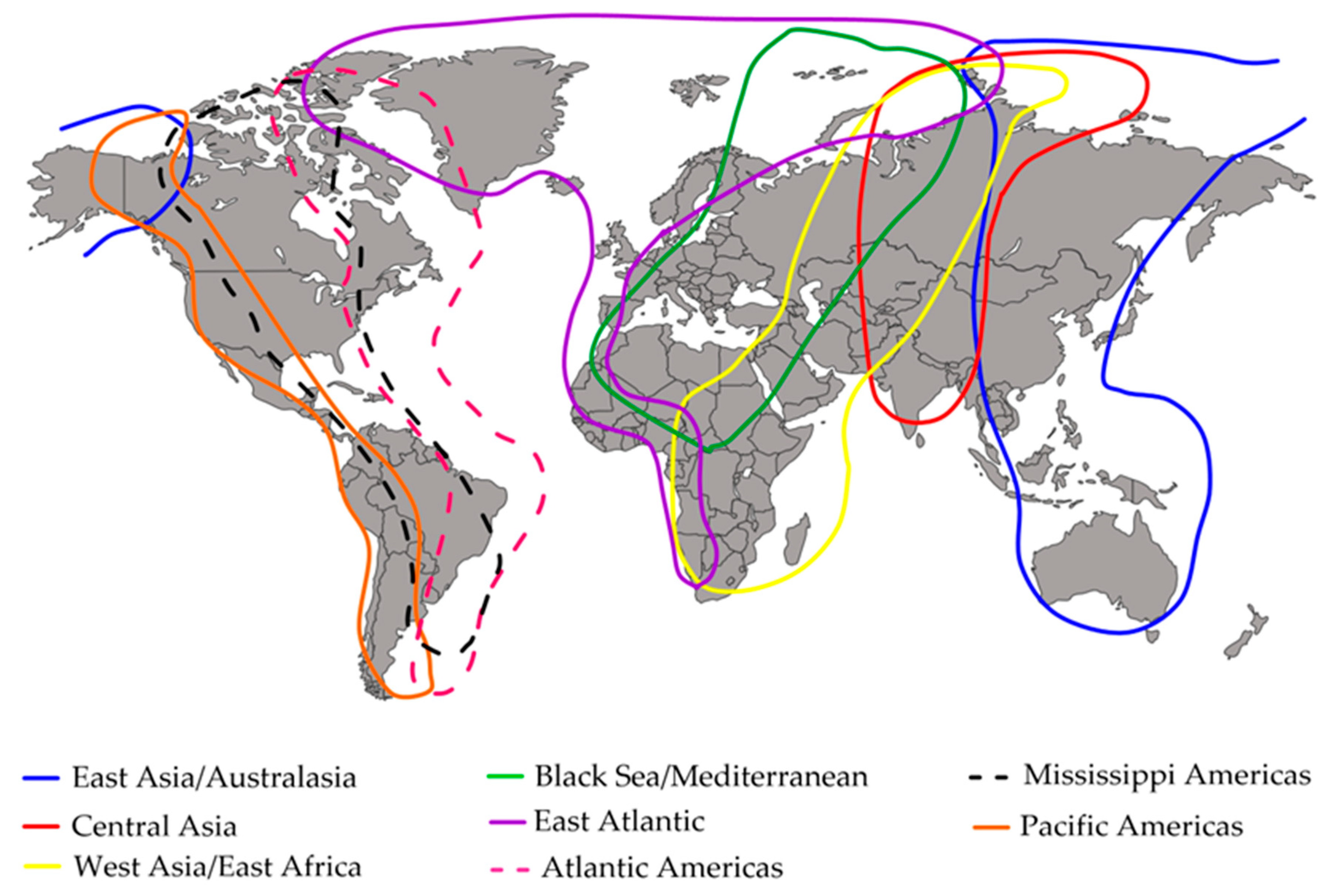
- Boere, G.; Stroud, D. The Flyway Concept: What it Is and What it Isn’t. In Waterbirds around the World; Boere, G.C., Galbraith, C.A., Stroud, D.A., Eds.; The Stationery Office: Edinburgh, UK, 2006; pp. 40–47. [Google Scholar]
- Chan, P.K. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 34 (Suppl. S2), S58–S64. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Donis, R.O.; World Health Organization/World Organisation for Animal Health/Food and Agriculture Organization (WHO/OIE/FAO) H5 Evolution Working Group. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Saad, M.D.; Ahmed, L.S.; Gamal-Eldein, M.A.; Fouda, M.K.; Khalil, F.; Yingst, S.L.; Parker, M.A.; Montevillel, M.R. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg. Infect. Dis. 2007, 13, 1120–1121. [Google Scholar] [CrossRef]
- Abdelwhab, E.M.; Hassan, M.K.; Abdel-Moneim, A.S.; Naguib, M.M.; Mostafa, A.; Hussein, I.T.M.; Arafa, A.; Erfan, A.M.; Kilany, W.H.; Agour, M.G.; et al. Introduction and enzootic of A/H5N1 in Egypt. Virus evolution, pathogenicity and vaccine efficacy ten years on. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 40, 80–90. [Google Scholar] [CrossRef]
- Salaheldin, A.H.; Veits, J.; Abd El-Hamid, H.S.; Harder, T.C.; Devrishov, D.; Mettenleiter, T.C.; Hafez, H.M.; Abdelwhab, E.M. Isolation and genetic characterization of a novel 2.2.1.2a H5N1 virus from a vaccinated meat-turkeys flock in Egypt. Virol. J. 2017, 14, 48. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Kandeil, A.; Bagato, O.; Maatouq, A.M.; Moatasim, Y.; Rubrum, A.; Song, M.S.; Webby, R.J.; Ali, M.A.; Kayali, G. Molecular characterization of avian influenza H5N1 virus in Egypt and the emergence of a novel endemic subclade. J. Gen. Virol. 2014, 95 Pt 7, 1444. [Google Scholar] [CrossRef]
- Fusaro, A.; Monne, I.; Salviato, A.; Valastro, V.; Schivo, A.; Amarin, N.M.; Gonzalez, C.; Ismail, M.M.; Al-Ankari, A.R.; Al-Blowi, M.H.; et al. Phylogeography and evolutionary history of reassortant H9N2 viruses with potential human health implications. J. Virol. 2011, 85, 8413–8421. [Google Scholar] [CrossRef]
- Pusch, E.A.; Suarez, D.L. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Vet. Sci. 2018, 5, 82. [Google Scholar] [CrossRef]
- El-Zoghby, E.F.; Arafa, A.S.; Hassan, M.K.; Aly, M.M.; Selim, A.; Kilany, W.H.; Selim, U.; Nasef, S.; Aggor, M.G.; Abdelwhab, E.M.; et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch. Virol. 2012, 157, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Freidl, G.S.; Meijer, A.; de Bruin, E.; de Nardi, M.; Munoz, O.; Capua, I.; Breed, A.C.; Harris, K.; Hill, A.; Kosmider, R.; et al. Influenza at the animal-human interface: A review of the literature for virological evidence of human infection with swine or avian influenza viruses other than A(H5N1). Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2014, 19, 20793. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Peng, X.; Peng, X.; Cheng, L.; Lu, X.; Jin, C.; Xie, T.; Yao, H.; Wu, N. Genetic and molecular characterization of H9N2 and H5 avian influenza viruses from live poultry markets in Zhejiang Province, eastern China. Sci. Rep. 2015, 5, 17508. [Google Scholar] [CrossRef]
- Nagy, A.; Mettenleiter, T.C.; Abdelwhab, E.M. A brief summary of the epidemiology and genetic relatedness of avian influenza H9N2 virus in birds and mammals in the Middle East and North Africa. Epidemiol. Infect. 2017, 145, 3320–3333. [Google Scholar] [CrossRef]
- Anderson, T.; Capua, I.; Dauphin, G.; Donis, R.; Fouchier, R.; Mumford, E.; Peiris, M.; Swayne, D.; Thiermann, A. FAO-OIE-WHO Joint Technical Consultation on Avian Influenza at the Human-Animal Interface. Influenza Other Respir. Viruses 2010, 4, 1–29. [Google Scholar]
- Li, R.; Adel, A.; Bohlin, J.; Lundkvist, Å.; Olsen, B.; Pettersson, J.H.; Naguib, M.M. Phylogeographic Dynamics of Influenza A(H9N2) Virus Crossing Egypt. Front. Microbiol. 2020, 11, 392. [Google Scholar] [CrossRef]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Shi, W.; Huang, L.; Xia, W.; Liu, D.; Li, H.; Chen, S.; Lei, F.; Cao, L.; et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Eurosurveillance 2014, 19, 20841. [Google Scholar] [CrossRef]
- Pu, J.; Wang, S.; Yin, Y.; Zhang, G.; Carter, R.A.; Wang, J.; Xu, G.; Sun, H.; Wang, M.; Wen, C.; et al. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proc. Natl. Acad. Sci. USA 2015, 112, 548–553. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, W.; Li, X.; Bo, H.; Zhang, Y.; Zou, S.; Gao, R.; Dong, J.; Zhao, X.; Chen, W.; et al. Genesis and Dissemination of Highly Pathogenic H5N6 Avian Influenza Viruses. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Harder, T.; Maurer-Stroh, S.; Pohlmann, A.; Starick, E.; Horeth-Bontgen, D.; Albrecht, K.; Pannwitz, G.; Teifke, J.; Gunalan, V.; Lee, R.T.; et al. Influenza A(H5N8) Virus Similar to Strain in Korea Causing Highly Pathogenic Avian Influenza in Germany. Emerg. Infect. Dis. 2015, 21, 860–863. [Google Scholar] [CrossRef]
- Lee, D.H.; Kwon, J.H.; Noh, J.Y.; Park, J.K.; Yuk, S.S.; Erdene-Ochir, T.O.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Lee, S.W.; et al. Pathogenicity of the Korean H5N8 highly pathogenic avian influenza virus in commercial domestic poultry species. Avian Pathol. J. WVPA 2016, 45, 208–211. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Bertran, K.; DeJesus, E.; Smith, D.; Swayne, D.E. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet. Res. 2017, 48, 33. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.S.; Russier, M.; Jeevan, T.; Marathe, B.; Govorkova, E.A.; Russell, C.J.; Kim-Torchetti, M.; Choi, Y.K.; Brown, I.; Saito, T.; et al. Novel Highly Pathogenic Avian A(H5N2) and A(H5N8) Influenza Viruses of Clade 2.3.4.4 from North America Have Limited Capacity for Replication and Transmission in Mammals. mSphere 2016, 1, 10–1128. [Google Scholar] [CrossRef]
- Yuk, S.S.; Lee, D.H.; Park, J.K.; Tseren-Ochir, E.O.; Kwon, J.H.; Noh, J.Y.; Song, C.S. Experimental infection of dogs with highly pathogenic avian influenza virus (H5N8). J. Vet. Sci. 2017, 18, 381–384. [Google Scholar] [CrossRef]
- Choi, W.S.; Baek, Y.H.; Kwon, J.J.; Jeong, J.H.; Park, S.J.; Kim, Y.I.; Yoon, S.W.; Hwang, J.; Kim, M.H.; Kim, C.J.; et al. Rapid acquisition of polymorphic virulence markers during adaptation of highly pathogenic avian influenza H5N8 virus in the mouse. Sci. Rep. 2017, 7, 40667. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Sun, X.; Creager, H.M.; Zeng, H.; Belser, J.A.; Maines, T.R.; Tumpey, T.M. Pathogenesis and Transmission of Novel Highly Pathogenic Avian Influenza H5N2 and H5N8 Viruses in Ferrets and Mice. J. Virol. 2015, 89, 10286–10293. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Herfst, S.; Fouchier, R.A. Infectious disease. How a virus travels the world. Science 2015, 347, 616–617. [Google Scholar] [CrossRef]
- Pohlmann, A.; Starick, E.; Harder, T.; Grund, C.; Hoper, D.; Globig, A.; Staubach, C.; Dietze, K.; Strebelow, G.; Ulrich, R.G.; et al. Outbreaks among Wild Birds and Domestic Poultry Caused by Reassorted Influenza A(H5N8) Clade 2.3.4.4 Viruses, Germany, 2016. Emerg. Infect. Dis. 2017, 23, 633–636. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Bergervoet, S.A.; Harders, F.; Bossers, A.; Koch, G. Multiple Reassorted Viruses as Cause of Highly Pathogenic Avian Influenza A(H5N8) Virus Epidemic, the Netherlands, 2016. Emerg. Infect. Dis. 2017, 23, 1974–1981. [Google Scholar] [CrossRef]
- Fusaro, A.; Monne, I.; Mulatti, P.; Zecchin, B.; Bonfanti, L.; Ormelli, S.; Milani, A.; Cecchettin, K.; Lemey, P.; Moreno, A.; et al. Genetic Diversity of Highly Pathogenic Avian Influenza A(H5N8/H5N5) Viruses in Italy, 2016-17. Emerg. Infect. Dis. 2017, 23, 1543–1547. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Slaterus, R.; Bodewes, R.; Rijks, J.M.; Spierenburg, M.A.H.; Beerens, N.; Kelder, L.; Poen, M.J.; Stegeman, J.A.; Fouchier, R.A.M.; et al. Deaths among Wild Birds during Highly Pathogenic Avian Influenza A(H5N8) Virus Outbreak, the Netherlands. Emerg. Infect. Dis. 2017, 23, 2050–2054. [Google Scholar] [CrossRef]
- Scoizec, A.; Niqueux, E.; Thomas, R.; Daniel, P.; Schmitz, A.; Le Bouquin, S. Airborne Detection of H5N8 Highly Pathogenic Avian Influenza Virus Genome in Poultry Farms, France. Front. Vet. Sci. 2018, 5, 15. [Google Scholar] [CrossRef]
- Pohlmann, A.; Starick, E.; Grund, C.; Hoper, D.; Strebelow, G.; Globig, A.; Staubach, C.; Conraths, F.J.; Mettenleiter, T.C.; Harder, T.; et al. Swarm incursions of reassortants of highly pathogenic avian influenza virus strains H5N8 and H5N5, clade 2.3.4.4b, Germany, winter 2016/17. Sci. Rep. 2018, 8, 15. [Google Scholar] [CrossRef]
- Salaheldin, A.H.; El-Hamid, H.S.; Elbestawy, A.R.; Veits, J.; Hafez, H.M.; Mettenleiter, T.C.; Abdelwhab, E.M. Multiple Introductions of Influenza A(H5N8) Virus into Poultry, Egypt, 2017. Emerg. Infect. Dis. 2018, 24, 943. [Google Scholar] [CrossRef]
- Grund, C.; Hoffmann, D.; Ulrich, R.; Naguib, M.; Schinköthe, J.; Hoffmann, B.; Harder, T.; Saenger, S.; Zscheppang, K.; Tönnies, M.; et al. A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg. Microbes Infect. 2018, 7, 132. [Google Scholar] [CrossRef]
- Peyre, M.; Samaha, H.; Makonnen, Y.J.; Saad, A.; Abd-Elnabi, A.; Galal, S.; Ettel, T.; Dauphin, G.; Lubroth, J.; Roger, F.; et al. Avian influenza vaccination in Egypt: Limitations of the current strategy. J. Mol. Genet. Med. Int. J. Biomed. Res. 2009, 3, 198–204. [Google Scholar]
- Kim, J.-K.; Kayali, G.; Walker, D.; Forrest, H.L.; Ellebedy, A.H.; Griffin, Y.S.; Rubrum, A.; Bahgat, M.M.; Kutkat, M.A.; Ali, M.A.A.; et al. Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proc. Natl. Acad. Sci. USA 2010, 107, 11044–11049. [Google Scholar] [CrossRef]
- Guyonnet, V.; Peters, A.R. Are current avian influenza vaccines a solution for smallholder poultry farmers? Gates Open Res. 2020, 4, 122. [Google Scholar] [CrossRef]
- Kandeil, A.; Mostafa, A.; El-Shesheny, R.; El-Taweel, A.N.; Gomaa, M.; Galal, H.; Kayali, G.; Ali, M.A. Avian influenza H5N1 vaccination efficacy in Egyptian backyard poultry. Vaccine 2017, 35, 6195–6201. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Awad, A.M.; Sedeik, M.E. Examination of the protective efficacy of two avian influenza H5 vaccines against clade 2.3.4.4b H5N8 highly pathogenic avian influenza virus in commercial broilers. Res. Vet. Sci. 2021, 140, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Maatouq AM, Cai Z, McKenzie PP, Webby RJ, El Refaey S, Kandeel A et al: Avian Influenza A(H5N1) Virus in Egypt. Emerg Infect Dis 2016, 22, 379–388. [CrossRef]
- Salaheldin, A.H.; Elbestawy, A.R.; Abdelkader, A.M.; Sultan, H.A.; Ibrahim, A.A.; Abd El-Hamid, H.S.; Abdelwhab, E.M. Isolation of Genetically Diverse H5N8 Avian Influenza Viruses in Poultry in Egypt, 2019–2021. Viruses 2022, 14, 1431. [Google Scholar] [CrossRef] [PubMed]
- Elsafty, M.M.; Morsy, A.R.I.; Elsayed, M.F.; Soliman, R.A.; Abotaleb, M.M. Efficacy of some avian influenza H5 vaccines against local highly pathogenic avian influenza viruses subtype H5N8 isolated in 2018 and 2020 in Egypt. VacciMonitor 2023, 32, e03123. [Google Scholar]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.L.; Palese, P. Susceptibility of different strains of influenza A virus to the inhibitory effects of 2-deoxy-2,3-dehydro-n-trifluoracetylneuraminic acid (FANA). Virology 1975, 63, 98–104. [Google Scholar] [CrossRef]
- Hurt, A.C.; Selleck, P.; Komadina, N.; Shaw, R.; Brown, L.; Barr, I.G. Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral. Res. 2007, 73, 228–231. [Google Scholar] [CrossRef]
- Dufrasne, F. Baloxavir Marboxil: An Original New Drug against Influenza. Pharmaceuticals 2021, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, Y.; Ma, C.; Jing, X.; Balannick, V.; Pinto, L.H.; Lamb, R.A. An amantadine-sensitive chimeric BM2 ion channel of influenza B virus has implications for the mechanism of drug inhibition. Proc. Natl. Acad. Sci. USA 2009, 106, 18775–18779. [Google Scholar] [CrossRef]
- Crosby, N.; Deane, K.H.; Clarke, C.E. Amantadine in Parkinson’s disease. Cochrane Database Syst. Rev. 2003, 2003, Cd003468. [Google Scholar] [CrossRef]
- Bright, R.A.; Medina, M.J.; Xu, X.; Perez-Oronoz, G.; Wallis, T.R.; Davis, X.M.; Povinelli, L.; Cox, N.J.; Klimov, A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet 2005, 366, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- WHO. Clinical Management of Human Infection with Avian Influenza A (H5N1) Virus; World Health Organization: Geneva, Switzerland, 2007.
- Cyranoski, D. China’s chicken farmers under fire for antiviral abuse. Nature 2005, 435, 1009. [Google Scholar] [CrossRef] [PubMed]
- Hussein, I.; Abdelwhab, E. Why the Veterinary Use of Antivirals in Egypt should Stop? Nature Publishing Group, Nature Middle East: Dubai, United Arab Emirates, 2016. [Google Scholar]
- Belshe, R.B.; Smith, M.H.; Hall, C.B.; Betts, R.; Hay, A.J. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J. Virol. 1988, 62, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Peng, C.; Luo, J.; Wang, C.; Han, L.; Wu, B.; Ji, G.; He, H. Adamantane-resistant influenza a viruses in the world (1902-2013): Frequency and distribution of M2 gene mutations. PLoS ONE 2015, 10, e0119115. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Rayner, J.M.; Smith, G.J.D.; Wang, P.; Naipospos, T.S.P.; Zhang, J.; Yuen, K.-Y.; Webster, R.G.; Peiris, J.S.M.; Guan, Y.; et al. Distribution of Amantadine-Resistant H5N1 Avian Influenza Variants in Asia. J. Infect. Dis. 2006, 193, 1626–1629. [Google Scholar] [CrossRef]
- Rohaim, M.A.; El-Naggar, .R.F.; Hamoud, M.M.; Nasr, S.A.; Ismael, E.; Laban, S.E.; Ahmed, H.A.; Munir, M. Re-Emergence of a Novel H5N1 Avian Influenza Virus Variant Subclade 2.2.1.1 in Egypt During 2014. Transbound. Emerg. Dis. 2017, 64, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Younan, M.; Poh, M.K.; Elassal, E.; Davis, T.; Rivailler, P.; Balish, A.L.; Simpson, N.; Jones, J.; Deyde, V.; Loughlin, R.; et al. Microevolution of highly pathogenic avian influenza A(H5N1) viruses isolated from humans, Egypt, 2007–2011. Emerg. Infect. Dis. 2013, 19, 43–50. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Bagato, O.; Kandeil, A.; Mostafa, A.; Mahmoud, S.H.; Hassanneen, H.M.; Webby, R.J.; Ali, M.A.; Kayali, G. Re-emergence of amantadine-resistant variants among highly pathogenic avian influenza H5N1 viruses in Egypt. Infect. Genet. Evol. 2016, 46, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Arafa, A.-S.A.; El-Kady, M.F.; Selim, A.A.; Gunalan, V.; Maurer-Stroh, S.; Goller, K.V.; Hassan, M.K.; Beer, M.; Abdelwhab, E.M.; et al. Evolutionary trajectories and diagnostic challenges of potentially zoonotic avian influenza viruses H5N1 and H9N2 co-circulating in Egypt. Infect. Genet. Evol. 2015, 34, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.H.P.; James, J.; Sealy, J.E.; Iqbal, M. A Global Perspective on H9N2 Avian Influenza Virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Monne, I.; Hussein, H.A.; Fusaro, A.; Valastro, V.; Hamoud, M.M.; Khalefa, R.A.; Dardir, S.N.; Radwan, M.I.; Capua, I.; Cattoli, G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir. Viruses 2013, 7, 240–243. [Google Scholar] [CrossRef]
- Hai, R.; Schmolke, M.; Leyva-Grado, V.H.; Thangavel, R.R.; Margine, I.; Jaffe, E.L.; Krammer, F.; Solórzano, A.; García-Sastre, A.; Palese, P.; et al. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat. Commun. 2013, 4, 2854. [Google Scholar] [CrossRef]
- Moscona, A. Neuraminidase inhibitors for influenza. N. E. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Skehel, J.J. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J. Biol. Chem. 2010, 285, 28403–28409. [Google Scholar] [CrossRef]
- Ferraris, O.; Lina, B. Mutations of neuraminidase implicated in neuraminidase inhibitors resistance. J. Clin. Virol. 2008, 41, 13–19. [Google Scholar] [CrossRef] [PubMed]
- McKimm-Breschkin, J.L.; Selleck, P.W.; Usman, T.B.; Johnson, M.A. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg. Infect. Dis. 2007, 13, 1354–1357. [Google Scholar] [CrossRef]
- Yen, H.L.; Ilyushina, N.A.; Salomon, R.; Hoffmann, E.; Webster, R.G.; Govorkova, E.A. Neuraminidase inhibitor-resistant recombinant A/Vietnam/1203/04 (H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo. J. Virol. 2007, 81, 12418–12426. [Google Scholar] [CrossRef]
- Orozovic, G.; Orozovic, K.; Lennerstrand, J.; Olsen, B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS ONE 2011, 6, e16028. [Google Scholar] [CrossRef] [PubMed]
- Yegani, S.; Shoushtari, A.H.; Eshratabadi, F.; Molouki, A. Full sequence analysis of hemagglutinin and neuraminidase genes and proteins of highly pathogenic avian influenza H5N1 virus detected in Iran, 2015. Trop. Anim. Health Prod. 2019, 51, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Skog, E.; Nykvist, M.; Naguib, M.M.; Wille, M.; Bröjer, C.; Agarwal, V.; Ellström, P.; Westman, G.; Lundkvist, Å.; Järhult, J.D. An oseltamivir-resistant avian H1N1 influenza A virus can transmit from mallards to chickens similarly to a wild-type strain: Implications for the risk of resistance transmission to humans. J. Gen. Virol. 2023, 104, 001835. [Google Scholar] [CrossRef]
- Bröjer, C.; Järhult, J.D.; Muradrasoli, S.; Söderström, H.; Olsen, B.; Gavier-Widén, D. Pathobiology and virus shedding of low-pathogenic avian influenza virus (A/H1N1) infection in mallards exposed to oseltamivir. J. Wildl. Dis. 2013, 49, 103–113. [Google Scholar] [CrossRef]
- Takashita, E.; Daniels, R.S.; Fujisaki, S.; Gregory, V.; Gubareva, L.V.; Huang, W.; Hurt, A.C.; Lackenby, A.; Nguyen, H.T.; Pereyaslov, D.; et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2017-2018. Antiviral. Res. 2020, 175, 104718. [Google Scholar] [CrossRef] [PubMed]
- Hurt, A.C. The epidemiology and spread of drug resistant human influenza viruses. Curr. Opin. Virol. 2014, 8, 22–29. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Fry, A.M.; Gubareva, L.V. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir. Ther. 2012, 17 Pt B, 159–173. [Google Scholar] [CrossRef]
- Trebbien, R.; Pedersen, S.S.; Vorborg, K.; Franck, K.T.; Fischer, T.K. Development of oseltamivir and zanamivir resistance in influenza A(H1N1)pdm09 virus, Denmark, 2014. Eurosurveillance 2017, 22, 30445. [Google Scholar] [CrossRef] [PubMed]
- Hurt, A.C.; Holien, J.K.; Parker, M.; Kelso, A.; Barr, I.G. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J. Virol. 2009, 83, 10366–10373. [Google Scholar] [CrossRef] [PubMed]
- Gubareva, L.V.; Matrosovich, M.N.; Brenner, M.K.; Bethell, R.C.; Webster, R.G. Evidence for Zanamivir Resistance in an Immunocompromised Child Infected with Influenza B Virus. J. Infect. Dis. 1998, 178, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Noshi, T.; Kitano, M.; Taniguchi, K.; Yamamoto, A.; Omoto, S.; Baba, K.; Hashimoto, T.; Ishida, K.; Kushima, Y.; Hattori, K.; et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral. Res. 2018, 160, 109–117. [Google Scholar] [CrossRef]
- Nakauchi, M.; Takashita, E.; Fujisaki, S.; Shirakura, M.; Ogawa, R.; Morita, H.; Miura, H.; Saito, S.; Watanabe, S.; Odagiri, T.; et al. Rapid detection of an I38T amino acid substitution in influenza polymerase acidic subunit associated with reduced susceptibility to baloxavir marboxil. Influenza Other Respir. Viruses 2020, 14, 436–443. [Google Scholar] [CrossRef]
- Gubareva, L.V.; Mishin, V.P.; Patel, M.C.; Chesnokov, A.; Nguyen, H.T.; De La Cruz, J.; Spencer, S.; Campbell, A.P.; Sinner, M.; Reid, H.; et al. Assessing baloxavir susceptibility of influenza viruses circulating in the United States during the 2016/17 and 2017/18 seasons. Eurosurveillance 2019, 24, 1800666. [Google Scholar] [CrossRef]
- Omoto, S.; Speranzini, V.; Hashimoto, T.; Noshi, T.; Yamaguchi, H.; Kawai, M.; Kawaguchi, K.; Uehara, T.; Shishido, T.; Naito, A.; et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 2018, 8, 9633. [Google Scholar] [CrossRef]
- Soga, T.; Duong, C.; Pattinson, D.; Sakai-Tagawa, Y.; Tokita, A.; Izumida, N.; Nishino, T.; Hagiwara, H.; Wada, N.; Miyamoto, Y.; et al. Characterization of influenza A(H1N1)pdm09 viruses isolated in the 2018-2019 and 2019-2020 influenza seasons in Japan. Viruses 2023, 15, 535. [Google Scholar] [CrossRef]
- Wit Ed Munster, V.J.; Riel Dv Beyer, W.E.P.; Rimmelzwaan, G.F.; Kuiken, T.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Molecular Determinants of Adaptation of Highly Pathogenic Avian Influenza H7N7 Viruses to Efficient Replication in the Human Host. J. Virol. 2010, 84, 1597–1606. [Google Scholar]
- Wagner, R.; Matrosovich, M.; Klenk, H.-D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 2002, 12, 159–166. [Google Scholar] [CrossRef]
- Steel, J.; Lowen, A.C. Influenza A virus reassortment. Curr. Top. Microbiol. Immunol. 2014, 385, 377–401. [Google Scholar]
- Cauldwell, A.V.; Long, J.S.; Moncorgé, O.; Barclay, W.S. Viral determinants of influenza A virus host range. J. Gen. Virol. 2014, 95, 1193–1210. [Google Scholar] [PubMed]
- Watanabe, Y.; Ibrahim, M.S.; Suzuki, Y.; Ikuta, K. The changing nature of avian influenza A virus (H5N1). Trends. Microbiol. 2012, 20, 11–20. [Google Scholar] [PubMed]
- Matrosovich, M.; Tuzikov, A.; Bovin, N.; Gambaryan, A.; Klimov, A.; Castrucci, M.R.; Donatelli, I.; Kawaoka, Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 2000, 74, 8502–8512. [Google Scholar]
- Oshansky, C.M.; Pickens, J.A.; Bradley, K.C.; Jones, L.P.; Saavedra-Ebner, G.M.; Barber, J.P.; Crabtree, J.M.; Steinhauer, D.A.; Tompkins, S.M.; Tripp, R.A. Avian influenza viruses infect primary human bronchial epithelial cells unconstrained by sialic acid α2,3 residues. PLoS ONE 2011, 6, e21183. [Google Scholar]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. At the centre: Influenza A virus ribonucleoproteins. Nat. Rev. Microbiol. 2015, 13, 28–41. [Google Scholar] [PubMed]
- Naffakh, N.; Tomoiu, A.; Rameix-Welti, M.A.; van der Werf, S. Host restriction of avian influenza viruses at the level of the ribonucleoproteins. Annu. Rev. Microbiol. 2008, 62, 403–424. [Google Scholar]
- Herfst, S.; Schrauwen, E.J.; Linster, M.; Chutinimitkul, S.; de Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef]
- Boivin, S.; Cusack, S.; Ruigrok, R.W.; Hart, D.J. Influenza A virus polymerase: Structural insights into replication and host adaptation mechanisms. J. Biol. Chem. 2010, 285, 28411–28417. [Google Scholar] [PubMed]
- Gabriel, G.; Fodor, E. Molecular determinants of pathogenicity in the polymerase complex. Curr. Top. Microbiol. Immunol. 2014, 385, 35–60. [Google Scholar]
- Stevaert, A.; Naesens, L. The Influenza Virus Polymerase Complex: An Update on Its Structure, Functions, and Significance for Antiviral Drug Design. Med. Res. Rev. 2016, 36, 1127–1173. [Google Scholar] [PubMed]
- Yang, R.; Sun, H.; Gao, F.; Luo, K.; Huang, Z.; Tong, Q.; Song, H.; Han, Q.; Liu, J.; Lan, Y. Human infection of avian influenza A H3N8 virus and the viral origins: A descriptive study. Lancet Microbe 2022, 3, e824–e834. [Google Scholar] [CrossRef]
- Huang, S.-W.; Wang, S.-F. The effects of genetic variation on H7N9 avian influenza virus pathogenicity. Viruses 2020, 12, 1220. [Google Scholar] [CrossRef]
- He, J.; Wu, Q.; Yu, J.-L.; He, L.; Sun, Y.; Shi, Y.-L.; Chen, Q.-Q.; Ge, Y.-L.; Zhang, Z.-H.; Li, W.-W. Sporadic occurrence of H9N2 avian influenza infections in human in Anhui province, eastern China: A notable problem. Microb. Pathog. 2020, 140, 103940. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Kawashita, N.; Ibrahim, M.S.; Elgendy, E.M.; Daidoji, T.; Ono, T.; Takagi, T.; Nakaya, T.; Matsumoto, K.; Watanabe, Y. PB2 mutations arising during H9N2 influenza evolution in the Middle East confer enhanced replication and growth in mammals. PLoS Pathog. 2019, 15, e1007919. [Google Scholar]
- Subbarao, E.K.; London, W.; Murphy, B.R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993, 67, 1761–1764. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, J.; Zou, R.; Pan, J.; Jin, T.; Li, L.; Liu, P.; Zhao, Y.; Yu, X.; Wang, H. Dynamic PB2-E627K substitution of influenza H7N9 virus indicates the in vivo genetic tuning and rapid host adaptation. Proc. Natl. Acad. Sci. USA 2020, 117, 23807–23814. [Google Scholar]
- Sun, X.; Belser, J.A.; Maines, T.R. Adaptation of H9N2 Influenza Viruses to Mammalian Hosts: A Review of Molecular Markers. Viruses 2020, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Everest, H.; Billington, E.; Daines, R.; Burman, A.; Iqbal, M. The Emergence and Zoonotic Transmission of H10Nx Avian Influenza Virus Infections. Mbio 2021, 12, e01785-21. [Google Scholar] [CrossRef]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef]
- Hatta, M.; Hatta, Y.; Kim, J.H.; Watanabe, S.; Shinya, K.; Nguyen, T.; Lien, P.S.; Le, Q.M.; Kawaoka, Y. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog. 2007, 3, 1374–1379. [Google Scholar] [CrossRef]
- Bussey, K.A.; Bousse, T.L.; Desmet, E.A.; Kim, B.; Takimoto, T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J. Virol. 2010, 84, 4395–4406. [Google Scholar] [CrossRef]
- Mänz, B.; Schwemmle, M.; Brunotte, L. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J. Virol. 2013, 87, 7200–7209. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, W.B.; Xu, K.; He, Y.X.; Wang, T.Y.; Chen, Z.; Li, T.X.; Liu, J.H.; Buchy, P.; Sun, B. Amino acids 473V and 598P of PB1 from an avian-origin influenza A virus contribute to polymerase activity, especially in mammalian cells. J. Gen. Virol. 2012, 93 Pt 3, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Dankar, S.K.; Forbes, N.E.; Jia, J.-J.; Brown, E.G. Adaptive mutation in influenza A virus non-structural gene is linked to host switching and induces a novel protein by alternative splicing. Emerg. Microbes Infect. 2012, 1, e42. [Google Scholar] [CrossRef] [PubMed]
- Suttie, A.; Deng, Y.M.; Greenhill, A.R.; Dussart, P.; Horwood, P.F.; Karlsson, E.A. Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes 2019, 55, 739–768. [Google Scholar] [CrossRef]
- Zhu, W.; Feng, Z.; Chen, Y.; Yang, L.; Liu, J.; Li, X.; Liu, S.; Zhou, L.; Wei, H.; Gao, R. Mammalian-adaptive mutation NP-Q357K in Eurasian H1N1 swine influenza viruses determines the virulence phenotype in mice. Emerg. Microbes Infect. 2019, 8, 989–999. [Google Scholar] [CrossRef]
- Mänz, B.; Dornfeld, D.; Götz, V.; Zell, R.; Zimmermann, P.; Haller, O.; Kochs, G.; Schwemmle, M. Pandemic Influenza A Viruses Escape from Restriction by Human MxA through Adaptive Mutations in the Nucleoprotein. PLOS Pathog. 2013, 9, e1003279. [Google Scholar] [CrossRef]
- Valley-Omar, Z.; Cloete, A.; Pieterse, R.; Walaza, S.; Salie-Bassier, Y.; Smith, M.; Govender, N.; Seleka, M.; Hellferscee, O.; Mtshali, P.S. Human surveillance and phylogeny of highly pathogenic avian influenza A (H5N8) during an outbreak in poultry in South Africa, 2017. Influenza Other Respir. Viruses 2020, 14, 266–273. [Google Scholar] [CrossRef]
- Mullick, J.; Cherian, S.S.; Potdar, V.A.; Chadha, M.S.; Mishra, A.C. Evolutionary dynamics of the influenza A pandemic (H1N1) 2009 virus with emphasis on Indian isolates: Evidence for adaptive evolution in the HA gene. Infect. Genet. Evol. 2011, 11, 997–1005. [Google Scholar] [CrossRef]
- Evseev, D.; Magor, K.E. Molecular evolution of the influenza A virus non-structural protein 1 in interspecies transmission and adaptation. Front. Microbiol. 2021, 12, 693204. [Google Scholar] [CrossRef]
- Lloren, K.K.S.; Lee, T.; Kwon, J.J.; Song, M.S. Molecular Markers for Interspecies Transmission of Avian Influenza Viruses in Mammalian Hosts. Int J Mol Sci 2017, 18, 2706. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Blixt, O.; Tumpey, T.M.; Taubenberger, J.K.; Paulson, J.C.; Wilson, I.A. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 2006, 312, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ibrahim, M.S.; Ellakany, H.F.; Kawashita, N.; Mizuike, R.; Hiramatsu, H.; Sriwilaijaroen, N.; Takagi, T.; Suzuki, Y.; Ikuta, K. Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog. 2011, 7, e1002068. [Google Scholar] [CrossRef]
- Auewarakul, P.; Suptawiwat, O.; Kongchanagul, A.; Sangma, C.; Suzuki, Y.; Ungchusak, K.; Louisirirotchanakul, S.; Lerdsamran, H.; Pooruk, P.; Thitithanyanont, A.; et al. An avian influenza H5N1 virus that binds to a human-type receptor. J. Virol. 2007, 81, 9950–9955. [Google Scholar] [CrossRef]
- Ayora-Talavera, G.; Shelton, H.; Scull, M.A.; Ren, J.; Jones, I.M.; Pickles, R.J.; Barclay, W.S. Mutations in H5N1 influenza virus hemagglutinin that confer binding to human tracheal airway epithelium. PLoS ONE 2009, 4, e7836. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, A.; Tuzikov, A.; Pazynina, G.; Bovin, N.; Balish, A.; Klimov, A. Evolution of the receptor binding phenotype of influenza A (H5) viruses. Virology 2006, 344, 432–438. [Google Scholar] [CrossRef]
- Yamada, S.; Suzuki, Y.; Suzuki, T.; Le, M.Q.; Nidom, C.A.; Sakai-Tagawa, Y.; Muramoto, Y.; Ito, M.; Kiso, M.; Horimoto, T.; et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006, 444, 378–382. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, T.; Hatta, M.; Das, S.C.; Ozawa, M.; Shinya, K.; Zhong, G.; Hanson, A.; Katsura, H.; Watanabe, S.; et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012, 486, 420–428. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Shinya, K.; Deng, G.; Jiang, Y.; Li, Z.; Guan, Y.; Tian, G.; Li, Y.; Shi, J.; et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009, 5, e1000709. [Google Scholar] [CrossRef]
- Xu, W.; Dai, Y.; Hua, C.; Wang, Q.; Zou, P.; Deng, Q.; Jiang, S.; Lu, L. Genomic signature analysis of the recently emerged highly pathogenic A(H5N8) avian influenza virus: Implying an evolutionary trend for bird-to-human transmission. Microbes Infect. 2017, 19, 597–604. [Google Scholar] [CrossRef]
- Gao, R.; Gu, M.; Liu, K.; Li, Q.; Li, J.; Shi, L.; Li, X.; Wang, X.; Hu, J.; Liu, X.; et al. T160A mutation-induced deglycosylation at site 158 in hemagglutinin is a critical determinant of the dual receptor binding properties of clade 2.3.4.4 H5NX subtype avian influenza viruses. Vet. Microbiol. 2018, 217, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Sorrell, E.M.; Song, H.; Hossain, M.J.; Ramirez-Nieto, G.; Monne, I.; Stevens, J.; Cattoli, G.; Capua, I.; Chen, L.M.; et al. Replication and transmission of H9N2 influenza viruses in ferrets: Evaluation of pandemic potential. PLoS ONE 2008, 3, e2923. [Google Scholar] [CrossRef]
- Peiris, J.S.; Guan, Y.; Markwell, D.; Ghose, P.; Webster, R.G.; Shortridge, K.F. Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: Potential for genetic reassortment? J. Virol. 2001, 75, 9679–9686. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Krauss, S.; Webster, R.G. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 2001, 281, 156–162. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.; Guo, J.; Deng, G.; Zhang, Q.; Wang, J.; He, X.; Wang, K.; Chen, J.; Li, Y.; et al. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 Avian Influenza viruses. PLoS Pathog. 2014, 10, e1004508. [Google Scholar] [CrossRef] [PubMed]
- Mashaal, D.; Mahmoud, S.H.; Müller, C.; Abo Shama, N.M.; Kamer, A.A.; Abdelaziz, A.A.; Ali, M.A.; Pleschka, S.; Mostafa, A. Differential Impact of Specific Amino Acid Residues on the Characteristics of Avian Influenza Viruses in Mammalian Systems. Pathogens 2022, 11, 1385. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Moatasim, Y.; El Taweel, A.; El Sayes, M.; Rubrum, A.; Jeevan, T.; McKenzie, P.P.; Webby, R.J.; Ali, M.A.; Kayali, G. Genetic and antigenic characteristics of highly pathogenic avian influenza A (H5N8) viruses circulating in domestic poultry in Egypt, 2017–2021. Microorganisms 2022, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Chang, S.C.; Mok, C.K.; Lo, Y.L.; Kung, Y.N.; Huang, J.H.; Shih, Y.H.; Wang, J.Y.; Chiang, C.; Chen, C.J.; et al. Genomic signatures of human versus avian influenza A viruses. Emerg. Infect. Dis. 2006, 12, 1353. [Google Scholar] [CrossRef]
- Wen L, Chu H, Wong BH-Y, Wang D, Li C, Zhao X, Chiu M-C, Yuan S, Fan Y, Chen H et al: Large-scale sequence analysis reveals novel human-adaptive markers in PB2 segment of seasonal influenza A viruses. Emerg. Microbes Infect. 2018, 7, 1–12.
- Shaw, M.; Cooper, L.; Xu, X.; Thompson, W.; Krauss, S.; Guan, Y.; Zhou, N.; Klimov, A.; Cox, N.; Webster, R.; et al. Molecular changes associated with the transmission of avian influenza a H5N1 and H9N2 viruses to humans. J. Med. Virol. 2002, 66, 107–114. [Google Scholar] [CrossRef]
- Kandeil, A.; El-Shesheny, R.; Maatouq, A.M.; Moatasim, Y.; Shehata, M.M.; Bagato, O.; Rubrum, A.; Shanmuganatham, K.; Webby, R.J.; Ali, M.A. Genetic and antigenic evolution of H9N2 avian influenza viruses circulating in Egypt between 2011 and 2013. Arch. Virol. 2014, 159, 2861–2876. [Google Scholar] [CrossRef]
- Guilligay, D.; Tarendeau, F.; Resa-Infante, P.; Coloma, R.; Crepin, T.; Sehr, P.; Lewis, J.; Ruigrok, R.W.H.; Ortin, J.; Hart, D.J.; et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 2008, 15, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Cattoli, G.; Monne, I.; Fusaro, A.; Joannis, T.M.; Lombin, L.H.; Aly, M.M.; Arafa, A.S.; Sturm-Ramirez, K.M.; Couacy-Hymann, E.; Awuni, J.A. Highly pathogenic avian influenza virus subtype H5N1 in Africa: A comprehensive phylogenetic analysis and molecular characterization of isolates. PLoS ONE 2009, 4, e4842. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Moatasim, Y.; Mahmoud, S.H.; Song, Y.; El Taweel, A.; Gomaa, M.; Kamel, M.N.; Sayes, M.E.; Kandeil, A.; Lam, T.T. Highly Pathogenic Avian Influenza A (H5N1) Virus Clade 2.3. 4.4 b in Wild Birds and Live Bird Markets, Egypt. Pathogens 2022, 12, 36. [Google Scholar] [CrossRef]
- Li, J.; Ishaq, M.; Prudence, M.; Xi, X.; Hu, T.; Liu, Q.; Guo, D. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 2009, 144, 123–129. [Google Scholar] [CrossRef]
- Samir, A.; Adel, A.; Arafa, A.; Sultan, H.; Hussein Ahmed, H.A. Molecular pathogenic and host range determinants of reassortant Egyptian low pathogenic avian influenza H9N2 viruses from backyard chicken. Int. J. Vet. Sci. Med. 2019, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Ma, W.; Sun, N.; Huang, L.; Li, Y.; Zeng, Z.; Wen, Y.; Zhang, Z.; Li, H.; Li, Q.; et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 2016, 6, 19474. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.P.; Lee, H.H.Y.; Lestra, M.; Nicholls, J.M.; Chan, M.C.W.; Sia, S.F.; Zhu, H.; Poon, L.L.M.; Guan, Y.; Peiris, J.S.M. Amino Acid Substitutions in Polymerase Basic Protein 2 Gene Contribute to the Pathogenicity of the Novel A/H7N9 Influenza Virus in Mammalian Hosts. J. Virol. 2014, 88, 3568–3576. [Google Scholar] [CrossRef]
- Elgendy, E.M.; Watanabe, Y.; Daidoji, T.; Arai, Y.; Ikuta, K.; Ibrahim, M.S.; Nakaya, T. Genetic characterization of highly pathogenic avian influenza H5N1 viruses isolated from naturally infected pigeons in Egypt. Virus Genes 2016, 52, 867–871. [Google Scholar] [CrossRef]
- Finkelstein, D.B.; Mukatira, S.; Mehta, P.K.; Obenauer, J.C.; Su, X.; Webster, R.G.; Naeve, C.W. Persistent host markers in pandemic and H5N1 influenza viruses. J. Virol. 2007, 81, 10292–10299. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Hu, Y.; Chang, G.; Sun, W.; Yang, Y.; Kang, X.; Wu, X.; Zhu, Q. PB1-mediated virulence attenuation of H5N1 influenza virus in mice is associated with PB2. J. Gen. Virol. 2011, 92, 1435–1444. [Google Scholar] [CrossRef]
- Bogs, J.; Kalthoff, D.; Veits, J.; Pavlova, S.; Schwemmle, M.; Mänz, B.; Mettenleiter, T.C.; Stech, J. Reversion of PB2-627E to -627K during replication of an H5N1 Clade 2.2 virus in mammalian hosts depends on the origin of the nucleoprotein. J. Virol. 2011, 85, 10691–10698. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Xu, Q.; Tan, Y.; Pu, J.; Yang, H.; Brown, E.G.; Liu, J. Mouse-Adapted H9N2 Influenza A Virus PB2 Protein M147L and E627K Mutations Are Critical for High Virulence. PLoS ONE 2012, 7, e40752. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.; Abdelmagid, M.A.; Mohamed, A.A.-E.; Wasberg, A.; Mosaad, Z.; Selim, K.; Shaaban, A.; Tarek, M.; Hagag, N.M.; Lundkvist, Å. Genetic Variations among Different Variants of G1-like Avian Influenza H9N2 Viruses and Their Pathogenicity in Chickens. Viruses 2022, 14, 1030. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, T.; Kise, D.; Yoshida, H.; Horita, T.; Murazaki, Y.; Nishimura, A.; Echigo, N.; Utsunomiya, H.; Tsuge, H. Structural Basis of the Influenza A Virus RNA Polymerase PB2 RNA-binding Domain Containing the Pathogenicity-determinant Lysine 627 Residue. J. Biol. Chem. 2009, 284, 6855–6860. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Zhang, X.; Xu, D.; Zhou, J.; Dai, X.; Chen, Z.; Li, Z. Characterization of an H3N2 canine influenza virus isolated from Tibetan mastiffs in China. Vet. Microbiol. 2013, 162, 345–352. [Google Scholar] [CrossRef]
- Elgendy, E.M.; Arai, Y.; Kawashita, N.; Daidoji, T.; Takagi, T.; Ibrahim, M.S.; Nakaya, T.; Watanabe, Y. Identification of polymerase gene mutations that affect viral replication in H5N1 influenza viruses isolated from pigeons. J. Gen. Virol. 2017, 98, 6–17. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Reid, A.H.; Lourens, R.M.; Wang, R.; Jin, G.; Fanning, T.G. Characterization of the 1918 influenza virus polymerase genes. Nature 2005, 437, 889–893. [Google Scholar] [CrossRef]
- Taft, A.S.; Ozawa, M.; Fitch, A.; Depasse, J.V.; Halfmann, P.J.; Hill-Batorski, L.; Hatta, M.; Friedrich, T.C.; Lopes, T.J.; Maher, E.A. Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus. Nat. Commun. 2015, 6, 7491. [Google Scholar] [CrossRef]
- Li, J.; Dohna, H.Z.; Cardona, C.J.; Miller, J.; Carpenter, T.E. Emergence and genetic variation of neuraminidase stalk deletions in avian influenza viruses. PLoS ONE 2011, 6, e14722. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, M.; Manicassamy, B.; Pena, L.; Sutton, T.; Hai, R.; Varga, Z.T.; Hale, B.G.; Steel, J.; Perez, D.R.; García-Sastre, A. Differential contribution of PB1-F2 to the virulence of highly pathogenic H5N1 influenza A virus in mammalian and avian species. PLoS Pathog. 2011, 7, e1002186. [Google Scholar] [CrossRef]
- Conenello, G.M.; Zamarin, D.; Perrone, L.A.; Tumpey, T.; Palese, P. A Single Mutation in the PB1-F2 of H5N1 (HK/97) and 1918 Influenza A Viruses Contributes to Increased Virulence. PLOS Pathog. 2007, 3, e141. [Google Scholar] [CrossRef] [PubMed]
- Wanitchang, A.; Jengarn, J.; Jongkaewwattana, A. The N terminus of PA polymerase of swine-origin influenza virus H1N1 determines its compatibility with PB2 and PB1 subunits through a strain-specific amino acid serine 186. Virus Res. 2011, 155, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Yamada, S.; Fukuyama, S.; Murakami, S.; Zhao, D.; Uraki, R.; Watanabe, T.; Tomita, Y.; Macken, C.; Neumann, G.; et al. Virulence-Affecting Amino Acid Changes in the PA Protein of H7N9 Influenza A Viruses. J. Virol. 2014, 88, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tang, G.; Huang, Y.; Yu, C.; Li, S.; Zhuang, L.; Fu, L.; Wang, S.; Li, N.; Li, X.; et al. A returning migrant worker with avian influenza A (H7N9) virus infection in Guizhou, China: A case report. J. Med. Case Rep. 2015, 9, 109. [Google Scholar] [CrossRef]
- Brown, E.G.; Liu, H.; Kit, L.C.; Baird, S.; Nesrallah, M. Pattern of mutation in the genome of influenza A virus on adaptation to increased virulence in the mouse lung: Identification of functional themes. Proc. Natl. Acad. Sci. USA 2001, 98, 6883–6888. [Google Scholar] [CrossRef]
- Yamaji, R.; Yamada, S.; Le, M.Q.; Ito, M.; Sakai-Tagawa, Y.; Kawaoka, Y. Mammalian Adaptive Mutations of the PA Protein of Highly Pathogenic Avian H5N1 Influenza Virus. J. Virol. 2015, 89, 4117–4125. [Google Scholar] [CrossRef]
- Hulse-Post, D.J.; Franks, J.; Boyd, K.; Salomon, R.; Hoffmann, E.; Yen, H.L.; Webby, R.J.; Walker, D.; Nguyen, T.D.; Webster, R.G. Molecular Changes in the Polymerase Genes (PA and PB1) Associated with High Pathogenicity of H5N1 Influenza Virus in Mallard Ducks. J. Virol. 2007, 81, 8515–8524. [Google Scholar] [CrossRef]
- Gabriel, G.; Dauber, B.; Wolff, T.; Planz, O.; Klenk, H.D.; Stech, J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. USA 2005, 102, 18590–18595. [Google Scholar] [CrossRef]
- Timofeeva, T.A.; Sadykova, G.K.; Lomakina, N.F.; Gambaryan, A.S.; Rudneva, I.A.; Timofeeva, E.B.; Shilov, A.A.; Boravleva, E.Y.; Zhuravleva, M.M.; Ivanov, P.A.; et al. The Effect of I155T, K156Q, K156E and N186K Mutations in Hemagglutinin on the Virulence and Reproduction of Influenza A/H5N1 Viruses. Mol. Biol. 2020, 54, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Maines, T.R.; Chen, L.M.; Van Hoeven, N.; Tumpey, T.M.; Blixt, O.; Belser, J.A.; Gustin, K.M.; Pearce, M.B.; Pappas, C.; Stevens, J.; et al. Effect of receptor binding domain mutations on receptor binding and transmissibility of avian influenza H5N1 viruses. Virology 2011, 413, 139–147. [Google Scholar] [CrossRef]
- Poucke, S.V.; Doedt, J.; Baumann, J.; Qiu, Y.; Matrosovich, T.; Klenk, H.-D.; Reeth, K.V.; Matrosovich, M. Role of Substitutions in the Hemagglutinin in the Emergence of the 1968 Pandemic Influenza Virus. J. Virol. 2015, 89, 12211–12216. [Google Scholar] [CrossRef] [PubMed]
- Chutinimitkul, S.; van Riel, D.; Munster, V.J.; van den Brand, J.M.; Rimmelzwaan, G.F.; Kuiken, T.; Osterhaus, A.D.; Fouchier, R.A.; de Wit, E. In Vitro Assessment of Attachment Pattern and Replication Efficiency of H5N1 Influenza A Viruses with Altered Receptor Specificity. J. Virol. 2010, 84, 6825–6833. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Herfst, S.; Sorrell, E.; Schrauwen, E.; Linster, M.; De Graaf, M.; Fouchier, R.; Kawaoka, Y. Transmission of influenza A/H5N1 viruses in mammals. Virus Res. 2013, 178, 15–20. [Google Scholar] [CrossRef]
- Wan, H.; Perez, D.R. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J. Virol. 2007, 81, 5181–5191. [Google Scholar] [CrossRef]
- Atim, G.; Tugume, T.; Ukuli, Q.A.; Erima, B.; Mubiru, A.; Kibuuka, H.; Mworozi, E.; McKenzie, P.; Turner, J.C.M.; Walker, D.; et al. Genetic Evolution of Avian Influenza A (H9N2) Viruses Isolated from Domestic Poultry in Uganda Reveals Evidence of Mammalian Host Adaptation, Increased Virulence and Reduced Sensitivity to Baloxavir. Viruses 2022, 14, 2074. [Google Scholar] [CrossRef]
- Tada, T.; Suzuki, K.; Sakurai, Y.; Kubo, M.; Okada, H.; Itoh, T.; Tsukamoto, K. Emergence of avian influenza viruses with enhanced transcription activity by a single amino acid substitution in the nucleoprotein during replication in chicken brains. J. Virol. 2011, 85, 10354–10363. [Google Scholar] [CrossRef]
- Alkie, T.N.; Lopes, S.; Hisanaga, T.; Xu, W.; Suderman, M.; Koziuk, J.; Fisher, M.; Redford, T.; Lung, O.; Joseph, T.; et al. A threat from both sides: Multiple introductions of genetically distinct H5 HPAI viruses into Canada via both East Asia-Australasia/Pacific and Atlantic flyways. Virus Evol. 2022, 8, veac077. [Google Scholar] [CrossRef]
- Sang, X.; Wang, A.; Ding, J.; Kong, H.; Gao, X.; Li, L.; Chai, T.; Li, Y.; Zhang, K.; Wang, C. Adaptation of H9N2 AIV in guinea pigs enables efficient transmission by direct contact and inefficient transmission by respiratory droplets. Sci. Rep. 2015, 5, 15928. [Google Scholar] [CrossRef]
- Katz, J.M.; Lu, X.; Tumpey, T.M.; Smith, C.B.; Shaw, M.W.; Subbarao, K. Molecular Correlates of Influenza A H5N1 Virus Pathogenesis in Mice. J. Virol. 2000, 74, 10807–10810. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Deng, G.; Song, J.; Tian, G.; Suo, Y.; Jiang, Y.; Guan, Y.; Bu, Z.; Kawaoka, Y.; Chen, H. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 2009, 384, 28–32. [Google Scholar] [CrossRef]
- Nao, N.; Kajihara, M.; Manzoor, R.; Maruyama, J.; Yoshida, R.; Muramatsu, M.; Miyamoto, H.; Igarashi, M.; Eguchi, N.; Sato, M. A single amino acid in the M1 protein responsible for the different pathogenic potentials of H5N1 highly pathogenic avian influenza virus strains. PLoS ONE 2015, 10, e0137989. [Google Scholar] [CrossRef]
- Soubies, S.M.; Volmer, C.; Croville, G.; Loupias, J.; Peralta, B.; Costes, P.; Lacroux, C.; Guérin, J.-L.; Volmer, R. Species-Specific Contribution of the Four C-Terminal Amino Acids of Influenza A Virus NS1 Protein to Virulence. J. Virol. 2010, 84, 6733–6747. [Google Scholar] [CrossRef]
- Jiao, P.; Tian, G.; Li, Y.; Deng, G.; Jiang, Y.; Liu, C.; Liu, W.; Bu, Z.; Kawaoka, Y.; Chen, H. A Single-Amino-Acid Substitution in the NS1 Protein Changes the Pathogenicity of H5N1 Avian Influenza Viruses in Mice. J. Virol. 2008, 82, 1146–1154. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Chen, Q.; Zhang, X.; Sun, Y.; Bi, Y.; Zhang, S.; Gu, J.; Li, J.; Liu, D. Three amino acid substitutions in the NS1 protein change the virus replication of H5N1 influenza virus in human cells. Virology 2018, 519, 64–73. [Google Scholar] [CrossRef]
- Kanrai, P.; Mostafa, A.; Madhugiri, R.; Lechner, M.; Wilk, E.; Schughart, K.; Ylösmäki, L.; Saksela, K.; Ziebuhr, J.; Pleschka, S. Identification of specific residues in avian influenza A virus NS1 that enhance viral replication and pathogenicity in mammalian systems. J. Gen. Virol. 2016, 97, 2135–2148. [Google Scholar] [CrossRef]
- Heui Seo, S.; Hoffmann, E.; Webster, R.G. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 2002, 8, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Dankar, S.K.; Wang, S.; Ping, J.; Forbes, N.E.; Keleta, L.; Li, Y.; Brown, E.G. Influenza A virus NS1 gene mutations F103L and M106I increase replication and virulence. Virol. J. 2011, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ayllon, J.; Domingues, P.; Rajsbaum, R.; Miorin, L.; Schmolke, M.; Hale, B.G.; García-Sastre, A. A single amino acid substitution in the novel H7N9 influenza A virus NS1 protein increases CPSF30 binding and virulence. J. Virol. 2014, 88, 12146–12151. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Jiao, P.; Wang, A.; Zhao, F.; Tian, G.; Wang, X.; Yu, K.; Bu, Z.; Chen, H. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J. Virol. 2006, 80, 11115–11123. [Google Scholar] [CrossRef]
- Forbes, N.E.; Ping, J.; Dankar, S.K.; Jia, J.-J.; Selman, M.; Keleta, L.; Zhou, Y.; Brown, E.G. Multifunctional Adaptive NS1 Mutations Are Selected upon Human Influenza Virus Evolution in the Mouse. PLoS ONE 2012, 7, e31839. [Google Scholar] [CrossRef]
- Reuther, P.; Giese, S.; Götz, V.; Kilb, N.; Mänz, B.; Brunotte, L.; Schwemmle, M. Adaptive mutations in the nuclear export protein of human-derived H5N1 strains facilitate a polymerase activity-enhancing conformation. J. Virol. 2014, 88, 263–271. [Google Scholar] [CrossRef]
- Guo, F.; Li, Y.; Yu, S.; Liu, L.; Luo, T.; Pu, Z.; Xiang, D.; Shen, X.; Irwin, D.M.; Liao, M.; et al. Adaptive Evolution of Human-Isolated H5Nx Avian Influenza A Viruses. Front. Microbiol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Yamaji, R.; Yamada, S.; Le, M.Q.; Li, C.; Chen, H.; Qurnianingsih, E.; Nidom, C.A.; Ito, M.; Sakai-Tagawa, Y.; Kawaoka, Y. Identification of PB2 mutations responsible for the efficient replication of H5N1 influenza viruses in human lung epithelial cells. J. Virol. 2015, 89, 3947–3956. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Hatta, M.; Kim, J.H.; Halfmann, P.; Imai, M.; Macken, C.A.; Le, M.Q.; Nguyen, T.; Neumann, G.; Kawaoka, Y. Novel residues in avian influenza virus PB2 protein affect virulence in mammalian hosts. Nat. Commun. 2014, 5, 5021. [Google Scholar] [CrossRef]
- Neumann, G. H5N1 influenza virulence, pathogenicity and transmissibility: What do we know? Future Virol. 2015, 10, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.; Czudai-Matwich, V.; Klenk, H.D. Adaptive mutations in the H5N1 polymerase complex. Virus Res 2013, 178, 53–62. [Google Scholar] [CrossRef]
- Wei, K.; Liu, X. Phylogenetic Analysis and Functional Characterization of the Influenza a H5N1 PB2 Gene. Transbound. Emerg. Dis. 2017, 64, 374–388. [Google Scholar] [CrossRef]
- Guo, F.; Shen, X.; Irwin, D.M.; Shen, Y. Avian influenza A viruses H5Nx (N1, N2, N6 and N8) show different adaptations of their codon usage patterns to their hosts. J. Infect. 2019, 79, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Rolling, T.; Koerner, I.; Zimmermann, P.; Holz, K.; Haller, O.; Staeheli, P.; Kochs, G. Adaptive mutations resulting in enhanced polymerase activity contribute to high virulence of influenza A virus in mice. J. Virol. 2009, 83, 6673–6680. [Google Scholar] [CrossRef]
- Lee, C.-Y.; An, S.-H.; Choi, J.-G.; Lee, Y.-J.; Kim, J.-H.; Kwon, H.-J. Rank orders of mammalian pathogenicity-related PB2 mutations of avian influenza A viruses. Sci. Rep. 2020, 10, 5359. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.; Herwig, A.; Klenk, H.D. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS Pathog. 2008, 4, e11. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, T.; Bai, X.; Liang, B.; Deng, L.; Zheng, Y.; Yu, M.; Li, Y.; Ping, J. Comprehensive analysis of the key amino acid substitutions in the polymerase and NP of avian influenza virus that enhance polymerase activity and affect adaptation to mammalian hosts. Vet. Microbiol. 2023, 282, 109760. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. E. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Arai, Y.; Kawashita, N.; Daidoji, T.; Ibrahim, M.S.; El-Gendy, E.M.; Takagi, T.; Takahashi, K.; Suzuki, Y.; Ikuta, K.; Nakaya, T.; et al. Novel Polymerase Gene Mutations for Human Adaptation in Clinical Isolates of Avian H5N1 Influenza Viruses. PLoS Pathog. 2016, 12, e1005583. [Google Scholar] [CrossRef]
- Czudai-Matwich, V.; Otte, A.; Matrosovich, M.; Gabriel, G.; Klenk, H.D. PB2 mutations D701N and S714R promote adaptation of an influenza H5N1 virus to a mammalian host. J. Virol. 2014, 88, 8735–8742. [Google Scholar] [CrossRef]
- Jiao, P.; Wei, L.; Song, Y.; Cui, J.; Song, H.; Cao, L.; Yuan, R.; Luo, K.; Liao, M. D701N mutation in the PB2 protein contributes to the pathogenicity of H5N1 avian influenza viruses but not transmissibility in guinea pigs. Front. Microbiol. 2014, 5, 642. [Google Scholar] [CrossRef]
- Sun, H.; Cui, P.; Song, Y.; Qi, Y.; Li, X.; Qi, W.; Xu, C.; Jiao, P.; Liao, M. PB2 segment promotes high-pathogenicity of H5N1 avian influenza viruses in mice. Front. Microbiol. 2015, 6, 73. [Google Scholar] [CrossRef][Green Version]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]
- Mertens, E.; Dugan, V.G.; Stockwell, T.B.; Lindsay, L.L.; Plancarte, M.; Boyce, W.M. Evaluation of phenotypic markers in full genome sequences of avian influenza isolates from California. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 521–536. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Z.; Shi, J.; Deng, G.; Kong, H.; Tao, S.; Li, C.; Liu, L.; Guan, Y.; Chen, H. Glycine at Position 622 in PB1 Contributes to the Virulence of H5N1 Avian Influenza Virus in Mice. J. Virol. 2016, 90, 1872–1879. [Google Scholar] [CrossRef]
- Na, E.J.; Kim, Y.S.; Lee, S.Y.; Kim, Y.J.; Park, J.S.; Oem, J.K. Genetic Characteristics of Avian Influenza Virus Isolated from Wild Birds in South Korea, 2019–2020. Viruses 2021, 13, 381. [Google Scholar] [CrossRef]
- Zhong, G.; Le, M.Q.; Lopes, T.J.S.; Halfmann, P.; Hatta, M.; Fan, S.; Neumann, G.; Kawaoka, Y. Mutations in the PA Protein of Avian H5N1 Influenza Viruses Affect Polymerase Activity and Mouse Virulence. J. Virol. 2018, 92, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Feng, H.; Xu, J.; Zhao, D.; Shi, J.; Li, Y.; Deng, G.; Jiang, Y.; Li, X.; Zhu, P.; et al. The PA protein directly contributes to the virulence of H5N1 avian influenza viruses in domestic ducks. J. Virol. 2011, 85, 2180–2188. [Google Scholar] [CrossRef]
- Lee, I.; Il Kim, J.; Park, S.; Bae, J.Y.; Yoo, K.; Yun, S.H.; Lee, J.Y.; Kim, K.; Kang, C.; Park, M.S. Single PA mutation as a high yield determinant of avian influenza vaccines. Sci. Rep. 2017, 7, 40675. [Google Scholar] [CrossRef]
- Siddique, N.; Naeem, K.; Abbas, M.A.; Ahmed, Z.; Malik, S.A. Sequence and phylogenetic analysis of highly pathogenic avian influenza H5N1 viruses isolated during 2006-2008 outbreaks in Pakistan reveals genetic diversity. Virol. J. 2012, 9, 300. [Google Scholar] [CrossRef]
- Lee, Y.N.; Lee, D.H.; Cheon, S.H.; Park, Y.R.; Baek, Y.G.; Si, Y.J.; Kye, S.J.; Lee, E.K.; Heo, G.B.; Bae, Y.C.; et al. Genetic characteristics and pathogenesis of H5 low pathogenic avian influenza viruses from wild birds and domestic ducks in South Korea. Sci. Rep. 2020, 10, 12151. [Google Scholar] [CrossRef]
- Mehle, A.; Dugan, V.G.; Taubenberger, J.K.; Doudna, J.A. Reassortment and mutation of the avian influenza virus polymerase PA subunit overcome species barriers. J. Virol. 2012, 86, 1750–1757. [Google Scholar] [CrossRef]
- Hu, J.; Hu, Z.; Song, Q.; Gu, M.; Liu, X.; Wang, X.; Hu, S.; Chen, C.; Liu, H.; Liu, W.; et al. The PA-gene-mediated lethal dissemination and excessive innate immune response contribute to the high virulence of H5N1 avian influenza virus in mice. J. Virol. 2013, 87, 2660–2672. [Google Scholar] [CrossRef]
- Griffin, E.F.; Tompkins, S.M. Fitness Determinants of Influenza A Viruses. Viruses 2023, 15, 1959. [Google Scholar]
- Kim, J.H.; Hatta, M.; Watanabe, S.; Neumann, G.; Watanabe, T.; Kawaoka, Y. Role of host-specific amino acids in the pathogenicity of avian H5N1 influenza viruses in mice. J. Gen. Virol. 2010, 91 Pt 5, 1284–1289. [Google Scholar]
- Mostafa, A.; Mahmoud, S.H.; Shehata, M.; Müller, C.; Kandeil, A.; El-Shesheny, R.; Nooh, H.Z.; Kayali, G.; Ali, M.A.; Pleschka, S. PA from a Recent H9N2 (G1-Like) Avian Influenza a Virus (AIV) Strain Carrying Lysine 367 Confers Altered Replication Efficiency and Pathogenicity to Contemporaneous H5N1 in Mammalian Systems. Viruses 2020, 12, 1046. [Google Scholar]
- Nguyen, H.T.; Chesnokov, A.; De La Cruz, J.; Pascua, P.N.Q.; Mishin, V.P.; Jang, Y.; Jones, J.; Di, H.; Ivashchenko, A.A.; Killian, M.L.; et al. Antiviral susceptibility of clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) viruses isolated from birds and mammals in the United States, 2022. Antivir. Res. 2023, 217, 105679. [Google Scholar] [PubMed]
- Hu, M.; Chu, H.; Zhang, K.; Singh, K.; Li, C.; Yuan, S.; Chow, B.K.; Song, W.; Zhou, J.; Zheng, B.J. Amino acid substitutions V63I or A37S/I61T/V63I/V100A in the PA N-terminal domain increase the virulence of H7N7 influenza A virus. Sci. Rep. 2016, 6, 37800. [Google Scholar] [PubMed]
- Xu, G.; Zhang, X.; Gao, W.; Wang, C.; Wang, J.; Sun, H.; Sun, Y.; Guo, L.; Zhang, R.; Chang, K.C.; et al. Prevailing PA Mutation K356R in Avian Influenza H9N2 Virus Increases Mammalian Replication and Pathogenicity. J. Virol. 2016, 90, 8105–8114. [Google Scholar] [PubMed]
- Linster, M.; van Boheemen, S.; de Graaf, M.; Schrauwen, E.J.A.; Lexmond, P.; Manz, B.; Bestebroer, T.M.; Baumann, J.; van Riel, D.; Rimmelzwaan, G.F.; et al. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 2014, 157, 329–339. [Google Scholar] [PubMed]
- Yang, Z.Y.; Wei, C.J.; Kong, W.P.; Wu, L.; Xu, L.; Smith, D.F.; Nabel, G.J. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 2007, 317, 825–828. [Google Scholar] [PubMed]
- Young, S.G.; Kitchen, A.; Kayali, G.; Carrel, M. Unlocking pandemic potential: Prevalence and spatial patterns of key substitutions in avian influenza H5N1 in Egyptian isolates. BMC Infect. Dis. 2018, 18, 314. [Google Scholar]
- Chen, L.M.; Blixt, O.; Stevens, J.; Lipatov, A.S.; Davis, C.T.; Collins, B.E.; Cox, N.J.; Paulson, J.C.; Donis, R.O. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 2012, 422, 105–113. [Google Scholar]
- Hanson, A.; Imai, M.; Hatta, M.; McBride, R.; Imai, H.; Taft, A.; Zhong, G.; Watanabe, T.; Suzuki, Y.; Neumann, G.; et al. Identification of Stabilizing Mutations in an H5 Hemagglutinin Influenza Virus Protein. J. Virol. 2015, 90, 2981–2992. [Google Scholar] [PubMed]
- Han, P.F.; Li, J.; Hu, Y.; Sun, W.; Zhang, S.; Yang, Y.H.; Li, Y.C.; Kang, X.P.; Wu, X.Y.; Zhu, S.Y.; et al. H5N1 influenza A virus with K193E and G225E double mutations in haemagglutinin is attenuated and immunogenic in mice. J. Gen. Virol. 2015, 96, 2522–2530. [Google Scholar] [PubMed]
- Timofeeva, T.A.; Sadykova, G.K.; Rudneva, I.A.; Boravleva, E.Y.; Gambaryan, A.S.; Lomakina, N.F.; Mochalova, L.V.; Bovin, N.V.; Usachev, E.V.; Prilipov, A.G. Changes in the phenotypic properties of highly pathogenic influenza A virus of H5N1 subtype induced by N186I and N186T point mutations in hemagglutinin. Mol. Biol. 2016, 50, 855–862. [Google Scholar]
- Peng, W.; Bouwman, K.M.; McBride, R.; Grant, O.C.; Woods, R.J.; Verheije, M.H.; Paulson, J.C.; de Vries, R.P. Enhanced Human-Type Receptor Binding by Ferret-Transmissible H5N1 with a K193T Mutation. J. Virol. 2018, 92, 10–128. [Google Scholar]
- Dortmans, J.C.; Dekkers, J.; Wickramasinghe, I.N.; Verheije, M.H.; Rottier, P.J.; van Kuppeveld, F.J.; de Vries, E.; de Haan, C.A. Adaptation of novel H7N9 influenza A virus to human receptors. Sci. Rep. 2013, 3, 3058. [Google Scholar]
- Xiong, X.; Martin, S.R.; Haire, L.F.; Wharton, S.A.; Daniels, R.S.; Bennett, M.S.; McCauley, J.W.; Collins, P.J.; Walker, P.A.; Skehel, J.J.; et al. Receptor binding by an H7N9 influenza virus from humans. Nature 2013, 499, 496–499. [Google Scholar] [PubMed]
- Zaraket, H.; Bridges, O.A.; Duan, S.; Baranovich, T.; Yoon, S.W.; Reed, M.L.; Salomon, R.; Webby, R.J.; Webster, R.G.; Russell, C.J. Increased acid stability of the hemagglutinin protein enhances H5N1 influenza virus growth in the upper respiratory tract but is insufficient for transmission in ferrets. J. Virol. 2013, 87, 9911–9922. [Google Scholar]
- Schrauwen, E.J.; Richard, M.; Burke, D.F.; Rimmelzwaan, G.F.; Herfst, S.; Fouchier, R.A. Amino Acid Substitutions That Affect Receptor Binding and Stability of the Hemagglutinin of Influenza A/H7N9 Virus. J. Virol. 2016, 90, 3794–3799. [Google Scholar]
- Matrosovich, M.; Zhou, N.; Kawaoka, Y.; Webster, R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 1999, 73, 1146–1155. [Google Scholar]
- Shtyrya, Y.; Mochalova, L.; Voznova, G.; Rudneva, I.; Shilov, A.; Kaverin, N.; Bovin, N. Adjustment of receptor-binding and neuraminidase substrate specificities in avian-human reassortant influenza viruses. Glycoconj J. 2009, 26, 99–109. [Google Scholar]
- Abdelwhab, E.M.; Arafa, A.S.; Stech, J.; Grund, C.; Stech, O.; Graeber-Gerberding, M.; Beer, M.; Hassan, M.K.; Aly, M.M.; Harder, T.C.; et al. Diversifying evolution of highly pathogenic H5N1 avian influenza virus in Egypt from 2006 to 2011. Virus Genes 2012, 45, 14–23. [Google Scholar] [CrossRef]
- Neumann, G.; Macken, C.A.; Karasin, A.I.; Fouchier, R.A.; Kawaoka, Y. Egyptian H5N1 influenza viruses-cause for concern? PLoS Pathog. 2012, 8, e1002932. [Google Scholar] [CrossRef] [PubMed]
- Perovic, V.R.; Muller, C.P.; Niman, H.L.; Veljkovic, N.; Dietrich, U.; Tosic, D.D.; Glisic, S.; Veljkovic, V. Novel phylogenetic algorithm to monitor human tropism in Egyptian H5N1-HPAIV reveals evolution toward efficient human-to-human transmission. PLoS ONE 2013, 8, e61572. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Arai, Y.; Daidoji, T.; Kawashita, N.; Ibrahim, M.S.; El-Gendy Eel, D.; Hiramatsu, H.; Kubota-Koketsu, R.; Takagi, T.; Murata, T.; et al. Characterization of H5N1 influenza virus variants with hemagglutinin mutations isolated from patients. MBio 2015, 6, 10–1128. [Google Scholar] [CrossRef]
- Imai, H.; Shinya, K.; Takano, R.; Kiso, M.; Muramoto, Y.; Sakabe, S.; Murakami, S.; Ito, M.; Yamada, S.; Le, M.T.; et al. The HA and NS genes of human H5N1 influenza A virus contribute to high virulence in ferrets. PLoS Pathog. 2010, 6, e1001106. [Google Scholar] [CrossRef]
- Tang, D.J.; Lam, Y.M.; Siu, Y.L.; Lam, C.H.; Chu, S.L.; Peiris, J.S.; Buchy, P.; Nal, B.; Bruzzone, R. A single residue substitution in the receptor-binding domain of H5N1 hemagglutinin is critical for packaging into pseudotyped lentiviral particles. PLoS ONE 2012, 7, e43596. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, B.; Zhou, H.; Suguitan, A.L.; Jr Cheng, X.; Subbarao, K.; Kemble, G.; Jin, H. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J. Virol. 2010, 84, 6570–6577. [Google Scholar] [CrossRef]
- Arafa, A.; El-Masry, I.; Kholosy, S.; Hassan, M.K.; Dauphin, G.; Lubroth, J.; Makonnen, Y.J. Phylodynamics of avian influenza clade 2.2.1 H5N1 viruses in Egypt. Virol. J. 2016, 13, 49. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Luo, J.; Li, M.; Liu, H.; Zhao, N.; Huang, J.; Zhu, X.; Ma, G.; Yuan, G.; et al. Amino Acid Substitution K470R in the Nucleoprotein Increases the Virulence of H5N1 Influenza A Virus in Mammals. Front. Microbiol. 2017, 8, 1308. [Google Scholar]
- Gabriel, G.; Abram, M.; Keiner, B.; Wagner, R.; Klenk, H.D.; Stech, J. Differential polymerase activity in avian and mammalian cells determines host range of influenza virus. J. Virol. 2007, 81, 9601–9604. [Google Scholar] [CrossRef]
- Phanich, J.; Rungrotmongkol, T.; Kungwan, N.; Hannongbua, S. Role of R292K mutation in influenza H7N9 neuraminidase toward oseltamivir susceptibility: MD and MM/PB(GB)SA study. J. Comput. Aided. Mol. Des. 2016, 30, 917–926. [Google Scholar] [CrossRef]
- Govorkova, E.A.; Baranovich, T.; Seiler, P.; Armstrong, J.; Burnham, A.; Guan, Y.; Peiris, M.; Webby, R.J.; Webster, R.G. Antiviral resistance among highly pathogenic influenza A (H5N1) viruses isolated worldwide in 2002–2012 shows need for continued monitoring. Antivir. Res. 2013, 98, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Govorkova, E.A.; Ilyushina, N.A.; Marathe, B.M.; McClaren, J.L.; Webster, R.G. Competitive fitness of oseltamivir-sensitive and -resistant highly pathogenic H5N1 influenza viruses in a ferret model. J. Virol. 2010, 84, 8042–8050. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, Y.; Dong, L.; Wang, D.; Huang, W.; Xin, L.; Yang, L.; Zhao, X.; Li, Z.; Wang, W.; et al. A comprehensive surveillance of adamantane resistance among human influenza A virus isolated from mainland China between 1956 and 2009. Antivir. Ther. 2010, 15, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Reuther, P.; Giese, S.; Gotz, V.; Riegger, D.; Schwemmle, M. Phosphorylation of highly conserved serine residues in the influenza A virus nuclear export protein NEP plays a minor role in viral growth in human cells and mice. J. Virol. 2014, 88, 7668–7673. [Google Scholar] [CrossRef] [PubMed]
- Long, J.X.; Peng, D.X.; Liu, Y.L.; Wu, Y.T.; Liu, X.F. Virulence of H5N1 avian influenza virus enhanced by a 15-nucleotide deletion in the viral nonstructural gene. Virus Genes 2008, 36, 471–478. [Google Scholar] [CrossRef]
- Dankar, S.K.; Miranda, E.; Forbes, N.E.; Pelchat, M.; Tavassoli, A.; Selman, M.; Ping, J.; Jia, J.; Brown, E.G. Influenza A/Hong Kong/156/1997(H5N1) virus NS1 gene mutations F103L and M106I both increase IFN antagonism, virulence and cytoplasmic localization but differ in binding to RIG-I and CPSF30. Virol. J. 2013, 10, 243. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Zhang, H.; Xin, G.; Zhang, D.; Zeng, J.; Chen, X.; Xu, Y.; Cui, Y.; Li, K. Effects of NS1 variants of H5N1 influenza virus on interferon induction, TNFalpha response and p53 activity. Cell. Mol. Immunol. 2010, 7, 235–242. [Google Scholar] [CrossRef]
- Fan, S.; Macken, C.A.; Li, C.; Ozawa, M.; Goto, H.; Iswahyudi, N.F.; Nidom, C.A.; Chen, H.; Neumann, G.; Kawaoka, Y. Synergistic effect of the PDZ and p85beta-binding domains of the NS1 protein on virulence of an avian H5N1 influenza A virus. J. Virol. 2013, 87, 4861–4871. [Google Scholar] [CrossRef]
- Kniss, K.; Sumner, K.; Tastad, K.; Lewis, N.; Jansen, L.; Julian, D.; Reh, M.; Carlson, E.; Williams, R.; Koirala, S.; et al. Risk for Infection in Humans after Exposure to Birds Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus, United States, 2022. Emerg. Infect. Dis. J. 2023, 29, 1215. [Google Scholar] [CrossRef]

| Viral Protein | Residue | AIV H5N1 | AIV H5N8 | AIV H9N2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aa Position | AAMM 1 | MAMM 2 | References | AAMM 1 | MAMM 2 | References | AAMM 1 | MAMM 2 | References | |
| PB2 | 44 | - | - | - | A | S | [153,154,155,156] | A | S | [157] |
| 63 | I | T | [25] | - | - | - | - | - | - | |
| 64 | - | - | - | M | T | [153,158] | I/M | T | [157] | |
| 73 | K | R | [159] | - | - | - | - | - | - | |
| 81 | - | - | - | T | M | [153,156] | T | M | [157] | |
| 89 | V | V | [160,161] | - | - | - | - | - | - | |
| 199 | A | S | [154,159] | A | S | [153,154,156] | A | S | [157] | |
| 256 | D | G | [25] | - | - | - | - | - | - | |
| 309 | D | D | [160,161] | - | - | - | - | - | - | |
| 318 | - | - | - | - | - | - | K/S/R | R | [157,162] | |
| 339 | K | K | [160,161] | - | - | - | - | - | - | |
| 588 | A | V | [160,161,163] | - | - | - | - | - | - | |
| 591 | Q | K | [25] | Q | K | [153,164] | - | - | - | |
| 627 | E | K | [25,121,160,165,166,167,168] | E | K | [153,169] | E/V | K | [157,170] | |
| 661 | - | - | - | A/V | T | [153,171] | A | T | [157] | |
| 701 | D | N | [25,146,160] | D | N | [153,172] | D | N | [157] | |
| 702 | - | - | - | K | R | [153,171] | K | R | [157] | |
| PB1 | 3 | D | V | [173] | - | - | - | - | - | - |
| 13 | - | - | - | L | P | [153,174] | L | P | [157] | |
| 105 | N | S | [175] | - | - | - | - | - | - | |
| 207 | K | R | [25] | - | - | - | - | - | - | |
| 336 | - | - | - | V | I | [153,154] | V | I | [157] | |
| 374 | A | S | [165] | - | - | - | - | - | - | |
| 375 | - | - | - | N | S | [153,174] | N | S | [157] | |
| 436 | Y | H | [25] | - | - | - | - | - | - | |
| 677 | T | M | [25,176] | - | - | - | - | - | - | |
| 740 | F | L | [165] | - | - | - | - | - | - | |
| PB1-F2 | 66 | S | S | [25,160,177,178] | - | - | - | - | - | - |
| 68 | - | - | - | - | - | - | T/I | I | [157] | |
| 73 | - | - | - | - | - | - | K | R | [157] | |
| 76 | - | - | - | - | - | - | V | A | [157] | |
| 79 | R | Q | [154,159] | - | - | - | R | Q | [157] | |
| 82 | L | S | [154,159] | - | - | - | L | S | [157] | |
| PA | 28 | - | - | - | P | L | [153,179] | P | L | [157] |
| 55 | - | - | - | D | N | [153,154,156] | D | N | [157] | |
| 57 | - | - | - | R | Q | [153,154] | R | Q | [157] | |
| 97 | T | I | [175] | - | - | - | - | - | - | |
| 100 | V/I | A | [25,160,180] | V | A | [153,181] | V | A | [157] | |
| 133 | - | - | - | E | G | [153,182] | E | G | [157] | |
| 158 | K | R | [165] | - | - | - | - | - | - | |
| 225 | - | - | - | S | C | [153,166] | S | C | [157] | |
| 241 | - | - | - | C | Y | [153,183] | C | Y | [157] | |
| 268 | - | - | - | L | I | [153,166] | L | I | [157] | |
| 312 | - | - | - | - | - | - | K | R | [157] | |
| 356 | K | R | [25,154] | K | R | [153,154] | K | R | [157] | |
| 382 | - | - | - | E | D | [153,156] | E | D | [157,162] | |
| 400 | S/T/F | L | [25,156,159] | - | - | - | S | L | [157] | |
| 404 | - | - | - | A | S | [153,154] | A | S | [157] | |
| 409 | S | N | [25,154,160,180] | S | N | [153,154,156] | S | N | [157] | |
| 515 | A/T | T | [160,184] | - | - | - | - | - | - | |
| 552 | - | - | - | T | S | [153,166] | T | S | [157] | |
| 556 | - | - | - | - | - | - | Q | R | [157] | |
| 615 | - | - | - | K | L | [153,185] | K | L | [157] | |
| 712 | A | T | [165] | - | - | - | - | - | - | |
| HA | 125 | - | - | - | - | - | - | A | T | [162] |
| 155 | I | T | [186] | - | - | - | - | - | - | |
| 173 | - | - | - | - | - | - | Q | H | [162] | |
| 180 | - | - | - | - | - | - | V | E | [162] | |
| 198 | E | D | [160,187] | - | - | - | - | - | - | |
| 216 | - | - | - | - | - | - | Q/L | L | [162] | |
| 222 | - | - | - | Q | L | [153,188] | - | - | - | |
| 224 | - | - | - | G | S | [153,188] | - | - | - | |
| 226 | - | - | - | - | - | - | Q | L | [157,170] | |
| 228 | - | - | - | - | - | - | R | K | [162,170] | |
| 234 | Q | L | [160,189] | - | - | - | Q | L | [157,190,191] | |
| 236 | G | S | [160,189] | - | - | - | - | - | - | |
| NP | 31 | - | - | - | - | - | - | R | K | [157] |
| 33 | V/I | I | [25,154,156,159] | V/I/D | I | [153,154,156] | V | I | [157] | |
| 34 | - | - | - | - | - | - | D | N | [157] | |
| 61 | - | - | - | I | L | [153,156,166] | I | L | [157] | |
| 100 | - | - | - | - | - | - | R | V | [157] | |
| 105 | M | V | [9,192,193] | |||||||
| 109 | I/V | V | [25,154,156] | I | V | [153,154] | I | V | [157] | |
| 127 | - | - | - | - | - | - | E | D | [157] | |
| 136 | L | M | [25] | L | M | [153,156] | L | M | [157] | |
| 184 | K | K | [11,25,194] | - | - | - | - | - | - | |
| 214 | - | - | - | R | K | [153,154,156] | K/N | K | [157] | |
| 283 | - | - | - | - | - | - | L | P | [157] | |
| 293 | - | - | - | - | - | - | R | K | [157] | |
| 305 | - | - | - | - | - | - | R | K | [157] | |
| 313 | - | - | - | F | Y | [153,154,156] | F | Y | [157] | |
| 357 | - | - | - | Q | K | [153,154] | Q | K | [157] | |
| 372 | - | - | - | E | D | [153,154] | E | D | [157] | |
| 375 | - | - | - | - | - | - | D | G/E | [157] | |
| 398 | - | - | - | K | Q | [153] | K | Q | [157] | |
| 422 | - | - | - | - | - | - | R | K | [157] | |
| 434 | - | - | - | - | - | - | E | K | [195] | |
| 442 | - | - | - | - | - | - | T | A | [157] | |
| 455 | - | - | - | D/N/E | E | [153,154] | D/E | E | [157] | |
| M1 | 15 | V | I | [80] | V | I | [153,196] | V | I | [157] |
| 30 | N | D | [197] | - | - | - | - | - | - | |
| 43 | I | M | [198] | - | - | - | - | - | - | |
| 115 | - | - | - | V | V/I/T | [153,166] | V | I | [157] | |
| 121 | - | - | - | T | A | [153,166] | T | A | [157] | |
| 137 | - | - | - | T | A | [153,156,166] | T | A | [157] | |
| M2 | 11 | - | - | - | T | I | [153,154] | T | I | [157] |
| 16 | E | G/D | [80] | E/G | G/D | [153,156] | E/G | G/D | [157] | |
| 20 | - | - | - | S | N | [153,154,156] | S | N | [157] | |
| 28 | I | I/V | [80] | I | I/V | [153,156] | I | I/V | [157] | |
| 55 | - | - | - | L | F | [153,199] | L | F | [157] | |
| 57 | - | - | - | Y | H | [153,154] | Y | H | [157] | |
| 86 | - | - | - | V | A | [153,154] | V | A | [157] | |
| NS1 | 42 | P | S | [25,200] | - | - | - | - | - | - |
| 55 | K | E | [201] | - | - | - | - | - | - | |
| 66 | K | E | [201] | - | - | - | - | - | - | |
| 74 | D | N | [202] | - | - | - | - | - | - | |
| 92 | D | E | [203] | - | - | - | - | - | - | |
| 103 | F/L | L | [25,204] | - | - | - | - | - | - | |
| 106 | I | M | [205] | - | - | - | - | - | - | |
| 138 | C | F | [201] | - | - | - | - | - | - | |
| 149 | V | A | [25,160,206,207] | - | - | - | - | - | - | |
| 227 | E | K | [80] | G | K/R | [153,199] | E/K | K/R | [80,157,162] | |
| NS2/NEP | 16 | M | I | [208] | - | - | - | - | - | - |
| 41 | Y | C | [208] | - | - | - | - | - | - | |
| 75 | E | G | [208] | - | - | - | - | - | - | |
| Viral Protein | aa Changes | Subtype | Characteristics/Effects of Mutations | References |
|---|---|---|---|---|
| PB2 | I64M | H5N1 |
| [209] |
| T339M | H5N1 |
| [210] | |
| I147A K339T A588T | H5N1 |
| [211,212] | |
| E158G T271A | H5N1 |
| [212,213,214,215] | |
| L89V G309D R477G I495V | H5N1 |
| [214,216] | |
| Q591K/R | H1N1 H9N2 H5N1 |
| [212,215,217] | |
| E627K | H5N1 H7N9 H10N8 |
| [35,113,214,215,218,219,220,221] | |
| D701N S714R | H5N1 |
| [215,222,223] | |
| S715N | H5N1 |
| [224] | |
| E249G G309D T339M | H5N1 |
| [210,215] | |
| V108A E192K A274T N456D G727R T339M I451V S471F R369K | H5Nx |
| [209,215] | |
| PB1-F2 | N66S | H5N1 |
| |
| PB1 | V3D | H5N1 |
| [212,225] |
| [219] | ||||
| K363R A374S F740L | H5N1 |
| [219] | |
| H436Y D622G | H5N1 |
| [131,184,226,227,228] | |
| I57T E172D M179I S361G N375S K387R L598P | H5Nx |
| [215] | |
| P708S | H5N8 |
| [48] | |
| PA | K158R | H5N1 |
| [173,219] |
| A343S D347E | H5N1 |
| [173,219,229] | |
| A369V V602I A712T | H5N1 |
| [219] | |
| S224P N383D | H5N1 |
| [230] | |
| E31K | H5N1 |
| [231] | |
| S409N K356R V100A Q/T/S400L | H5N1 |
| [154,232,233] | |
| T552S T97I K142E I353R T515A P149S R266H L357I T515S | H5N1 |
| [184,215,217,234,235,236,237] | |
| V44I V127A C241Y A343T I573V | H5N1 |
| [215,236] | |
| R367K | H5N1 |
| [238] | |
| K615R | H5N1 |
| [215,234] | |
| A369V | H5N1 |
| [219] | |
| A37T I38M/T | H5N1 H1N1 H3N2 |
| [239] | |
| F4C M12I M86V F105L L226F E237K P257L N321K T369A V387I | H5Nx |
| [215] | |
| N409S V63I | H7Nx |
| [180,240] | |
| K356R | H9N2 |
| [241] | |
| HA | H110Y | H5N1 |
| [113,212,242] |
| S133A T188I | H5N1 |
| [243,244] | |
| N158D | H5N1 |
| [144,212] | |
| T160A | H5N1 |
| [113,212] | |
| Q196R Q226L G228S | H5N1 |
| [212,245] | |
| N224K Q226L | H5N1 |
| [144,212,246] | |
| Q226L G228S | H5N1 |
| [113,212] | |
| T318I | H5N1 |
| [144,212] | |
| K193E G225E | H5N1 |
| [247] | |
| S221P D95G | H5N1 |
| [246] | |
| N186I/T | H5N1 |
| [248] | |
| Q222L G224S | H5N1 |
| [189] | |
| K193T | H5N1 |
| [249] | |
| G186V | H7N9 |
| [250,251] | |
| K58I | H5N1 |
| [252] | |
| K58I G219S | H7N9 |
| [253] | |
| Q192R/H G222L Q224S | H5N1 |
| [244,254,255,256] | |
| Δ129 I151T | H5N1 |
| [139] | |
| Δ154–156 T318I H103Y N220K Q222L | H5N1 |
| [113,144,257] | |
| S223N K153D G272S | H5N1 |
| [7,215,258] | |
| S223I/N Δ129 I151T H125Y N94D A134V N182K T195I | H5N1 |
| [245,259,260] | |
| A134V G139R S155N E186G N193K V210I P235S E75K S123P I151T S133A T188I | H5N1 |
| [215,244,245,261] | |
| T156A | H5N1 |
| [242,262] | |
| D154N | H5N1 |
| [78,215,260,263] | |
| N182S R497k K189R | H5N1 |
| [215,244] | |
| E184G D376N | H5Nx |
| [215] | |
| Q30P D31N K35R D45N A86V D88G A127T A/T127S R140K M140T S141P R162I V174I A184E T195A T/N195I V210A V219I R310K R323K R326K I375M D387N E433G N476D E477K M479I E502G M532I V533I | H5Nx |
| [215] | |
| NP | K470R | H5N1 |
| [264] |
| A184K | H5N1 |
| [11] | |
| N319K | H5N1 H7N7 |
| [185,265] | |
| A284T | H5Nx |
| [215] | |
| R100I V343I R384K S413L P419S R452K P453S | H5Nx |
| [215] | |
| NA | R292K | H7N9 |
| [266] |
| H252Y |
| [267,268] | ||
| H274Y E119A V116A I117V/T K150N D198N I222V/T/M S246N N247S N295S | H5N1 H1N1 |
| [239,267,268] | |
| I222R | H5N1 H1N1 |
| [267] | |
| I222R H274Y | H5N1 |
| [267] | |
| I8T I/V16A V16I V33I N39S P45T K55R A58T K241R I243V N305T G318S P323S S364N G365E I380V V404I N430S/D G435S T441D | H5Nx |
| [215] | |
| M1 | N30D T215A | H5N1 |
| [197,244] |
| I43M | H5N1 |
| [198] | |
| T137A A239T C269Y V280I S283N D340N | H5Nx |
| [215] | |
| M2 | L26F/I V27A A30T/V S31N G34E L38F | HxNx |
| [75,244,267,269] |
| NS1 | F55C | H5N1 |
| [219,270] |
| D120N | H5N1 |
| [219] | |
| Δ263–277 D92E | H5N1 |
| [212,271] | |
| N200S G205R | H5N1 |
| [212,261] | |
| F103L M106I | H5N1 |
| [212,272] | |
| P42S | H5N1 |
| [200,212] | |
| Δ80–84 | H5N1 |
| [212,272,273] | |
| F138Y | H5N1 |
| [212,274] | |
| S205N | H5Nx |
| [215] | |
| G47S N48S R59H R67Q E70K T81I R88C V136A I137V D139N L185F D209N V209I L212F | H5Nx |
| [209,215] | |
| M/A14V A48T T/V115A | H5Nx |
| [209,215] | |
| NS2 | M/A14V A48T T/V115A |
| [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasiri, A.; Soltane, R.; Hegazy, A.; Khalil, A.M.; Mahmoud, S.H.; Khalil, A.A.; Martinez-Sobrido, L.; Mostafa, A. Vaccination and Antiviral Treatment against Avian Influenza H5Nx Viruses: A Harbinger of Virus Control or Evolution. Vaccines 2023, 11, 1628. https://doi.org/10.3390/vaccines11111628
Alasiri A, Soltane R, Hegazy A, Khalil AM, Mahmoud SH, Khalil AA, Martinez-Sobrido L, Mostafa A. Vaccination and Antiviral Treatment against Avian Influenza H5Nx Viruses: A Harbinger of Virus Control or Evolution. Vaccines. 2023; 11(11):1628. https://doi.org/10.3390/vaccines11111628
Chicago/Turabian StyleAlasiri, Ahlam, Raya Soltane, Akram Hegazy, Ahmed Magdy Khalil, Sara H. Mahmoud, Ahmed A. Khalil, Luis Martinez-Sobrido, and Ahmed Mostafa. 2023. "Vaccination and Antiviral Treatment against Avian Influenza H5Nx Viruses: A Harbinger of Virus Control or Evolution" Vaccines 11, no. 11: 1628. https://doi.org/10.3390/vaccines11111628
APA StyleAlasiri, A., Soltane, R., Hegazy, A., Khalil, A. M., Mahmoud, S. H., Khalil, A. A., Martinez-Sobrido, L., & Mostafa, A. (2023). Vaccination and Antiviral Treatment against Avian Influenza H5Nx Viruses: A Harbinger of Virus Control or Evolution. Vaccines, 11(11), 1628. https://doi.org/10.3390/vaccines11111628








