New-Onset Rheumatic Immune-Mediated Inflammatory Diseases Following SARS-CoV-2 Vaccinations until May 2023: A Systematic Review
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
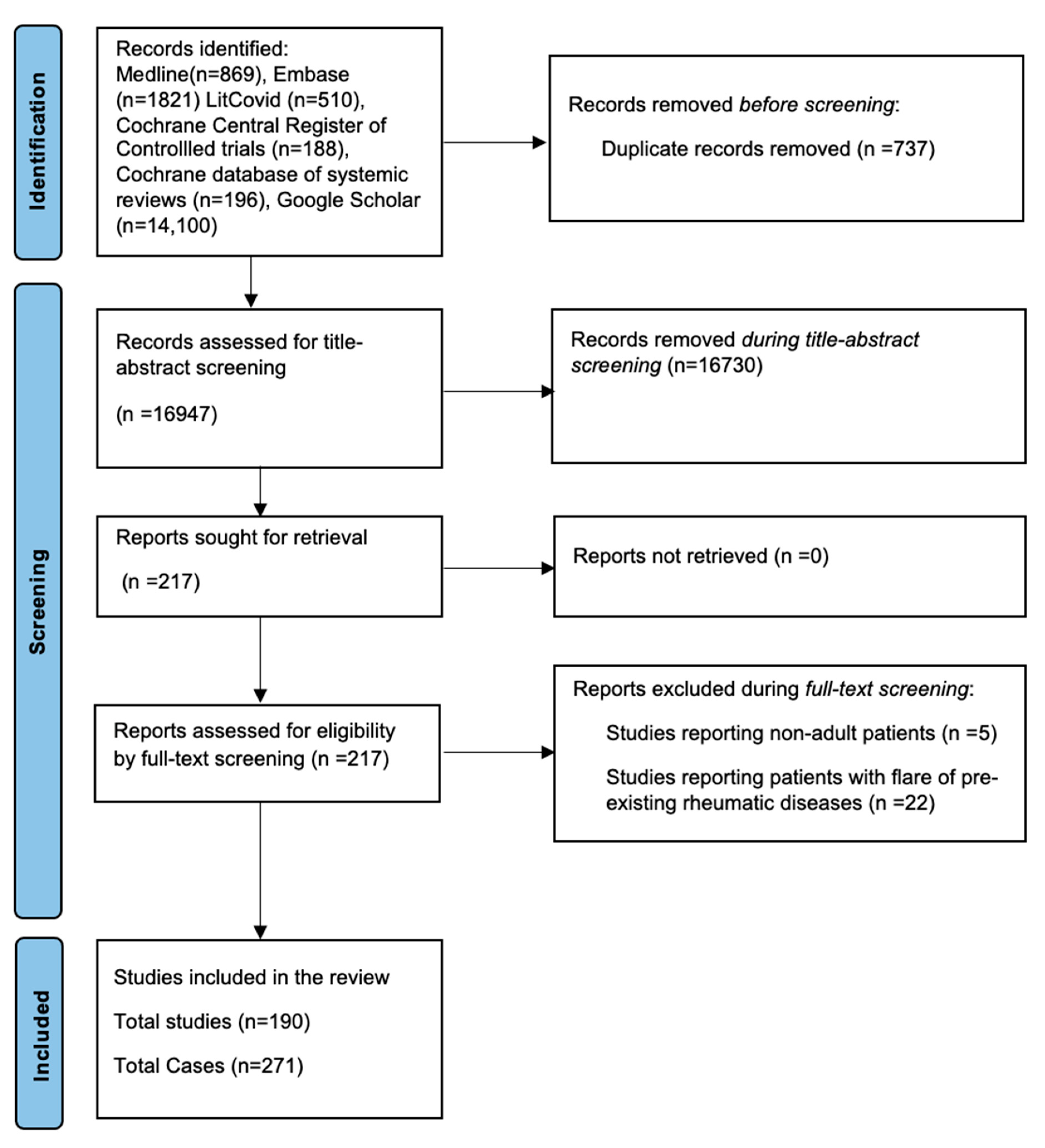
3.1. Identification of the Literature
3.2. Patient Demographics
3.3. Vaccination Characteristics
3.4. Clinical Presentations
3.5. Vasculitides
3.5.1. Small-vessel Vasculitis
3.5.2. Medium-Vessel Vasculitis
3.5.3. Large-Vessel Vasculitis
3.5.4. Giant-cell Arteritis
3.6. Connective Tissue Diseases
3.6.1. Idiopathic Inflammatory Myositis
3.6.2. Systemic Lupus Erythematosus
3.6.3. Subacute Cutaneous Lupus
3.7. Inflammatory Arthritis
3.7.1. Reactive Arthritis
3.7.2. Rheumatoid Arthritis
3.8. Polymyalgia Rheumatica
3.9. Adult-Onset Stills Disease
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Napoli, R.D. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls—NCBI Bookshelf: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (accessed on 15 September 2023).
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Drugs and Lactation Database (LactMed®®); COVID-19 Vaccines—NCBI Bookshelf: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565969/ (accessed on 15 September 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shimagami, H.; Yamaguchi, Y.; Kato, Y.; Kumanogoh, A. Marked Increase of Interferon-β after BNT162b2 mRNA Vaccination: A Case of Polyarthritis with Pleurisy. BMJ Case Rep. 2022, 15, e246533. [Google Scholar] [CrossRef]
- Osada, A.; Sakuragi, C.; Toya, C.; Mitsuo, A. New-Onset Polymyalgia Rheumatica Following the Administration of the Pfizer-BioNTech COVID-19 Vaccine. Intern. Med. 2022, 61, 749–753. [Google Scholar] [CrossRef]
- Padiyar, S.; Kamath, N.; Mathew, J.; Chandu, A.S.; Deodhar, D.; Shastry, B.A.; Shashikala, T.; Ganapati, A. New-Onset Adult-Onset Still’s Disease-like Syndrome after ChAdOx1 nCoV-19 Vaccination—A Case Series with Review of Literature. Clin. Rheumatol. 2022, 41, 1569–1575. [Google Scholar] [CrossRef]
- Unal Enginar, A. Arthritis Following COVID-19 Vaccination: Report of Two Cases. Int. Immunopharmacol. 2021, 101, 108256. [Google Scholar] [CrossRef]
- An, Q.; Qin, D.; Pei, J. Reactive Arthritis after COVID-19 Vaccination. Hum. Vaccines Immunother. 2021, 17, 2954–2956. [Google Scholar] [CrossRef]
- Hyun, H.; Song, J.Y.; Seong, H.; Yoon, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Polyarthralgia and Myalgia Syndrome after ChAdOx1 nCOV-19 Vaccination. J. Korean Med. Sci. 2021, 36, e245. [Google Scholar] [CrossRef]
- Luchetti Gentiloni, M.M.; Paci, V.; Marconi, V.; Gigli, M.; Benfaremo, D.; Sordillo, R.; Macchini, C.; Massaccesi, L.; Perna, G.P.; Offidani, A.M.; et al. SARS-CoV-2 Infection, Vaccination, and Immune-Mediated Diseases: Results of a Single-Center Retrospective Study. Front. Immunol. 2022, 13, 859550. [Google Scholar] [CrossRef]
- Kaur, I.; Zafar, S.; Capitle, E.; Khianey, R. COVID-19 Vaccination as a Potential Trigger for New-Onset Systemic Lupus Erythematosus. Cureus 2022, 14, e21917. [Google Scholar] [CrossRef]
- Molina-Rios, S.; Rojas-Martinez, R.; Estévez-Ramirez, G.M.; Medina, Y.F. Systemic Lupus Erythematosus and Antiphospholipid Syndrome after COVID-19 Vaccination. A Case Report. Mod. Rheumatol. Case Rep. 2022, 7, 43–46. [Google Scholar] [CrossRef]
- Saleh, A.M.; Khalid, A.; Alshaya, A.K.; Alanazi, S.M.M. Systemic Lupus Erythematosus with Acute Pancreatitis and Vasculitic Rash Following COVID-19 Vaccine: A Case Report and Literature Review. Clin. Rheumatol. 2022, 41, 1577–1582. [Google Scholar] [CrossRef]
- Báez-Negrón, L.; Vilá, L.M. New-Onset Systemic Lupus Erythematosus after mRNA SARS-CoV-2 Vaccination. Case Rep. Rheumatol. 2022, 2022, 6436839. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Patil, A. Systemic Lupus Erythematosus after COVID-19 Vaccination: A Case Report. J. Cosmet. Dermatol. 2021, 20, 3103–3104. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.; Padilla, C.; Krampe, N.; Doerfler, S.; Morgenlander, A.; Thiel, B.; Aggarwal, R. Systemic Lupus Erythematous after Pfizer COVID-19 Vaccine: A Case Report. Clin. Rheumatol. 2022, 41, 1597–1601. [Google Scholar] [CrossRef]
- Santiago, J.; Negron-Ocasio, G.; Ortiz-Troche, S.; Padilla Rodriguez, K.; Ramirez-Marquez, J.; Velazquez, L.; Da Silva Lugo, I.; Feliciano-Figueroa, J.; Colon-Marquez, J. Rare Expression of Systemic Sarcoidosis After A Novel Rna Vaccine. Chest 2021, 160, A1233–A1234. [Google Scholar] [CrossRef]
- Nune, A.; Iyengar, K.P.; Ish, P.; Varupula, B.; Musat, C.A.; Sapkota, H.R. The Emergence of New-Onset SLE Following SARS-CoV-2 Vaccination. QJM Int. J. Med. 2021, 114, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Licciardi-Fernandez, M.J.; Burmann, S.-N.; Burkert, B.; Oellig, F.; Michalowitz, A.-L. Induction and Exacerbation of Subacute Cutaneous Lupus Erythematosus Following mRNA-based or Adenoviral Vector-based SARS-CoV-2 Vaccination. Clin. Exp. Dermatol. 2021, 47, 161–163. [Google Scholar] [CrossRef]
- Zavala-Miranda, M.F.; González-Ibarra, S.G.; Pérez-Arias, A.A.; Uribe-Uribe, N.O.; Mejia-Vilet, J.M. New-Onset Systemic Lupus Erythematosus Beginning as Class V Lupus Nephritis after COVID-19 Vaccination. Kidney Int. 2021, 100, 1340–1341. [Google Scholar] [CrossRef]
- Hidaka, D.; Ogasawara, R.; Sugimura, S.; Fujii, F.; Kojima, K.; Nagai, J.; Ebata, K.; Okada, K.; Kobayashi, N.; Ogasawara, M.; et al. New-Onset Evans Syndrome Associated with Systemic Lupus Erythematosus after BNT162b2 mRNA COVID-19 Vaccination. Int. J. Hematol. 2021, 115, 424–427. [Google Scholar] [CrossRef]
- Raviv, Y.; Betesh-Abay, B.; Valdman-Grinshpoun, Y.; Boehm-Cohen, L.; Kassirer, M.; Sagy, I. First Presentation of Systemic Lupus Erythematosus in a 24-Year-Old Male Following mRNA COVID-19 Vaccine. Case Rep. Rheumatol. 2022, 2022, 9698138. [Google Scholar] [CrossRef] [PubMed]
- Zengarini, C.; Pileri, A.; Salamone, F.P.; Piraccini, B.M.; Vitale, G.; La Placa, M. Subacute Cutaneous Lupus Erythematosus Induction after SARS-CoV-2 Vaccine in a Patient with Primary Biliary Cholangitis. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e179. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Honda, H.; Tanaka, T.; Uraguchi, K.; Kawahara, M.; Hagiya, H. COVID-19 mRNA Vaccine–Associated Uveitis Leading to Diagnosis of Sarcoidosis: Case Report and Review of Literature. J. Investig. Med. High Impact Case Rep. 2022, 10, 232470962210864. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, J.-G.; Tampe, B.; Korsten, P. First Report of Two Cases of Löfgren’s Syndrome after SARS-CoV-2 Vaccination-Coincidence or Causality? Vaccines 2021, 9, 1313. [Google Scholar] [CrossRef]
- Izuka, S.; Komai, T.; Natsumoto, B.; Shoda, H.; Fujio, K. Self-Limited Polymyalgia Rheumatica-like Syndrome Following mRNA-1273 SARS-CoV-2 Vaccination. Intern. Med. 2022, 61, 903–906. [Google Scholar] [CrossRef]
- Ottaviani, S.; Juge, P.-A.; Forien, M.; Ebstein, E.; Palazzo, E.; Dieudé, P. Polymyalgia Rheumatica Following COVID-19 Vaccination: A Case-Series of Ten Patients. Jt. Bone Spine 2022, 89, 105334. [Google Scholar] [CrossRef]
- Nune, A.; Sapkota, H.; Iyengar, K. The Emergence of Rheumatic Immune-Mediated Inflammatory Disease Manifestations Following SARS-CoV-2 Vaccination. Indian J. Rheumatol. 2022, 17, 214. [Google Scholar] [CrossRef]
- Manzo, C.; Natale, M.; Castagna, A. Polymyalgia Rheumatica as Uncommon Adverse Event Following Immunization with COVID-19 Vaccine: A Case Report and Review of Literature. Aging Med. 2021, 4, 234–238. [Google Scholar] [CrossRef]
- Gambichler, T.; Krogias, C.; Tischoff, I.; Tannapfel, A.; Gold, R.; Susok, L. Bilateral Giant Cell Arteritis with Skin Necrosis Following SARS-CoV-2 Vaccination. Br. J. Dermatol. 2021, 186, e83. [Google Scholar] [CrossRef]
- Sauret, A.; Stievenart, J.; Smets, P.; Olagne, L.; Guelon, B.; Aumaître, O.; André, M.; Trefond, L. Case of Giant Cell Arteritis After SARS-CoV-2 Vaccination: A Particular Phenotype? J. Rheumatol. 2021, 49, 120. [Google Scholar] [CrossRef]
- Mejren, A.; Sørensen, C.; Gormsen, L.; Tougaard, R.; Nielsen, B. Large-Vessel Giant Cell Arteritis after COVID-19 Vaccine. Scandinavian J. Rheumatol. 2021, 51, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Anzola, A.M.; Trives, L.; Martínez-Barrio, J.; Pinilla, B.; Álvaro-Gracia, J.M.; Molina-Collada, J. New-Onset Giant Cell Arteritis Following COVID-19 mRNA (BioNTech/Pfizer) Vaccine: A Double-Edged Sword? Clin. Rheumatol. 2022, 41, 1623–1625. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sankhala, K.K.; Bose, S.; Gallemore, R.P. Combined Central Retinal Artery and Vein Occlusion with Ischemic Optic Neuropathy After COVID-19 Vaccination. Int. Med. Case Rep. J. 2022, 15, 7–14. [Google Scholar] [CrossRef]
- Fillon, A.; Sautenet, B.; Barbet, C.; Moret, L.; Thillard, E.M.; Jonville-Béra, A.P.; Halimi, J.M. De Novo and Relapsing Necrotizing Vasculitis after COVID-19 Vaccination. Clin. Kidney J. 2021, 15, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Gillion, V.; Jadoul, M.; Demoulin, N.; Aydin, S.; Devresse, A. Granulomatous Vasculitis after the AstraZeneca Anti–SARS-CoV-2 Vaccine. Kidney Int. 2021, 100, 706–707. [Google Scholar] [CrossRef]
- Feghali, E.J.; Zafar, M.; Abid, S.; Santoriello, D.; Mehta, S. De-Novo Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Following the mRNA-1273 (Moderna) Vaccine for COVID-19. Cureus 2021, 13, e19616. [Google Scholar] [CrossRef]
- Nakatani, K.; Sakata, E.; Fujihara, M.; Mizukawa, K.; Koyama, T. Systemic Vasculitis Following SARS-CoV-2 mRNA Vaccination Demonstrated on FDG PET/CT. Clin. Nucl. Med. 2022, 47, e403–e405. [Google Scholar] [CrossRef]
- Schierz, J.-H.; Merkel, C.; Kittner, T.; Ali, F. Vasculitis and Bursitis on [18F]FDG-PET/CT Following COVID-19 mRNA Vaccine: Post Hoc Ergo Propter Hoc? Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1086–1087. [Google Scholar] [CrossRef]
- Shakoor, M.T.; Birkenbach, M.P.; Lynch, M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 611–613. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, B. Case Report: ANCA-Associated Vasculitis Presenting with Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021, 12, 762006. [Google Scholar] [CrossRef]
- Baier, E.; Olgemöller, U.; Biggemann, L.; Buck, C.; Tampe, B. Dual-Positive MPO- and PR3-ANCA-Associated Vasculitis Following SARS-CoV-2 mRNA Booster Vaccination: A Case Report and Systematic Review. Vaccines 2022, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Hirooka, Y.; Sugiyama, M. Propylthiouracil-Induced Antineutrophil Cytoplasmic Antibody-Associated Vasculitis after COVID-19 Vaccination. Vaccines 2021, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Obata, S.; Hidaka, S.; Yamano, M.; Yanai, M.; Ishioka, K.; Kobayashi, S. MPO-ANCA-Associated Vasculitis after the Pfizer/BioNTech SARS-CoV-2 Vaccination. Clin. Kidney J. 2021, 15, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Suzuki, J.; Kuniyoshi, S.; Tanno, Y.; Fujii, H. Granulomatosis with Polyangiitis Following Pfizer-BioNTech COVID-19 Vaccination. Mod. Rheumatol. Case Rep. 2022, 7, 127–129. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Horn, B.J.M.; Terraccaine, W.; Jose, A.; Krishnan, P. A Case of Antineutrophil Cytoplasmic Antibodies (ANCA)-Associated Vasculitis Post COVID-19 Vaccination. Cureus 2022, 14, e23162. [Google Scholar] [CrossRef]
- So, D.; Min, K.-W.; Jung, W.Y.; Han, S.-W.; Yu, M.-Y. Microscopic Polyangiitis Following mRNA COVID-19 Vaccination: A Case Report. J. Korean Med. Sci. 2022, 37, e154. [Google Scholar] [CrossRef] [PubMed]
- Al-Allaf, A.-W.; Razok, A.; Al-Allaf, Y.; Aker, L. Post-COVID-19 Vaccine Medium-Vessel Vasculitis and Acute Anterior Uveitis, Causation vs Temporal Relation; Case Report and Literature Review. Ann. Med. Surg. 2022, 75, 103407. [Google Scholar] [CrossRef]
- Nappi, E.; De Santis, M.; Paoletti, G.; Pelaia, C.; Terenghi, F.; Pini, D.; Ciccarelli, M.; Selmi, C.F.; Puggioni, F.; Canonica, G.W.; et al. New Onset of Eosinophilic Granulomatosis with Polyangiitis Following mRNA-Based COVID-19 Vaccine. Vaccines 2022, 10, 716. [Google Scholar] [CrossRef]
- Sekar, A.; Campbell, R.; Tabbara, J.; Rastogi, P. ANCA Glomerulonephritis after the Moderna COVID-19 Vaccination. Kidney Int. 2021, 100, 473–474. [Google Scholar] [CrossRef]
- Prabhahar, A.; Naidu, G.S.R.S.N.K.; Chauhan, P.; Sekar, A.; Sharma, A.; Sharma, A.; Kumar, A.; Nada, R.; Rathi, M.; Kohli, H.S.; et al. ANCA-Associated Vasculitis Following ChAdOx1 nCoV19 Vaccination: Case-Based Review. Rheumatol. Int. 2022, 42, 749–758. [Google Scholar] [CrossRef]
- Ibrahim, H.; Alkhatib, A.; Meysami, A. Eosinophilic Granulomatosis with Polyangiitis Diagnosed in an Elderly Female After the Second Dose of mRNA Vaccine Against COVID-19. Cureus 2022, 14, e21176. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Hsu, H.; Chen, Y. Cutaneous Polyarteritis Nodosa Following ChAdOx1 nCoV-19 Vaccination. Int. J. Dermatol. 2022, 61, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, M.A.; Liu, M.; Saganas, C.; Montani, M.; Vogt, B.; Huynh-Do, U.; Fuster, D.G. De Novo Vasculitis after mRNA-1273 (Moderna) Vaccination. Kidney Int. 2021, 100, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Vutipongsatorn, K.; Isaacs, A.; Farah, Z. Inflammatory Myopathy Occurring Shortly after Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: Two Case Reports. J. Med. Case Rep. 2022, 16, 57. [Google Scholar] [CrossRef]
- Maramattom, B.V.; Philips, G.; Thomas, J.; Santhamma, S.G.N. Inflammatory Myositis after ChAdOx1 Vaccination. Lancet Rheumatol. 2021, 3, e747–e749. [Google Scholar] [CrossRef]
- Camargo Coronel, A.; Jiménez Balderas, F.J.; Quiñones Moya, H.; Hernández Zavala, M.R.; Mandinabeitia Rodríguez, P.; Hernández Vázquez, J.R.; Zamora Zarco, S.; Aguilar Castillo, S.D.J. Dermatomyositis Post Vaccine against SARS-CoV2. BMC Rheumatol. 2022, 6, 20. [Google Scholar] [CrossRef]
- Gouda, W.; Albasri, A.; Alsaqabi, F.; Al Sabah, H.Y.; Alkandari, M.; Abdelnaby, H. Dermatomyositis Following BNT162b2 mRNA COVID-19 Vaccination. J. Korean Med. Sci. 2022, 37, e32. [Google Scholar] [CrossRef]
- Tagini, F.; Carrel, L.; Fallet, B.; Gachoud, D.; Ribi, C.; Monti, M. Behçet’s-like Adverse Event or Inaugural Behçet’s Disease after SARS-CoV-2 mRNA-1273 Vaccination? Rheumatology 2021, 61, e112–e113. [Google Scholar] [CrossRef]
- Capassoni, M.; Ketabchi, S.; Cassisa, A.; Caramelli, R.; Molinu, A.A.; Galluccio, F.; Guiducci, S. AstraZeneca (AZD1222) COVID-19 Vaccine-associated Adverse Drug Event: A Case Report. J. Med. Virol. 2021, 93, 5718–5720. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, T.-J.; Chen, K.-H.; Sun, H.-Y.; Chen, W.-T.; Hsieh, S.-C.; Cheng, A.; Chen, Y.-C. Fatal Myositis, Rhabdomyolysis and Compartment Syndrome after ChAdOx1 nCoV-19 Vaccination. J. Microbiol. Immunol. Infect. 2022, 55, 1131–1133. [Google Scholar] [CrossRef]
- Theodorou, D.J.; Theodorou, S.J.; Axiotis, A.; Gianniki, M.; Tsifetaki, N. COVID-19 Vaccine-Related Myositis. QJM: Int. J. Med. 2021, 114, 424–425. [Google Scholar] [CrossRef]
- Ramalingam, S.; Arora, H.; Lewis, S.; Gunasekaran, K.; Muruganandam, M.; Nagaraju, S.; Padmanabhan, P. COVID-19 Vaccine-Induced Cellulitis and Myositis. Clevel. Clin. J. Med. 2021, 88, 648–650. [Google Scholar] [CrossRef]
- Gonzalez, D.; Gupta, L.; Murthy, V.; Gonzalez, E.B.; Williamson, K.A.; Makol, A.; Tan, C.L.; Sulaiman, F.N.; Shahril, N.S.; Isa, L.M.; et al. Anti-MDA5 Dermatomyositis after COVID-19 Vaccination: A Case-Based Review. Rheumatol. Int. 2022, 42, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Ikegami, T.; Igawa, K. Two Cases of anti-TIF1-γ Antibody Positive Dermatomyositis with Manifested Symptoms after SARS-CoV-19 Vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e517. [Google Scholar] [CrossRef]
- Gupta, K.; Shankar Sharma, G.; Kumar, A. COVID-19 Vaccination-Associated Anti-Jo-1 Syndrome. Rheumatology 2021, 59, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Ajima, S.; Ishikawa, Y. Emergence of Behçet’s Disease post-SARS-CoV2-vaccination: Two Clinical Cases in Japan. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e248–e249. [Google Scholar] [CrossRef] [PubMed]
- Baimukhamedov, C. Arthritis of the Left Elbow Joint after Vaccination against SARS-CoV-2 Infection. Int. J. Rheum. Dis. 2021, 24, 1218–1220. [Google Scholar] [CrossRef]
- Cole, A.; Thomas, R.; Goldman, N.; Howell, K.; Chakravarty, K.; Denton, C.P.; Ong, V.H. Diffuse Cutaneous Systemic Sclerosis Following SARS-Co V-2 Vaccination. J. Autoimmun. 2022, 128, 102812. [Google Scholar] [CrossRef]
- Oniszczuk, J.; Pagot, E.; Limal, N.; Hüe, S.; Audard, V.; Moktefi, A.; El Karoui, K. Scleroderma Renal Crisis Following mRNA Vaccination against SARS-CoV-2. Kidney Int. 2021, 100, 940–941. [Google Scholar] [CrossRef]
- Metin, Z.; Celepli, P. A Case of Morphea Following the COVID-19 mRNA Vaccine: On the Basis of Viral Spike Proteins. Int. J. Dermatol. 2022, 61, 639–641. [Google Scholar] [CrossRef]
- Wireko, F.W.; Khalafalla, S.; Jamshidi, T.; Mahgoub, S. Septic and Crystal-Induced Arthritis (Pseudogout) Post-COVID-19 Vaccination. Cureus 2022, 14, e23902. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, K.-H. Adult-Onset Still’s Disease after BNT162b2 mRNA COVID-19 Vaccine. J. Korean Med. Sci. 2021, 36, e344. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jung, J.-Y.; Suh, C.-H.; Kim, H.-A. Flare of Adult-Onset Still’s Disease Following mRNA COVID-19 Vaccination: A Case Report and Review of Literature. Clin. Rheumatol. 2022, 41, 1583–1589. [Google Scholar] [CrossRef]
- Iwata, J.; Kanetsuna, Y.; Takano, A.; Horiuchi, Y. A Case of Cutaneous Arteritis after Administration of mRNA Coronavirus Disease 2019 Vaccine. Dermatol. Ther. 2022, 35, e15618. [Google Scholar] [CrossRef]
- Azzazi, Y.; Abdelkader, H.A.; Khedr, H.; El-Komy, M.H.M. Extensive Cutaneous Leukocytoclastic Vasculitis after Sinopharm Vaccine: Case Report and Review of the Literature. J. Cutan. Pathol. 2022, 49, 736–742. [Google Scholar] [CrossRef]
- Fritzen, M.; Funchal, G.D.G.; Luiz, M.O.; Durigon, G.S. Leukocytoclastic Vasculitis after Exposure to COVID-19 Vaccine. An. Bras. Dermatol. 2022, 97, 118–121. [Google Scholar] [CrossRef]
- Oskay, T.; Isık, M. Leukocytoclastic Vasculitis after the Third Dose of CoronaVac Vaccination. Clin. Rheumatol. 2021, 41, 1931–1933. [Google Scholar] [CrossRef]
- Mücke, V.T.; Knop, V.; Mücke, M.M.; Ochsendorf, F.; Zeuzem, S. First Description of Immune Complex Vasculitis after COVID-19 Vaccination with BNT162b2: A Case Report. BMC Infect. Dis. 2021, 21, 958. [Google Scholar] [CrossRef]
- Altun, E.; Kuzucular, E. Leukocytoclastic Vasculitis after COVID-19 Vaccination. Dermatol. Ther. 2021, 35, e15279. [Google Scholar] [CrossRef]
- Uh, J.A.; Lee, S.K.; Kim, J.H.; Lee, J.H.; Kim, M.S.; Lee, U.H. Cutaneous Small-Vessel Vasculitis after ChAdOx1 COVID-19 Vaccination: A Report of Five Cases. Int. J. Low. Extrem. Wounds. 2022, 21, 193–196. [Google Scholar] [CrossRef]
- Fiorillo, G.; Pancetti, S.; Cortese, A.; Toso, F.; Manara, S.; Costanzo, A.; Borroni, R.G. Leukocytoclastic Vasculitis (Cutaneous Small-Vessel Vasculitis) after COVID-19 Vaccination. J. Autoimmun. 2022, 127, 102783. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.; Wollina, U.; Temiz, S.A.; Hasan, A. SARS-CoV-2 Vaccination-induced Cutaneous Vasculitis: Report of Two New Cases and Literature Review. Dermatol. Ther. 2022, 35, e15458. [Google Scholar] [CrossRef]
- Erler, A.; Fiedler, J.; Koch, A.; Heldmann, F.; Schütz, A. Leukocytoclastic Vasculitis After Vaccination with a SARS-CoV-2 Vaccine. Arthritis Rheumatol. 2021, 73, 2188. [Google Scholar] [CrossRef]
- Lebowitz, E.; Kim, J.S.; Magro, C. Reactive Arthritis Following COVID-19 Vaccination with BNT162b2. JAAD Case Rep. 2022, 24, 108–111. [Google Scholar] [CrossRef]
- Magliulo, D.; Narayan, S.; Ue, F.; Boulougoura, A.; Badlissi, F. Adult-Onset Still’s Disease after mRNA COVID-19 Vaccine. Lancet Rheumatol. 2021, 3, e680–e682. [Google Scholar] [CrossRef]
- Leone, F.; Cerasuolo, P.G.; Bosello, S.L.; Verardi, L.; Fiori, E.; Cocciolillo, F.; Merlino, B.; Zoli, A.; D’Agostino, M.A. Adult-Onset Still’s Disease Following COVID-19 Vaccination. Lancet Rheumatol. 2021, 3, e678–e680. [Google Scholar] [CrossRef]
- AlQudari, E.A.; Alabdan, L.I.; Alkhathami, A.A.; Alotaibi, M.D.; Alhamzi, H.A. Adult-Onset Still’s Disease After the ChAdOx1 nCoV-19 Vaccine. Cureus 2022, 14, e21279. [Google Scholar] [CrossRef]
- Sweeney, A.; Tracey, G.; Garnham, K. Adult-onset Still Disease Post-adenovirus Vector COVID-19 Vaccine. Intern. Med. J. 2021, 51, 2144–2145. [Google Scholar] [CrossRef]
- Muench, F.; Krusche, M.; Sander, L.E.; Rose, T.; Burmester, G.-R.; Schneider, U. Macrophage Activation Syndrome in a Patient with Adult-Onset Still’s Disease Following First COVID-19 Vaccination with BNT162b2. BMC Rheumatol. 2021, 5, 60. [Google Scholar] [CrossRef]
- Bindoli, S.; Giollo, A.; Galozzi, P.; Doria, A.; Sfriso, P. Hyperinflammation after Anti-SARS-CoV-2 mRNA/DNA Vaccines Successfully Treated with Anakinra: Case Series and Literature Review. Exp. Biol. Med. 2022, 247, 338–344. [Google Scholar] [CrossRef]
- Ungari, M.; Pezzarossa, E. Cutaneous Lymphocytic Vasculitis After Administration of the Second Dose of AZD1222 (Oxford–AstraZeneca) Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: Casuality or Causality? Am. J. Dermatopathol. 2021, 44, 80–82. [Google Scholar] [CrossRef]
- Yokote, A.; Fujioka, S.; Takahashi, N.; Mishima, T.; Tsuboi, Y. Polymyalgia Rheumatica Following COVID-19 Vaccination. Intern. Med. 2022, 61, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wong, C.K.; Yeung, W.Y.W.; Siu, C.W.; Yin, L. Case Report: Coronavirus Disease 2019 (COVID-19) Modified RNA Vaccination-Induced Adult-Onset Still’s Disease with Fulminant Myocarditis as Initial Presentation. Front. Cardiovasc. Med. 2023, 10, 1066699. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Ishida, T. Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis with Periaortitis That Developed After mRNA COVID-19 Vaccination. Cureus 2023, 15, e37480. [Google Scholar] [CrossRef] [PubMed]
- Alalem, N.; Yousaf, N. A Case Report of Reactive Arthritis After COVID-19 AstraZeneca Vaccination. Cureus 2023, 15, e35544. [Google Scholar] [CrossRef]
- Wojturska, W.; Nowakowski, J.; Pilch, W.; Biernikowicz, M.; Korkosz, M. Reactive Arthritis after Vaccination against SARS-CoV-2: A Case Series and a Mini-Review. Hum. Vaccines Immunother. 2023, 19, 2173912. [Google Scholar] [CrossRef]
- Weng, J.; Zhou, L.L.; Hahn, T.; Shojania, K.; Dutz, J. Adult-Onset Still Disease After ChAdOx1 nCOV-19 Vaccination. J. Rheumatol. 2022, 50, 290–291. [Google Scholar] [CrossRef]
- Wang, Y.; Hui, H.; Cheng, J.; Mao, H.; Shi, B. Subacute Cutaneous Lupus Erythematosus after COVID-19 Vaccine. Int. J. Rheum. Dis. 2023, 26, 1407–1409. [Google Scholar] [CrossRef]
- VanDerVeer, S.J.; Maier, K.D.; Hill, E.M. Rheum-CoV-2 Vaccination Case Series. JCR J. Clin. Rheumatol. 2022, 29, 105–108. [Google Scholar] [CrossRef]
- Tosunoğlu, B.; Güneş, H.N.; Çokal, B.G.; Yoldaş, T.K. Myositis Developing after COVID-19 mRNA Vaccine: Case Report. Acta Neurol. Taiwanica 2023, 32, 79–81. [Google Scholar]
- Sugimoto, T.; Yorishima, A.; Oka, N.; Masuda, S.; Nakamoto, N.; Kidoguchi, G.; Watanabe, H.; Yoshida, Y.; Mokuda, S.; Hirata, S. Appearance of Anti-MDA5 Antibody-Positive Dermatomyositis after COVID-19 Vaccination. Mod. Rheumatol. Case Rep. 2022, 7, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Srichawla, B.S. Polyarteritis Nodosa Following mRNA-1273 COVID-19 Vaccination: Case Study and Review of Immunological Mechanisms. Cureus 2023, 15, e33620. [Google Scholar] [CrossRef] [PubMed]
- Sogbe, M.; Blanco-Di Matteo, A.; Di Frisco, I.M.; Bastidas, J.F.; Salterain, N.; Gavira, J.J. Systemic Lupus Erythematosus Myocarditis after COVID-19 Vaccination. Reumatol. Clínica 2023, 19, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Peter, A.; Bhargava, J.K.; Arya, V.; Gupta, M.K.; Yadav, N.; Tiwari, P. Sarcoidosis Presenting as Bilateral Optic Neuritis after ChAdOx1 nCoV-19 Vaccination. Monaldi Arch. Chest Dis. 2022, 93. [Google Scholar] [CrossRef]
- Shokraee, K.; Rezaei, S.S.; Shahriarian, S.; Masoumi, M.; Parsaei, A.; Amini, B. Reactive Arthritis after Gam-COVID-Vac Vaccination: A Case Report. Mod. Rheumatol. Case Rep. 2023, 7, 368–372. [Google Scholar] [CrossRef]
- Sakai, M.; Takao, K.; Mizuno, M.; Ando, H.; Kawashima, Y.; Kato, T.; Kubota, S.; Hirose, T.; Hirota, T.; Horikawa, Y.; et al. Two Cases of Systemic Lupus Erythematosus after Administration of Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine. Mod. Rheumatol. Case Rep. 2023, 7, 378–382. [Google Scholar] [CrossRef]
- Saiz, L.C.; Villanueva Alcojol, M. Case Report: Granulomatosis with Polyangiitis (GPA) and Facial Paralysis after COVID-19 Vaccination. Med. Clínica 2023, 161, 84–85. [Google Scholar] [CrossRef]
- Rimmer, S.; Ly, L.; Boh, E. Subacute Cutaneous Lupus Erythematosus after mRNA-Based SARS-CoV-2 Vaccination. JAAD Case Rep. 2023, 33, 70–72. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Ohmura, S.; Ishihara, R.; Miyamoto, T. Possible Case of Polyarteritis Nodosa with Epididymitis Following COVID-19 Vaccination: A Case Report and Review of the Literature. Mod. Rheumatol. Case Rep. 2022, 7, 172–176. [Google Scholar] [CrossRef]
- Wang, S.; Noumi, B.; Malik, F.; Wang, S. A Rare Case of MDA-5-Positive Amyopathic Dermatomyositis with Rapidly Progressive Interstitial Lung Disease Following COVID-19 mRNA Vaccination—A Case Report. SN Compr. Clin. Med. 2022, 5, 18. [Google Scholar] [CrossRef]
- Mung, S.M.; Goh, T.L.; Hughes, M.; Jude, E.B. Inflammatory Arthritis Associated with COVID-19 Vaccination. Cureus 2023, 15, e35951. [Google Scholar] [CrossRef] [PubMed]
- Lo Sardo, L.; Parisi, S.; Ditto, M.C.; De Giovanni, R.; Maletta, F.; Grimaldi, S.; Brussino, L.; Fusaro, E. New Onset of Giant Cell Arteritis Following ChAdOx1-S (Vaxevria®®). Vaccine Adm. Vaccines 2023, 11, 434. [Google Scholar] [CrossRef]
- Koh, S.; Chen, H.; Hsu, C. Prolonged Peripheral Seronegative Spondyloarthritis Following BioNTech Coronavirus Disease 2019 Vaccination: A Case Report. Int. J. Rheum. Dis. 2022, 26, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Nakazawa, D.; Nishio, S.; Isozaki, T.; Komatsumoto, M.; Atsumi, T. Development of ANCA-Associated Vasculitis Followed by SARS-CoV-2 Vaccination in a Patient with HLA-DRB1*09:01 Allele. Mod. Rheumatol. Case Rep. 2023, 7, 426–430. [Google Scholar] [CrossRef]
- Katsouli, O.K.; Lainis, V.G.; Kapellos, G.G.; Vlachoyiannopoulos, P.G. Large Vessel Vasculitis After the Administration of Oxford-AstraZeneca COVID-19 Vaccine. Mediterr. J. Rheumatol. 2023, 34, 97. [Google Scholar] [CrossRef]
- Kan, A.K.; Yeung, W.W.; Lau, C.; Li, P.H. Adult-Onset Still’s Disease after mRNA COVID-19 Vaccination Presenting with Severe Myocarditis with Acute Heart Failure and Cardiogenic Shock: A Case Report. Hong Kong Med. J. 2023, 29, 162–164. [Google Scholar] [CrossRef]
- Iwata, M.; Ogawa, T.; Anju, K.; Okiyama, N.; Nomura, T. Adult-onset Still’s Disease Following mRNA-1273 Moderna COVID-19 Vaccination: A Case Report. J. Cutan. Immunol. Allergy 2022, 6, 66–67. [Google Scholar] [CrossRef]
- Haruna, K.; Shirota, S.; Nishioka, H. Polymyalgia Rheumatica (PMR) Lacking Shoulder Pain Following COVID-19 Vaccination. Cureus 2023, 15, e34714. [Google Scholar] [CrossRef]
- Golstein, M.A.; Fagnart, O.; Steinfeld, S.D. Reactive Arthritis after COVID-19 Vaccination: 17 Cases. Rheumatology 2023, kead169. [Google Scholar] [CrossRef]
- Gen, S.; Iwai, T.; Ohnari, S.; Nobe, K.; Ikeda, N. ANCA-Associated Vasculitis after Moderna COVID-19 Vaccination. Case Rep. Nephrol. 2023, 2023, 4906876. [Google Scholar] [CrossRef]
- Furr, T.; Garg, M. Rare Cases of Polymyalgia Rheumatica After Receiving COVID-19 Vaccinations. Cureus 2023, 15, e37782. [Google Scholar] [CrossRef]
- El Hasbani, G.; Uthman, I. ANCA-Associated Vasculitis Following the First Dose of Pfizer-BioNTech COVID-19 Vaccine. Nephron 2022, 147, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Durucan, I.; Guner, S.; Kilickiran Avci, B.; Unverengil, G.; Melikoglu, M.; Ugurlu, S. Post-COVID-19 Vaccination Inflammatory Syndrome: A Case Report. Mod. Rheumatol. Case Rep. 2022, 7, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Ansari, B.; Najafi, M.A.; Kheradmand, M.; Najafi, F.; Najafi, M.R. Immune-Mediated Necrotizing Myopathy Following Vaccination with the AstraZeneca (AZD1222) COVID-19 Vaccine: A Case Report and Brief Review. Rev. Neurol. 2023, 179, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.C.; Metze, D.; Steinbrink, K.; Böhm, M. Systemic Sarcoidosis with Cutaneous Tattoo Involvement Following COVID-19 Vaccination. Acta Derm.-Venereol. 2023, 103, adv6244. [Google Scholar] [CrossRef]
- Zamoner, W.; Scardini, J.B.; De Dio, B.J.; Marques, A.M.; Silva, V.D.S.; Garcia, A.L.; Dos Santos, D.C.; Viero, R.M. ANCA-Associated Vasculitis Following Oxford-AstraZeneca COVID-19 Vaccine in Brazil: Is There a Causal Relationship? A Case Report. Front. Med. 2022, 9, 1003332. [Google Scholar] [CrossRef]
- Yonezawa, H.; Ohmura, S.; Ohkubo, Y.; Miyamoto, T. New-Onset Seropositive Rheumatoid Arthritis Following COVID-19 Vaccination in a Patient with Seronegative Status. Intern. Med. 2022, 61, 3449–3452. [Google Scholar] [CrossRef]
- Yadav, R.; Shah, S.; Chhetri, S. ANCA-Associated Vasculitis Following Johnson and Johnson COVID-19 Vaccine. Ann. Med. Surg. 2022, 79, 104123. [Google Scholar] [CrossRef]
- Xia, C.; Edwards, R.; Omidvar, B. A Case of Giant Cell Arteritis with a Normal Erythrocyte Sedimentation Rate (ESR) Post ChAdOx1 nCoV-19 Vaccination. Cureus 2022, 14, e25388. [Google Scholar] [CrossRef]
- Wu, M.; Karim, M.; Ashinoff, R. COVID-19 Vaccine-Associated Dermatomyositis. JAAD Case Rep. 2022, 23, 58–60. [Google Scholar] [CrossRef]
- Watanabe, T.; Minaga, K.; Hara, A.; Yoshikawa, T.; Kamata, K.; Kudo, M. Case Report: New-Onset Rheumatoid Arthritis Following COVID-19 Vaccination. Front. Immunol. 2022, 13, 859926. [Google Scholar] [CrossRef]
- Wang, W.; Yu, S.; Hu, S.C. Subacute Cutaneous Lupus Erythematosus Following ChAdOx1 nCoV-19 Vaccination. J. Dermatol. 2022, 49, e401–e402. [Google Scholar] [CrossRef]
- Vanaskova, E.; Kelbich, P.; Novotny, T. Reactive Synovitis of the Knee Joint after COVID-19 Vaccination: The First Ultrastructural Analysis of Synovial Fluid. Int. J. Rheum. Dis. 2022, 25, 1324–1327. [Google Scholar] [CrossRef]
- Uddin, K.; Mohamed, K.H.; Agboola, A.A.; Naqvi, W.A.; Hussaini, H.; Mohamed, A.S.; Haseeb, M.; Nasir, H. Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Renal Vasculitis Following COVID-19 Vaccination: A Case Report and Literature Review. Cureus 2022, 14, e30206. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sekiguchi, Y.; Sasaki, M.; Inaba, S.; Oyama, S.; Inoue, Y.; Warabi, M.; Ohashi, K.; Inoshita, S. Antineutrophil Cytoplasmic Antibody-Associated Vasculitis after COVID-19 Vaccination with Pfizer-BioNTech. Intern. Med. 2022, 61, 2925–2929. [Google Scholar] [CrossRef]
- Seeley, E.A.; Zimmer, M.; Berghea, R. Suspected COVID-19 Immunization-Induced Probable Catastrophic Antiphospholipid Syndrome. Cureus 2022, 14, e27313. [Google Scholar] [CrossRef]
- Schoenardie, B.O.; Schoenardie, A.O.; Damke, J.P. Monoarthritis Induced by the Oxford-AstraZenecaR SARS-CoV-2 Vaccine. Rev. Colomb. De Reumatol. 2022, 29, S77–S79. [Google Scholar] [CrossRef]
- Park, Y.R.; Ben-Artz, A. New Onset of Inflammatory Arthritis Following Moderna COVID-19 Vaccination. Isr. Med. Assoc. J. 2022, 24, 333–334. [Google Scholar]
- Ohmura, S.; Ohkubo, Y.; Ishihara, R.; Otsuki, Y.; Miyamoto, T. Medium-Vessel Vasculitis Presenting with Myalgia Following COVID-19 Moderna Vaccination. Intern. Med. 2022, 61, 3453–3457. [Google Scholar] [CrossRef]
- Numakura, T.; Murakami, K.; Tamada, T.; Yamaguchi, C.; Inoue, C.; Ohkouchi, S.; Tode, N.; Sano, H.; Aizawa, H.; Sato, K.; et al. A Novel Development of Sarcoidosis Following COVID-19 Vaccination and a Literature Review. Intern. Med. 2022, 61, 3101–3106. [Google Scholar] [CrossRef]
- Mohaghegh, F.; Hatami, P.; Refaghat, A.; Matini, A.H.; Mohseni Afshar, Z.; Aryanian, Z. Unmasking Sarcoidosis Following SARS-CoV-2 Vaccination: A Case Report. Clin. Case Rep. 2022, 10, e6660. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Mukherjee, S.; Bhattacharya, M.; Detroja, R.; Merzon, E.; Blum, I.; Livoff, A.; Shlapobersky, M.; Baum, G.; Talisman, R.; et al. Clinical and Molecular Characterization of a Rare Case of BNT162b2 mRNA COVID-19 Vaccine-Associated Myositis. Vaccines 2022, 10, 1135. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, T.; Xu, G. ANCA-Associated Vasculitis Following the CoronaVac Vaccination. Ther. Adv. Chronic Dis. 2022, 13, 204062232211257. [Google Scholar] [CrossRef]
- Lourenço, C.; Pascoal, A.; Paiva, A.; Campos, I.; Pagaimo, J. Polymyalgia Rheumatica After ChAdOx1 nCov-19 Vaccine: A Case Report. Cureus 2022, 14, e25346. [Google Scholar] [CrossRef]
- Kreuter, A.; Lausch, S.; Burmann, S.-N.; Paschos, A.; Michalowitz, A.-L. Onset of Amyopathic Dermatomyositis Following mRNA -based SARS-CoV-2 Vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e669–e672. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, J.; Lee, S.-G.; Kim, G.-T. COVID-19 Vaccination-Related Small Vessel Vasculitis with Multiorgan Involvement. Z. Für Rheumatol. 2022, 81, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Woo, C.G. Clinicopathological Characteristics of Inflammatory Myositis Induced by COVID-19 Vaccine (Pfizer-BioNTech BNT162b2): A Case Report. J. Korean Med. Sci. 2022, 37, e91. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Kim, H.S.; Han, K.H.; Han, S.Y.; Jo, H.A. A Case Report of MPO-ANCA-Associated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination. J. Korean Med. Sci. 2022, 37, e204. [Google Scholar] [CrossRef]
- Khanna, U.; Oprea, Y.; Mir, A.; Halverstam, C. New Diagnosis of Systemic Lupus Erythematosus after COVID-19 Vaccination: A Case Report and Review of Literature. JAAD Case Rep. 2022, 30, 30–34. [Google Scholar] [CrossRef]
- Holzer, M.-T.; Krusche, M.; Ruffer, N.; Haberstock, H.; Stephan, M.; Huber, T.B.; Kötter, I. New-Onset Dermatomyositis Following SARS-CoV-2 Infection and Vaccination: A Case-Based Review. Rheumatol. Int. 2022, 42, 2267–2276. [Google Scholar] [CrossRef]
- Greb, C.S.; Aouhab, Z.; Sisbarro, D.; Panah, E. A Case of Giant Cell Arteritis Presenting After COVID-19 Vaccination: Is It Just a Coincidence? Cureus 2022, 14, e21608. [Google Scholar] [CrossRef] [PubMed]
- Godoy, I.R.B.; Rodrigues, T.C.; Skaf, A.Y. Bilateral Upper Extremity Myositis after COVID-19 Vaccination. BJR Case Rep. 2022, 8, 20220002. [Google Scholar] [CrossRef]
- Gamonal, S.B.L.; Marques, N.C.V.; Pereira, H.M.B.; Gamonal, A.C.C. New-onset Systemic Lupus Erythematosus after ChAdOX1 nCoV-19 and Alopecia Areata after BNT162b2 Vaccination against SARS-CoV-2. Dermatol. Ther. 2022, 35, e15677. [Google Scholar] [CrossRef]
- Farooq, M.; Mohammed, Y.; Zafar, M.; Dharmasena, D.; Rana, U.I.; Kankam, O. COVID-19 Vaccine-Induced Pneumonitis, Myositis and Myopericarditis. Cureus 2022, 14, e20979. [Google Scholar] [CrossRef] [PubMed]
- Essien, F.; Evans, J.; Kyle, A.; Urisman, A.; Adams, N. ‘Granulomatosis with Polyangiitis after Pfizer Vaccination’: A Case Report. Ther. Adv. Rare Dis. 2022, 3, 263300402211300. [Google Scholar] [CrossRef] [PubMed]
- Draz, H.E.; Khalifa, A.A. Reactive Arthritis (ReA) Following the First Dose of COVID-19 Vaccine; A Case Report. Rheumatology 2022, 30, 95–98. [Google Scholar] [CrossRef]
- Chua, X.-H.; Lin, W.-L.; Lee, Y.-T. Adult-Onset Still’s Disease Following Coronavirus 2 (SARS-CoV-2) Vaccination: A Case Report. Vaccines 2022, 10, 1687. [Google Scholar] [CrossRef]
- Christodoulou, M.; Iatridi, F.; Chalkidis, G.; Lioulios, G.; Nikolaidou, C.; Badis, K.; Fylaktou, A.; Papagianni, A.; Stangou, M. ANCA-Associated Vasculitis May Result as a Complication to Both SARS-CoV-2 Infection and Vaccination. Life 2022, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Chomičienė, A.; Černiauskas, K.; Linauskienė, K.; Meškauskas, R.; Malinauskienė, L. Two Case Reports of Skin Vasculitis Following the COVID-19 Immunization. Open Med. 2022, 17, 1944–1948. [Google Scholar] [CrossRef]
- Chaima, K.; Mariem, A.; Sana, B.; Khadija, S.; Mariem, R.; Massara, B.; Emna, B.; Sonia, B.; Abderrahman, M.; Hamida, T. Vaccine-induced Dermatomyositis Following COVID-19 Vaccination. Dermatol. Ther. 2022, 35, e15749. [Google Scholar] [CrossRef]
- Bose, P.; Goenka, U.; Moitra, S.; Majumdar, S.; Goenka, M.K.; Sen, S.G. COVID-19 Vaccine-associated Myositis—A Case Report. Clin. Case Rep. 2022, 10, e6314. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.; Sahoo, D.; Khangembam, A.; Jamil, M.; Ish, P. New-Onset Rheumatoid Arthritis Following ChAdOx1 nCoV-19 Vaccine Administration. Indian J. Rheumatol. 2022, 17, 323–324. [Google Scholar] [CrossRef]
- Ball-Burack, M.R.; Kosowsky, J.M. A Case of Leukocytoclastic Vasculitis Following SARS-CoV-2 Vaccination. J. Emerg. Med. 2022, 63, e62–e65. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Yamamoto, S.; Tochitani, K. Transient Large-Vessel Vasculitis after COVID-19 mRNA Vaccination. Intern. Med. 2022, 61, 2083–2084. [Google Scholar] [CrossRef] [PubMed]
- Ahmer, S.; Bourke, J.; Ardakani, N.M. Transient Cryoglobulinaemic Vasculitis Following ChAdOx1 nCoV-19 Vaccine. BMJ Case Rep. 2022, 15, e250913. [Google Scholar] [CrossRef]
- Villa, M.; Díaz-Crespo, F.; Pérez de José, A.; Verdalles, Ú.; Verde, E.; Almeida Ruiz, F.; Acosta, A.; Mijaylova, A.; Goicoechea, M. A Case of ANCA-Associated Vasculitis after AZD1222 (Oxford–AstraZeneca) SARS-CoV-2 Vaccination: Casualty or Causality? Kidney Int. 2021, 100, 937–938. [Google Scholar] [CrossRef]
- Türk, S.; Öztürk, Z.; Karataş, D.; Gönüllü, E. Inactivated COVID-19 Vaccine Can Induce Reactive Polyarthritis in Older Patients: Report of Two Cases. Georgian Med. News 2021, 319, 100–102. [Google Scholar]
- Sirufo, M.M.; Raggiunti, M.; Magnanimi, L.M.; Ginaldi, L.; De Martinis, M. Henoch-Schönlein Purpura Following the First Dose of COVID-19 Viral Vector Vaccine: A Case Report. Vaccines 2021, 9, 1078. [Google Scholar] [CrossRef]
- Risal, U.; Subedee, A.; Pangeni, R.; Pandey, R.; Pandey, S.; Adhikari, S.; Basnyat, B. Case Report: Adult Onset Still’s Disease after Vaccination against COVID-19. Wellcome Open Res. 2022, 6, 333. [Google Scholar] [CrossRef]
- Quattrini, L.; Verardi, L.; Caldarola, G.; Peluso, G.; De Simone, C.; D’Agostino, M. New Onset of Remitting Seronegative Symmetrical Synovitis with Pitting Oedema and Palmoplantar Psoriasis Flare-up after Sars-CoV-2 Vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e727. [Google Scholar] [CrossRef]
- Naitlho, A.; Lahlou, W.; Bourial, A.; Rais, H.; Ismaili, N.; Abousahfa, I.; Belyamani, L. A Rare Case of Henoch-Schönlein Purpura Following a COVID-19 Vaccine—Case Report. SN Compr. Clin. Med. 2021, 3, 2618–2621. [Google Scholar] [CrossRef]
- Liu, V.; Messenger, N.B. New-Onset Cutaneous Lupus Erythematosus after the COVID-19 Vaccine. Dermatol. Online J. 2022, 27, 1–4. [Google Scholar] [CrossRef]
- Kar, B.R.; Singh, B.S.; Mohapatra, L.; Agrawal, I. Cutaneous Small-vessel Vasculitis Following COVID-19 Vaccine. J. Cosmet. Dermatol. 2021, 20, 3382–3383. [Google Scholar] [CrossRef]
- Hines, A.M.; Murphy, N.; Mullin, C.; Barillas, J.; Barrientos, J.C. Henoch-Schönlein Purpura Presenting Post COVID-19 Vaccination. Vaccine 2021, 39, 4571–4572. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Pérez, L.; Puerta-Peña, M.; Falkenhain-López, D.; Montero-Menárguez, J.; Gutiérrez-Collar, C.; Rodríguez-Peralto, J.L.; Sanz-Bueno, J. Small-vessel Vasculitis Following Oxford-AstraZeneca Vaccination against SARS-CoV-2. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e741. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, H.-Y.; Lu, C.-C.; Lin, S.-H. Case Report: Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis with Acute Renal Failure and Pulmonary Hemorrhage May Occur After COVID-19 Vaccination. Front. Med. 2021, 8, 765447. [Google Scholar] [CrossRef] [PubMed]
- Chan-Chung, C.; Ong, C.S.; Chan, L.L.; Tan, E.K. Eosinophilic Granulomatosis with Polyangiitis after COVID-19 Vaccination. QJM Int. J. Med. 2021, 114, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.E.Z.; Irimpen, A. Severe but Self-Limiting Polyarthralgia with Functional Impairment Following ChAdOx1 nCov-19 Vaccination in an Elderly Recipient. Vaccines 2021, 9, 1220. [Google Scholar] [CrossRef]
- Berry, C.T.; Eliliwi, M.; Gallagher, S.; Panaccione, S.; Klein, W.M.; Healy, A.L.; Stoecker, B.; Kallas, R. Cutaneous Small Vessel Vasculitis Following Single-Dose Janssen Ad26.COV2.S Vaccination. JAAD Case Rep. 2021, 15, 11–14. [Google Scholar] [CrossRef]
- Dube, G.K.; Benvenuto, L.J.; Batal, I. Antineutrophil Cytoplasmic Autoantibody–Associated Glomerulonephritis Following the Pfizer-BioNTech COVID-19 Vaccine. Kidney Int. Rep. 2021, 6, 3087–3089. [Google Scholar] [CrossRef]
- Takenaka, T.; Matsuzaki, M.; Fujiwara, S.; Hayashida, M.; Suyama, H.; Kawamoto, M. Myeloperoxidase Anti-Neutrophil Cytoplasmic Antibody Positive Optic Perineuritis after mRNA Coronavirus Disease-19 Vaccine. QJM Int. J. Med. 2021, 114, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, S.; Al-Jobory, O.; Hallak, A.; Bharadwaj, T.; Patel, M. IgG4 Related Pleural Disease: Recurrent Pleural Effusion after COVID-19 Vaccination. Respirol. Case Rep. 2022, 10, e01026. [Google Scholar] [CrossRef]
- Aochi, S.; Uehara, M.; Yamamoto, M. IgG4-Related Disease Emerging after COVID-19 mRNA Vaccination. Intern. Med. 2023, 62, 1547–1551. [Google Scholar] [CrossRef]
- Matsuda, M.; Funakubo Asanuma, Y.; Yokota, K.; Sakai, S.; Yazawa, H.; Maruyama, T.; Tsuzuki Wada, T.; Araki, Y.; Mimura, T. New-Onset Adult-Onset Still’s Disease Following COVID-19 Vaccination: Three Case Reports and a Literature Review. Intern. Med. 2023, 62, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Avalos, C.; Ahmadzadeh, Y.; Gatsak, D.; Moosa, S.A.; Mozaffari, M.A.; Imas, A.S.; Miller, R. Cardiac Tamponade as a Complication of Microscopic Polyangiitis: A Case Associated with a COVID-19 mRNA Vaccine. Cureus 2023, 15, e37569. [Google Scholar] [CrossRef] [PubMed]
- Sagy, I.; Zeller, L.; Raviv, Y.; Porges, T.; Bieber, A.; Abu-Shakra, M. New-Onset Systemic Lupus Erythematosus Following BNT162b2 mRNA COVID-19 Vaccine: A Case Series and Literature Review. Rheumatol. Int. 2022, 42, 2261–2266. [Google Scholar] [CrossRef]
- Roy, R.; Mani, A.; John, J.; Antony, T.; Jacob, L. New-Onset Henoch–Schonlein Purpura after COVID-19 Vaccination. Indian J. Rheumatol. 2022, 17, 206. [Google Scholar] [CrossRef]
- Chan, A.R.; Cohen Tervaert, J.W.; Redmond, D.; Yacyshyn, E.; Ferrara, G.; Hwang, P.M.; Osman, M.; Gniadecki, R. A Case Series of Dermatomyositis Following SARS-CoV-2 Vaccination. Front. Med. 2022, 9, 1013378. [Google Scholar] [CrossRef]
- Nahra, V.; Makandura, M.; Mattar, M.; Anthony, D.D. A Case Series on the COVID-19 Vaccines and Possible Immune-Related Adverse Events: A New Challenge for the Rheumatologists. Cureus 2022, 14, e29660. [Google Scholar] [CrossRef]
- Bansal, P.; Sonani, B.; Aslam, F. Progression from Palindromic Rheumatism to Rheumatoid Arthritis after COVID-19 Vaccination. ARP Rheumatol. 2022, 1, 100–101. [Google Scholar]
- Parperis, K.; Constantinou, M. Remitting Seronegative Symmetrical Synovitis with Pitting Oedema Following BNT162b2 mRNA COVID-19 Vaccination. BMJ Case Rep. 2021, 14, e244479. [Google Scholar] [CrossRef] [PubMed]
- Baimukhamedov, C.; Makhmudov, S.; Botabekova, A. Seropositive Rheumatoid Arthritis after Vaccination against SARS-CoV-2 Infection. Int. J. Rheum. Dis. 2021, 24, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, H.; Xia, S.-L. New-Onset Arthritis Following COVID-19 Vaccination: A Systematic Review of Case Reports. Vaccines 2023, 11, 665. [Google Scholar] [CrossRef]
- Ursini, F.; Ruscitti, P.; Raimondo, V.; De Angelis, R.; Cacciapaglia, F.; Pigatto, E.; Olivo, D.; Di Cola, I.; Galluccio, F.; Francioso, F.; et al. Spectrum of Short-Term Inflammatory Musculoskeletal Manifestations after COVID-19 Vaccine Administration: A Report of 66 Cases. Ann. Rheum. Dis. 2021, 81, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Ray, I.; Mascarenhas, D.; Kunal, S.; Sachdeva, R.A.; Ish, P. Myocarditis Post-SARS-CoV-2 Vaccination: A Systematic Review. QJM: Int. J. Med. 2022, 116, 7–25. [Google Scholar] [CrossRef]
- Safary, A.; Esalatmanesh, K.; Eftekharsadat, A.T.; Jafari Nakjavani, M.-R.; Khabbazi, A. Autoimmune Inflammatory Rheumatic Diseases Post-COVID-19 Vaccination. Int. Immunopharmacol. 2022, 110, 109061. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 Vaccines: Modes of Immune Activation and Future Challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Olivieri, B.; Betterle, C.; Zanoni, G. Vaccinations and Autoimmune Diseases. Vaccines 2021, 9, 815. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, L.; López-Hoyos, M.; Beares, I.; Mata, C.; Garcia-Unzueta, M.; Calvo-Alen, J.; Blanco, R.; Aurrecoechea, E.; Tripathi, G.; Martinez-Taboada, V.M. Toll-like receptor 4 gene polymorphisms in polymyalgia rheumatica and elderly-onset rheumatoid arthritis. Clin. Exp. Rheumatol. 2011, 29, 795–800. [Google Scholar]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems Vaccinology of the BNT162b2 mRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Sachinidis, A.; Garyfallos, A. COVID-19 Vaccination Can Occasionally Trigger Autoimmune Phenomena, Probably via Inducing Age-associated B Cells. Int. J. Rheum. Dis. 2021, 25, 83–85. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification. Available online: https://apps.who.int/iris/handle/10665/259959 (accessed on 15 September 2023).
| SN | Article | Country | Age | Sex | Vaccine Received | R-IMID Diagnosis | Immunosuppressive Drugs Used | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Shimagami et al. [5] | Japan | 90 | F | PfizerBioNTech | Tenosynovitis and pleural effusion | Prednisolone | Clinical improvement |
| 70 | M | PfizerBioNtech | Tenosynovitis | Prednisolone | Clinical improvement | |||
| 2 | Osada A et al. [6] | Japan | 80 | F | Pfizer BioNTech | PMR | Prednisolone | Clinical improvement |
| 3 | Padiyar et al. [7] | India | 20 | F | Oxford-AstraZeneca | AOSD | corticosteroids, naproxen, and tocilizumab | Clinical improvement |
| 47 | M | Oxford-AstraZeneca | AOSD | Naproxen | Clinical improvement | |||
| 35 | F | Oxford-AstraZeneca | AOSD | Steroid, tocilizumab | Clinical improvement | |||
| 4 | Unal Enginar et al. [8] | Tukey | 74 | F | Sinovac | Seronegative RA | Prednisolone | Clinical improvement |
| 76 | M | Sinovac | Seronegative RA | Prednisolone | Clinical improvement | |||
| 5 | An et al. [9] | China | 23 | F | CoronaVac | Reactive arthritis | IA Betamethasone | Remission |
| 6 | Hyun et al. [10] | South Korea | 68 | F | OxfordAstraZen | PMR | NSAID, and possiblly Prednisolone (it was not mentioned who received these) | Remission |
| 67 | F | OxfordAstraZen | PMR | Remission | ||||
| 67 | F | OxfordAstraZen | PMR | Remission | ||||
| 25 | F | OxfordAstraZen | PMR | Remission | ||||
| 70 | F | OxfordAstrazen | PMR | Remission | ||||
| 7 | Gentiloni et al. [11] | Italy | 71 | F | PfizerBioNTech | Seronegative RA | Prednisolone | Remission |
| 72 | M | PfizerBioNTech | Seronegative RA | Prednisolone | Remission | |||
| 61 | M | PfizerBioNtech | Seronegative RA | Prednisolone | Remission | |||
| 68 | M | PfizerBioNTech | Seronegative RA | Prednisolone | Remission | |||
| 72 | M | PfizerBioNTech | PMR | Prednisolone | Remission | |||
| 38 | F | PfizerBioNTech | Undifferentiated CTD | Prednisolone | Remission | |||
| 46 | F | PfizerBioNTech | DM | Prednisolone | Remission | |||
| 78 | F | PfizerBioNTech | Cutaneous vasculitis | Prednisolone | Remission | |||
| 8 | Santiago et al. [18] | n/a | 32 | M | Pfizer BioNTech | Sarcoidosis | Prednisolone and azathioprine | Clinical improvement |
| 9 | Nune et al. [19] | United Kingdom | 24 | M | Pfizer BioNTech | SLE | Prednisolone and methotrexate | Clinical improvement |
| 10 | Kreuter et al. [20] | Germany | 79 | M | Pfizer BioNTech | SLE | HCQ and prednisolone | Remission |
| 11 | Zavala-Miranda et al. [21] | Mexico | 23 | F | Oxford-AstraZeneca | SLE | Mycopheolate, HCQ, glucocorticosteroids, | Clinical improvement |
| 12 | Hidaka et al. [22] | Japan | 53 | F | Pfizer BioNTech | SLE | Prednisolone | Clinical improvement |
| 13 | Raviv et al. [23] | Israel | 24 | M | Pfizer BioNTech | SLE | Hydroxychloroquine, topical steroids and NSAID | Clinical improvement |
| 14 | Zengarini et al. [24] | Italy | 30 | F | Pfizer BioNTech | SLE | Prednisolone | Clinical improvement |
| 15 | Matsuo et al. [25] | Japan | 34 | F | Pfizer BioNTech | Sarcoidosis | Prednisolone | Remission |
| 16 | Rademacher et al. [26] | Germany | 21 | F | Oxford-AstraZeneca | Sarcoidosis | Prednisolone | Clinical improvement |
| 27 | M | Oxford-AstraZeneca | Sarcoidosis | Prednisolone | Clinical improvement | |||
| 17 | Kaur et al. [12] | United States | 54 | M | PfizerBioNTech | SLE | Prednisolone | Remission |
| 18 | Molina-Rios et al. [13] | Colombia | 42 | F | PfizerBioNTech | SLE | Prednisolone, HCQ and azathioprine | Remission |
| 19 | Arshadi Mousa et al. [14] | Saudi Arabia | 22 | F | PfizerBioNTech | SLE | Prednisolone, HCQ and azathioprine | Remission |
| 20 | Báez-Negrón et al. [15] | Puerto Rico | 27 | F | Moderna | SLE | Prednisolone, HCQ and MMF | No improvement |
| 21 | Patil and Patil. [16] | India | 22 | F | Covishield | SLE | Prednisolone, HCQ and MMF | Clinical improvement |
| 22 | Lemoine et al. [17] | United States | 68 | F | Pfizer BioNTech | SLE | Methotrexate and prednisone | Clinical improvement |
| 23 | Izuka et al. [27] | Japan | 70 | M | Moderna | PMR | Acetaminophen, was self-limited before commencing steroids | Clinical improvement |
| 24 | Ottaviani et al. [28] | France | 74 | F | PfizerBioNTech | PMR | Glucocorticoids | Clinical improvement |
| 70 | F | PfizerBioNTech | PMR | Glucocorticoids and methotrexate | Clinical improvement | |||
| 74 | F | PfizerBioNTech | PMR | Glucocorticoids | Clinical improvement | |||
| 77 | F | PfizerBioNTech | PMR | Glucocorticoids and methotrexate | Clinical improvement | |||
| 65 | M | Moderna | PMR | Glucocorticoids | Clinical improvement | |||
| 78 | F | PfizerBioNTech | PMR | Glucocorticoids | Clinical improvement | |||
| 73 | F | PfizerBioNTech | PMR | Glucocorticoids | Clinical improvement | |||
| 75 | F | PfizerBioNTech | PMR | Glucocorticoids and tocilizumab | Clinical improvement | |||
| 77 | M | PfizerBioNTech | PMR | Shoulder corticosteroid injections | Clinical improvement | |||
| 89 | M | PfizerBioNTech | PMR | Glucocorticoids and methotrexate | Clinical improvement | |||
| 25 | Manzo et al. [30] | Italy | 69 | F | Pfizer BioNTech | PMR | Prednisolone | Clinical improvement |
| 26 | Gambichler et al. [31] | Germany | 82 | M | Pfizer BioNTech | GCA | Not reported | Not Reported |
| 27 | Sauret et al. [32] | France | 70 | M | Oxford-AstraZeneca | GCA | Prednisolone | Remission |
| 28 | Mejren et al. [33] | Spain | 62 | F | Pfizer BioNTech | GCA | Prednisolone | Clinical improvement |
| 29 | Anzola et al. [34] | Spain | 83 | F | Pfizer BioNTech | GCA | Pulse steroids and methotrexate | Remission |
| 30 | Lee et al. [35] | United States | 34 | M | Pfizer BioNTech | GCA | IV methyl prednisolone, and oral prednisolone | Clinical improvement |
| 31 | Fillon et al. [36] | France | 73 | M | Pfizer BioNTech | PAN | Cyclophosphamide and steroids | No improvement |
| 32 | Gillion et al. [37] | Belgium | 77 | M | Oxford-AstraZeneca | ANCA-negative vasculitis | Methylprednisolone | Remission |
| 33 | Feghali et al. [38] | United States | 58 | M | Moderna | AAV | Prednisolone, cyclophosphamide and rituximab | Remission |
| 34 | Nakatani et al. [39] | Japan | 80 | M | Pfizer BioNTech | LVV | Not reported | Not reported |
| 35 | Schierz et al. [40] | Germany | 78 | F | Moderna | LVV | Not reported | Not reported |
| 36 | Shakoor et al. [41] | United States | 78 | F | Pfizer BioNTech | AAV | Rituximab and prednisone | Clinical improvement |
| 37 | Hakroush et al. [42] | Germany | 79 | F | Pfizer BioNTech | AAV | Prednisone and cyclophosphamide | Clinical improvement |
| 38 | Baier et al. [43] | Germany | 57 | F | Pfizer BioNTech | AAV | Methylprednisolone and prednisone | Clinical improvement |
| 39 | Okuda et al. [44] | Japan | 37 | F | Pfizer BioNTech | AAV | Prednisolone | Clinical improvement |
| 40 | Obata et al. [45] | Japan | 84 | M | Pfizer BioNTech | AAV | Methylprednisolone and prednisolone | Clinical Improvement |
| 41 | Shirai et al. [46] | Japan | 63 | F | Pfizer BioNTech | AAV | Rituximab, prednisolone and cyclophosphamide | Clinical improvement |
| 42 | Al-Yafeai et al. [47] | United States | 62 | F | Pfizer BioNTech | AAV | Rituximab, cyclophosphamide and plasmapheresis | Clinical improvement |
| 43 | So et al. [48] | Korea | 42 | M | Pfizer BioNTech | AAV | Methylprednisolone, oral prednisolone, rituximab and plasma exchange | Clinical improvement |
| 44 | Al-Allaf et al. [49] | Qatar | 46 | M | Pfizer BioNTech | AAV | Azathioprine and prednisolone | Clinical improvement |
| 45 | Nappi et al. [50] | Italy | 63 | M | Moderna | AAV | Methylprednisolone, prednisolone and cyclophosamide | Clinical improvement |
| 46 | Sekar et al. [51] | USA | 52 | M | Moderna | AAV | Rituximab, cyclophosphamide and prednisone | No improvement |
| 47 | Ibrahim et al. [53] | USA | 79 | F | Moderna | AAV | Prednisone and azathioprine | Clinical improvement |
| 48 | Prabhahar et al. [52] | India | 51 | M | Oxford-AstraZeneca | AAV | Prednisolone and rituximab | Remission |
| 49 | Su et al. [54] | Taiwan | 52 | F | Oxford-AstraZeneca | Cutaneous PAN | Prednisolone and methotrexate | Remission |
| 50 | Anderegg et al. [55] | Switzerland | 39 | M | Moderna | ANCA negative vasculitis | Glucocorticoids and cyclophosphamide | No improvement |
| 81 | M | Moderna | AAV | Glucocorticoids, cyclophosphamide and plasmapheresis | Remission | |||
| 51 | Yokote et al. [94] | Japan | 71 | F | Pfizer BioNTech | PMR | Prednisolone | Clinical improvement |
| 52 | Nune et al. [29] | United Kingdom | 70 | F | PfizerBioNTech | Seronegative RA | Prednisolone | Clinical improvement |
| 44 | F | OxfordAstrazenic | PMR | Prednisolone | Clinical improvement | |||
| 53 | Vutipongsatorn et al. [56] | United Kingdom | 55 | F | Pfizer BioNTech | DM | IV methyl prednisolone, IVIG, cyclophosphamide and MMF | Clinical improvement |
| 72 | F | Pfizer BioNTech | PM | IV methyl prednisolone and IVIG | Clinical improvement | |||
| 54 | Maramattom et al. [57] | India | 74 | M | OxfordAstraZen | PM | Prednisolone | Remission |
| 75 | F | OxfordAstraZen | PM | Prednisolone and mycophenolate | Remission | |||
| 80 | F | OxfordAstraZen | PM | Prednisolone | Remission | |||
| 55 | Coronel et al. [58] | Mexico | 76 | F | Pfizer BioNTech | DM | Corticosteroids and methotrexate | Clinical improvement |
| 56 | Gouda et al. [59] | Egypt | 43 | F | Pfizer BioNTech | DM | Prednisolone, MMF and HCQ | Clinical improvement |
| 57 | Capassoni et al. [61] | Italy | 37 | F | Oxford-AstraZeneca | PM | IV methyl prednisone | Clinical improvement |
| 58 | Tagini et al. [60] | Switzerland | 20 | F | Moderna | Behcet’s | Colchicine, prednisone and azathioprine | Clinical improvement |
| 59 | Huang et al. [62] | Taiwan | 44 | M | Oxford-AstraZeneca | DM | IV methylprednisolone and cyclophosphamide | ICU hospitalization and death |
| 60 | Theodorou et al. [63] | Greece | 56 | F | mRNA | Focal myositis | Cryotherapy, compression, and NSAIDs | Remission |
| 61 | Ramalingam et al. [64] | Mexico | 81 | M | Moderna | Focal myositis | Methylprednisolone | Clinical improvement |
| 62 | Gonzalez et al. [65] | United States | 45 | M | Moderna | DM | Methylprednisolone, rituximab, IVIG, and methotrexate | Remission |
| 58 | F | Covishield | DM | MMF, HCQ, cyclophosphamide, rituximab, tofacitinib, tacrolimus, and plasma exchange | Clinical improvement | |||
| 45 | F | PfizerBioNTech | DM | Corticosteroids, HCQ, and MMF | Clinical improvement | |||
| 28 | F | PfizerBioNTech | DM | Prednisolone and cyclophosphamide | Clinical improvement | |||
| 51 | F | PfizerBioNTech | DM | Corticosteroids, rituximab, tacrolimus and IVIG | Clinical improvement | |||
| 54 | F | PfizerBioNTech | DM | Azathioprine which was changed to MMF due to intolerance and prednisolone | Clinical improvement | |||
| 63 | Yoshida et al. [66] | Japan | 81 | F | PfizerBioNTech | DM | Prednisolone | Clinical improvement |
| 87 | F | PfizerBioNTech | DM | Prednisolone and IVIG | Clinical improvement | |||
| 64 | Gupta et al. [67] | India | 46 | F | Oxford-AstraZeneca | Anti-synthetase syndrome | Prednisolone and methotrexate | Clinical improvement |
| 65 | Hashizume et al. [68] | Japan | 29 | F | PfizerBioNTech | Behcets | Colchicine | Clinical improvement |
| 59 | M | PfizerBioNTech | Behcets | Corticosteroid and Colchicine | Clinical improvement | |||
| 66 | Lebowitz et al. [86] | United States | 49 | M | Pfizer BioNTech | Reactive arthritis | Prednisolone | Clinical improvement |
| 67 | Baimukhamedov et al. [69] | Kazakhstan | 58 | M | SPUTNIK-V | Reactive arthritis | IA corticosteroid and NSAIDs | No improvement |
| 68 | Cole et al. [70] | United Kingdom | 70 | M | Oxford-AstraZeneca | Systemic sclerosis | Not recorded | Not reported |
| 69 | Oniszczuk et al. [71] | France | 34 | F | Pfizer BioNTech | Systemic sclerosis | Antihypertensives and ACE inhibitors | Clinical improvement |
| 70 | Metin et al. [72] | Turkey | 55 | F | Pfizer BioNTech | Localised scleroderma | Clobetasol pomade and calcipotriol pomade | Remission |
| 71 | Wireko et al. [73] | United States | 69 | M | Pfizer BioNTech | Pseudogout and septic arthritis | Ceftriaxone | Clinical improvement |
| 72 | Magliulo et al. [87] | United States | 45 | F | Moderna (2) | AOSD | Prednisolone | Remission |
| 73 | Leone et al. [88] | Italy | 36 | M | Oxford-AstraZeneca | AOSD | Methylprednisolone and anakinra | Clinical improvement |
| 74 | AlQudari et al. [89] | Saudi Arabia | 29 | M | Oxford-AstraZeneca | AOSD | Methylprednisolone | Clinical improvement |
| 75 | Park et al. [74] | Korea | 36 | F | Pfizer BioNTech | AOSD | Methylprednisolone and tocilizumab | Clinical improvement |
| 76 | Sweeney et al. [90] | Australia | 53 | M | Oxford-AstraZeneca | AOSD | Prednisolone | Remission |
| 77 | Muench et al. [91] | Germany | 26 | F | Pfizer BioNTech | AOSD | Methylprednisolone, IVIG, and anakinra | Clinical improvement |
| 78 | Bindoli et al. [92] | Italy | 65 | M | OxfordAstraZeneca | AOSD | Anakira and prednisone | Clinical improvement |
| 57 | F | PfizerBioNTech | AOSD | Anakinra and prednisolone | Clinical improvement | |||
| 53 | F | PfizerBioNTech | AOSD | Dexamethasone and anakinra | Clinical improvement | |||
| 50 | F | PfizerBioNTech | AOSD | Prednisolone, anakinra, and cyclosporine | Clinical improvement | |||
| 79 | Iwata et al. [76] | Japan | 53 | F | Pfizer BioNTech | Cutaneous vasculitis | Betamethasone | Remission |
| 80 | Azzazi et al. [77] | n/a | 57 | F | Sinopharm | Cutaneous vasculitis | Oral prednisolone | Clinical improvement |
| 81 | Fritzen et al. [78] | Brazil | 60 | F | Oxford-AstraZeneca | Cutaneous vasculitis | Oral Prednisone | Remission |
| 82 | Ungari et al. [93] | USA | 64 | M | Oxford-AstraZeneca | Cutaneous vasculitis | Systemic antihistamine and local steroid therapy | Remission |
| 83 | Oskay et al. [79] | Turkey | 77 | M | CoronaVac | Cutaneous vasculitis | Prednisolone | Remission |
| 84 | Mucke et al. [80] | Germany | 76 | M | Pfizer BioNTech | Cutaneous vasculitis | Prednisolone | Remission |
| 85 | Altun et al. [81] | Turkey | 38 | M | Pfizer BioNTech | Cutaneous vasculitis | Prednisolone | Clinical improvement |
| 86 | Uh et al. [82] | Korea | 64 | F | OxfordAstraZeneca | Cutaneous Vasculitis | Antihistamines and topical steroids | Remission |
| 44 | F | OxfordAstraZeneca | Cutaneous Vasculitis | Methylprednisolone, antihistamine | Remission | |||
| 68 | F | OxfordAstraZeneca | Cutaneous Vasculitis | Methylprednisolone, antihistamine, topical steroid | Remission | |||
| 67 | F | OxfordAstraZeneca | Cutaneous Vasculitis | Methylprednisolone, antihistamine, topical steroid | Remission | |||
| 59 | F | OxfordAstraZeneca | Cutaneous Vasculitis | Methylprednisolone, antihistamine, and topical steroid | Clinical improvement | |||
| 87 | Fiorillo et al. [83] | Italy | 71 | F | Oxford-AstraZeneca | Cutaneous vasculitis | Prednisone | Remission |
| 88 | Abdelmaksoud et al. [84] | Italy | 17 | F | PfizerBioNTech | Cutaneous IgA vasculitis | Systemic corticosteroids | Remission |
| 48 | M | PfizerBioNTech | Cutaneous IgA vasculitis | Systemic corticosteroids | Remission | |||
| 89 | Erler et al. [85] | Germany | 42 | F | Pfizer BioNTech | Cutaneous vasculitis | Prednisolone | Remission |
| 90 | Zhou et al. [95] | Hong Kong | 72 | F | Pfizer BioNTech | AOSD with myocarditis and heart failure | Prednisolone, indomethacin, hydrocortisone and methotrexate | Remission |
| 91 | Yoshino et al. [96] | Japan | 56 | M | Pfizer BioNTech | AAV with periaortitis (MPO) and GN | Methylprednisolone, cyclophosphamide, and methotrexate | Remission |
| 92 | Alalem et al. [97] | Saudi Arabia | 24 | M | OxfordAstraZen | Reactive arthritis (monoarthritis) | Ibuprofen, naproxen, and IA triamcinolone | Symptoms improved |
| 93 | Wojturska et al. [98] | Poland | 33 | M | OxfordAstraZen | Reactive arthritis (monoarthritis) | Diclofenac | Remission |
| 39 | M | Moderna | Reactive arthritis (polyarthritis) | Celecoxib | Remission | |||
| 67 | F | OxfordAstraZen | Reactive arthritis (polyarthritis) | Methylpredinosolone | Remission | |||
| 94 | Weng et al. [99] | Canada | 51 | F | OxfordAstraZen | AOSD | Prednisone and Celecoxib | Symptoms improved |
| 95 | Wang et al. [100] | Australia | 47 | F | Sinopharm | SCLE | Hydroxychloroquine | Not reported |
| 96 | VanDerVeer et al. [101] | USA | 66 | F | Pfizer BioNTech | RA | Indomethacin | Symptoms improved |
| 61 | F | Pfizer BioNTech | RA | Prednisolone and methotrexate | Clinical remission | |||
| 36 | F | Morderna | RA | Adalimumab | Clinical remission | |||
| 72 | F | Pfizer BioNTech | RA | Prednisolone, leflunomide, and golimumab | Clinical remission | |||
| 69 | M | Pfizer BioNTech | PMR | Prednisolone | Symptoms improved | |||
| 97 | Tosunoğlu et al. [102] | Taiwan | 21 | F | Pfizer BioNTech | PM | Methylprednisolone and IVIG | Symptoms improved |
| 98 | Sugimoto et al. [103] | Japan | 62 | F | Pfizer BioNTech | DM (anti-MDA-5) | Methylprednisolone, oral tacrolimus, IV cyclophosphamide, and plasmapheresis | Patient died |
| 99 | Srichawla et al. [104] | USA | 59 | F | Moderna | PAN | Methylprednisolone, and methotrexate | Remission |
| 100 | Sogbe et al. [105] | Spain | 72 | F | Pfizer BioNTech | SLE with myocarditis | prednisolone | Remission |
| 101 | Shukla et al. [106] | India | 56 | F | OxfordAstraZen | Sarcoidosis | prednisolone | Symptoms improved |
| 102 | Shokraee et al. [107] | Iran | 41 | M | Sputnik V | Reactive arthritis (monoarthritis) | IA injection of triamcinolone, and oral prednisolone | Symptoms improved |
| 103 | Sakai et al. [108] | Japan | 26 | F | Pfizer BioNTech | SLE | Methylprednisolone, HCQ, MMF, and belimumab | Not reported |
| 62 | M | Morderna | SLE | Methylprednisolone, prednisolone, and IVcyclophosphamide | Symptom improved | |||
| 104 | Saiz et al. [109] | Spain | 48 | F | Moderna | AAV (PR3 positive) | corticosteroids, methotrexate, and rituximab | Symptoms improved |
| 105 | Rimmer et al. [110] | USA | 79 | F | Moderna | SCLE | HCQ, prednisolone, IVIG, and mycophenolate | Remission |
| 106 | Ohkubo et al. [111] | Japan | 61 | M | Pfizer BioNTech | PAN | Prednisolone | Remission |
| 107 | Wang et al. [112] | USA | 60 | F | Pfizer BioNTech | Amyopathic DM (anti-MDA-5) | Prednisolone | Not reported |
| 108 | Mung et al. [113] | UK | 71 | M | OxfordAstraZen | Seronegative RA | Prednisolone and HCQ | Symptoms improved |
| 109 | Lo Sardo et al. [114] | Italy | 78 | M | OxfordAstraZen | GCA | Prednisolone and Tocilizumab | Symptoms improved |
| 110 | Koh et al. [115] | Taiwan | 17 | F | Pfizer BioNTech | Seronegative RA | Oral NSAIDs, arthrocentesis, etanercept, and sulfasalazine | Symptoms improved |
| 111 | Kawamura et al. [116] | Japan | 71 | F | Pfizer BioNTech | AAV(MPO) | Corticosteroids and IV cyclophosphamide | Symptoms improved |
| 112 | Katsouli et al. [117] | Greece | 52 | F | OxfordAstraZen | LVV | Prednisolone and tocilizumab | Remission |
| 113 | Kan et al. [118] | China | 72 | F | Pfizer BioNTech | AOSD with myocarditis and heart failure | Prednisolone | Remission |
| 114 | Iwata et al. [119] | Japan | 53 | F | Pfizer BioNTech | Cutaneous vasculitis | Betamethasone | Remission |
| 115 | Haruna et al. [120] | Japan | 77 | F | Pfizer BioNTech | PMR without shoulder symptoms | Prednisolone | Symptoms improved |
| 116 | Golstein et al. [121] | Belgium | 48 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved |
| 61 | M | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 59 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 53 | F | Moderna | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 57 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 66 | F | Oxford-Astrazeneca | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 31 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 44 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 80 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 68 | M | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 45 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 21 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 67 | M | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 55 | M | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 52 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 44 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 29 | F | Pfizer-BioNTech | Reactive arthritis (oligoarticular) | Prednisolone | symptom improved | |||
| 117 | Gen et al. [122] | Japan | 82 | F | Moderna | AAV (MPO) | Prednisolone | Symptoms improved |
| 118 | Furr et al. [123] | USA | 69 | F | Moderna | PMR | Prednisolone | Symptoms improved |
| 74 | M | Pfizer-BioNTech | PMR | Prednisolone | Symptom improved | |||
| 119 | El Hasbani et al. [124] | USA | 47 | F | Pfizer-BioNTech | AAV (MPO) | Methylprednisolone, prednisone and azathioprine | Symptoms improved |
| 120 | Durucan et al. [125] | Turkey | 24 | M | Pfizer-BioNTech | PM and myocarditis | Myorelaxant, and NSAIDs | Remission |
| 121 | Ansari et al. [126] | Iran | 28 | M | Oxford-Astrazeneca | IMNM | Methylprednisolone, prednisone, and azathioprine. | Remission |
| 122 | Albers et al. [127] | Germany | 41 | M | Pfizer-BioNTech | Sarcoidosis | topical corticosteroids, tacrolimus, and HCQ | Symptoms improved |
| 123 | Alalem et al. [97] | Saudi Arabia | 24 | M | Oxford-Astrazeneca | Reactive arthritis (monoarthritis) | Ibuprofen, Naproxen, and IA triamcinolone | Symptoms improved |
| 124 | Zamoner et al. [128] | Brazil | 58 | F | Oxford-Astrazeneca | AAV (MPO) with crescentic GN | Methylprednisolone, prednisone, IV cyclophosphamide, and azathioprine | Not reported |
| 125 | Yonezawa et al. [129] | Japan | 54 | M | Pfizer-BioNTech | RA | Methylprednisolone, and iguratimod | Symptoms improved |
| 126 | Yadav et al. [130] | Nepal | 52 | F | Johnson and Johnson | AAV (C-ANCA positive) with rapidly progressing GN | Cyclophophaide and methylprednisolone | Not reported |
| 127 | Xia et al. [131] | Australia | 68 | M | Oxford-Astrazeneca | GCA(AION) | Methylprednisolone and tocilizumab | Not reported |
| 128 | Wu et al. [132] | USA | 77 | F | Pfizer-BioNTech | DM (anti-TIF positive) | IV methylprednisolone, IVIG, MMF, and prednisolone | Symptoms improved |
| 129 | Watanabe et al. [133] | Japan | 53 | M | Pfizer-BioNTech | Seropositive RA | Prednisolone, methotrexate, and tocilizumab | Remission |
| 130 | Wang et al. [134] | Taiwan | 81 | M | Oxford-Astrazeneca | SCLE (positive Ro, ANA) | Prednisolone | Symptoms improved |
| 131 | Vanaskova et al. [135] | Czech Republic | 53 | M | Pfizer-BioNTech | Reactive arthritis (monoarthritis) | Dexamethasone | Symptoms improved |
| 132 | Uddin et al. [136] | Pakistan | 59 | M | Pfizer-BioNTech | AAV(PR3) | Methylprednisolone, rituximab, and prednisone | Symptoms improved |
| 133 | Suzuki et al. [137] | Japan | 72 | M | Pfizer-BioNTech | AAV (MPO) | Methylprednisolone, prednisolone, and rituximab | Symptoms improved |
| 134 | Seeley et al. [138] | USA | 35 | F | Pfizer-BioNTech | APL syndrome (catastrophic) | IV dexamethasone, HCQ, and prednisolone | Symptoms improved |
| 135 | Schoenardie et al. [139] | Brazil | 25 | F | Oxford-Astrazeneca | Seronegative RA (monoarthritis) | NSAID and prednisolone | Symptoms improved |
| 136 | Park et al. [140] | USA | 64 | M | Moderna | Seronegative RA (monoarthritis) | Naproxen | Symptoms improved |
| 73 | F | Moderna | Dactylitis | Celecoxib | Symptoms improved | |||
| 137 | Ohmura et al. [141] | Japan | 41 | F | Moderna | MVV (negative ANCA) | Ibuprofen and prednisolone | Symptoms improved |
| 138 | Numakura et al. [142] | Japan | 61 | M | Pfizer-BioNTech | Sarcoidosis | IA steroid injection | Not reported |
| 139 | Mohaghegh et al. [143] | Iran | 65 | F | COVIran Barekat | Sarcoidosis | Methotrexate and prednisolone | Symptoms improved |
| 140 | Magen et al. [144] | Israel | 34 | F | Pfizer-BioNTech | PM | IV methylprednisolone, prednisone, IVIG, and azathioprine | Symptoms persists |
| 141 | Ma et al. [145] | China | 70 | F | CoronaVac | AAV(MPO) GN | IV Glucocorticoids, IV cyclophosphamide, and low-dose steroids maintenance therapy | Symptoms improved |
| 142 | Lourenço et al. [146] | Portugal | 75 | F | Oxford-Astrazeneca | PMR | IM betamethasone and prednisolone | Symptoms improved |
| 143 | Kreuter et al. [147] | Germany | 68 | F | Pfizer-BioNTech | IIM | IV glucocorticosteroids | Symptoms improved |
| 144 | Kim, Y et al. [148] | South Korea | 77 | F | Pfizer-BioNTech | SVV with crescentic GN | Methylprednisolone | Remission |
| 145 | Kim, J et al. [149] | South Korea | 30 | M | Pfizer-BioNTech | DM | Glucocorticoid and azathioprine | Not reported |
| 146 | Kim B, et al. [150] | South Korea | 72 | F | Moderna | AAV (MPO +ve) with crescentic GN | Plasmapheresis, IV cyclophosphamide and IV methylprednisolone | Constitutional symptoms improved |
| 147 | Khanna et al. [151] | USA | 18 | F | Pfizer-BioNTech | SLE, with neutrophilic urticarial dermatosis | Prednisone and HCQ | Remission |
| 148 | Holzer et al. [152] | Germany | 19 | M | Pfizer-BioNTech | DM | Glucocorticoids, IVIG, Tofacitinib, MMF, Rituximab, Ciclosporin A, Anakinra, Nintedanib and Daratumumab | Not reported |
| 57 | F | Pfizer-BioNTech | DM | Glucocorticoids, HCQ, and Azathioprine | Not reported | |||
| 51 | M | Pfizer-BioNTech | DM | Glucocorticoids, SC Methotrexate, HCQ, and Azathioprine | Not reported | |||
| 149 | Greb et al. [153] | USA | 79 | M | Pfizer-BioNTech | GCA | Prednisolone | Remission |
| 150 | Godoy et al. [154] | Brazil | 64 | F | Oxford-Astrazeneca | PM | Corticoid and immunosuppressant therapy | Symptoms improved |
| 151 | Gamonal et al. [155] | Brazil | 27 | F | Oxford-Astrazeneca | SLE, with alopecia areata | Prednisolone and hydroxychloroquine | Not reported |
| 152 | Farooq et al. [156] | UK | 63 | M | Oxford-Astrazeneca | Inflammatory myositis with myocarditis and pneumonitis | Methylprednisolone and prednisolone | Symptoms improved |
| 153 | Essien et al. [157] | USA | 27 | M | Pfizer-BioNTech | AAV | Prednisolone and rituximab | Relapse |
| 154 | Draz et al. [158] | Egypt | 54 | M | Pfizer-BioNTech | Reactive arthritis | IM dexamethasone and NSAID | Symptoms improved |
| 155 | Chua et al. [159] | Taiwan | 30 | F | Moderna | AOSD | methylprednisolone, prednisolone, and naproxen | Remission |
| 156 | Christodoulou et al. [160] | Greece | 72 | F | Moderna | AAV (MPO) with pulmonary-renal syndrome | Prednisolone, cyclophosphamide and plasmapheresis | Remission |
| 157 | Chomičienė et al. [161] | Lithuania | 49 | F | Pfizer-BioNTech | HSP (IgA vasculitis) | Methylprednisolone and plasmapheresis | Remission |
| 65 | F | Pfizer-BioNTech | Urticarial vasculitis | IVdexamethasone, plasmapheresis and oral methylprednisolone | Remission | |||
| 158 | Chaima et al. [162] | Tunisia | 52 | F | Pfizer-BioNTech | DM | Prednisolone | Remission |
| 159 | Bose et al. [163] | India | 53 | M | Oxford-Astrazeneca | Focal myositis | NSAIDa | Symptoms improved |
| 160 | Barman et al. [164] | India | 38 | F | Oxford-Astrazeneca | RA | prednisolone, methotrexate, HCQ, and sulfasalazine | Symptom improved |
| 161 | Ball-Burack et al. [165] | USA | 22 | M | Johnson and Johnson | LCC vasculitis | NSAIDs | Symptoms improved |
| 162 | Aoki et al. [166] | Japan | 81 | M | Pfizer-BioNTech | LVV | Naproxen | Symptoms improved |
| 163 | Ahmer et al. [167] | Australia | 50 | F | Oxford-Astrazeneca | Cryoglobulinemic vasculitis | Nil | Remission |
| 164 | Villa et al. [168] | Spain | 63 | M | Moderna | AAV (MPO) with crescentic GN | IV glucocorticoids, prednisone, and cyclophosphamide | Symptoms improved |
| 165 | Türk et al. [169] | Turkey | 72 | F | Sinovac | Reactive arthritis | Prednisolone | Symptoms improved |
| 79 | F | Sinovac | Reactive arthritis | Methylprednisolone | Symptoms persisted | |||
| 166 | Sirufo et al. [170] | Italy | 76 | F | Oxford-Astrazeneca | HSP | Deflazacort | Symptoms improved |
| 167 | Risal et al. [171] | Nepal | 47 | F | Oxford-Astrazeneca | AOSD | Prednisolone and methotrexate | Symptoms improved |
| 168 | Quattrini et al. [172] | Italy | 83 | F | Pfizer-BioNTech | RS3PE | Prednisolone and methotrexate | Rapid clinical improvement |
| 169 | Naitlho et al. [173] | Morroco | 62 | M | Oxford-Astrazeneca | HSP | Prednisolone | Symptoms improved |
| 170 | Liu et al. [174] | USA | 70 | M | Pfizer-BioNTech | SACL | Topical steroids | Symptoms improved |
| 171 | Kar et al. [175] | India | 46 | M | COVAXIN | Cutaneous small vessel vasculitis | Nil | Symptoms improved |
| 172 | Hines et al. [176] | USA | 40 | F | Pfizer-BioNTech | HSP | Nil | Symptoms improved |
| 173 | Guzmán-Pérez et al. [177] | Spain | 57 | F | Oxford-Astrazeneca | Cutaneous small-vessel vasculitis | Nil | Not reported |
| 174 | Chen et al. [178] | Taiwan | 70 | F | Moderna | AAV (MPO) with pulmonary-renal syndrome | Plasma exchange, corticosteroid, and anti-CD20 therapy | Symptoms persisted |
| 175 | Chan-Chung et al. [179] | Singapore | 62 | F | Pfizer-BioNTech | EGPA (MPO-positive) | IV methylprednisolone and rituximab | Symptoms improved |
| 176 | Chan et al. [180] | Australia | 79 | F | Oxford-Astrazeneca | Polyarthralgia | Nil | Symptoms improved |
| 177 | Berry et al. [181] | USA | 65 | M | Janssen | Cutaneous small-vessel vasculitis | Prednisolone | Remission |
| 178 | Dube et al. [182] | USA | 29 | F | Pfizer-BioNTech | AAV (PR3 positive) with GN | Methylprednisolone, rituximab, and cyclophosphamide | Not reported |
| 179 | Takenaka et al. [183] | Japan | 75 | F | Pfizer-BioNTech | AAV (MPO positive) optic perineuritis | Methylprednisolone | Not reported |
| 180 | Tasnim et al. [184] | USA | 71 | M | Pfizer-BioNTech | IgG4 disease | Nil | Not reported |
| 181 | Aochi et al. [185] | Japan | 78 | F | Pfizer-BioNTech | IgG4 disease | Prednisolone | Not reported |
| 65 | M | Pfizer-BioNTech | IgG4 disease | Prednisolone | Not reported | |||
| 63 | M | Pfizer-BioNTech | IgG4 disease | Prednisolone | Not reported | |||
| 182 | Matsuda et al. [186] | Japan | 59 | F | Pfizer-BioNTech | AOSD | Corticosteroid and tocilizumab | Not reported |
| 77 | F | Pfizer-BioNTech | AOSD | IV methylprednisolone, prednisolone, and tocilizumab | Not reported | |||
| 35 | M | Moderna | AOSD | prednisolone | Not reported | |||
| 183 | Avalos et al. [187] | USA | 74 | F | Pfizer-BioNTech | Microscopic polyangiitis | Methylprednisolone and rituximab | Not reported |
| 184 | Sagy et al. [188] | Israel | 24 | M | Pfizer-BioNTech | SLE | HCQ, topical steroid, etoricoxib | Not reported |
| 24 | M | Pfizer-BioNTech | SLE | HCQ, prednisolone, azathioprine | Not reported | |||
| 56 | M | Pfizer-BioNTech | SLE | HCQ and etoricoxib | Not reported | |||
| 185 | Roy et al. [189] | India | 60 | F | Covishield | HSP | Nil | Not reported |
| 186 | Chan et al. [190] | Canada | 53 | F | Oxford-Astrazeneca | DM (PL12- positive) with ILD and Myocarditis | Prednisolone, MMF, methotrexate, and HCQ | Symptoms improved |
| 76 | F | Pfizer-BioNTech | DM (SAE-1 positive) with ILD and Myocarditis | Prednisolone, MMF, and IVIG | Symptoms improved | |||
| 187 | Nahra et al. [191] | USA | 71 | M | Pfizer-BioNTech | Seronegative RA | NSAID and prednisolone | Symptoms improved |
| 74 | M | Pfizer-BioNTech | Seropositive RA | Prednisolone and leflunomide | Symptoms improved | |||
| 188 | Bansal et al. [192] | USA | 40 | F | Pfizer-BioNTech | Seropositive RA | Methotrexate and HCQ | Not reported |
| 189 | Parperis et al. [193] | Greece | 80 | M | Pfizer-BioNTech | RS3PE | Prednisolone | Symptoms improved |
| 190 | Baimukhamedov et al. [194] | Kazakhstan | 38 | F | SPUTNIK-V | Seropositive RA | NSAIDs, methylprednisolone, and methotrexate | Not reported |
| Variable | Number (N = 271) |
|---|---|
| Age | 56 mean (±18.9 SD) |
| Gender | |
| Male | 101 (37.1%) |
| Female | 170 (62.5%) |
| Country | |
| United States | 47 (17.3%) |
| Japan | 36 (13.3%) |
| Italy | 23 (8.5%) |
| Belgium | 18 (6.6%) |
| Germany | 15 (5.5%) |
| South Korea | 15 (5.5%) |
| India | 14 (5.1%) |
| France | 13 (4.8%) |
| United Kingdom | 8 (2.9%) |
| Turkey | 8 (2.9%) |
| Taiwan | 7 (2.6%) |
| Spain | 6 (2.2%) |
| Israel | 5 (1.8%) |
| Brazil | 5 (1.8%) |
| Australia | 5 (1.8%) |
| Greece | 4 (1.5%) |
| Mexico | 3 (1.1%) |
| Canada | 3 (1.1%) |
| Switzerland | 3 (1.1%) |
| Poland | 3 (1.1%) |
| Saudi Arabia | 3 (1.1%) |
| Iran | 3 (1.1%) |
| China | 3 (1.1%) |
| Moraco | 1(0.4%) |
| Kazakhstan | 2 (0.7%) |
| Egypt | 2 (0.7%) |
| Nepal | 2 (0.7%) |
| Lithuania | 2 (0.7%) |
| Portugal | 1 (0.4%) |
| Singapore | 1 (0.4%) |
| Hongkong | 1 (0.4%) |
| Qatar | 1 (0.4%) |
| Puerto Rico | 1 (0.4%) |
| Pakistan | 1 (0.4%) |
| Columbia | 1 (0.4%) |
| Tunisia | 1 (0.4%) |
| Czech Republic | 1 (0.4%) |
| Unknown | 3 (1.1%) |
| Variable | Number (N = 271) |
|---|---|
| Vaccination types | |
| Pfizer BioNTech | 153 (56.5%) |
| Oxford-AstraZeneca | 61 (22.5%) |
| Moderna | 33 (12.2%) |
| Corona Vac/Sinovac | 07 (2.6%) |
| Covishield | 03 (1.1%) |
| Sputnik-V | 03 (1.1%) |
| Johnson and Johnson | 02 (0.7%) |
| Sinopharm | 02 (0.7%) |
| Covaxin | 01 (0.4%) |
| Janssen | 01 (0.4%) |
| COVIran Barekat | 01 (0.4%) |
| Information not available | 04 (1.5%) |
| Number of doses | |
| One | 119 (43.9%) |
| Two | 123 (45.4%) |
| Three | 11 (4.1%) |
| Not reported | 18 (6.6%) |
| R-IMID | Number (N = 271) | % |
|---|---|---|
| Vasculitides | 86 | 31.7% |
| Small-vessel vasculitis | 64 | 23.6% |
| (1) ANCA-associated vasculitis | 30 | 11.1% |
| (a) Eosinophilic granulomatosis with polyangiitis (EGPA) | 3 | 1.1% |
| (2) ANCA-negative vasculitis | 3 | 1.1% |
| (3) Cutaneous vasculitis | 22 | 8.1% |
| (4) Henoch-Schonlein purpura (HSP) | 5 | 1.8% |
| (5) Cryoglubulinemia | 1 | 0.4% |
| Medium-vessel vasculitis | 6 | 2.2% |
| Large-vessel vasculitis | 8 | 3.0% |
| 1. IGG4 disease | 4 | 1.5% |
| Giant-cell arteritis | 8 | 3.0% |
| Connective tissue diseases | 66 | 24.4% |
| 1. Idiopathic inflammatory myositis (IIM) | 37 | 13.7% |
| (a) Polymyositis (PM) | 9 | 3.3% |
| (b) Dermatomyositis (DM) | 24 | 8.9% |
| (c) Focal myositis | 3 | 1.1% |
| (d) Immune-mediated necrotising myopathy (IMNM) | 1 | 0.4% |
| 2. Antisynthetase syndrome | 1 | 0.4% |
| 3. Systemic lupus erythematosus (SLE) | 24 | 8.9% |
| (a) Subacute cutaneous lupus (SCLE) | 6 | 2.2% |
| 4. Antiphospholipid syndrome | 1 | 0.4% |
| 5. Undifferentiated CTD | 1 | 0.4% |
| 6. Systemic sclerosis | 2 | 0.7% |
| Inflammatory arthritis | 55 | 20.3% |
| 1. Reactive arthritis | 30 | 11.1% |
| 2. Rheumatoid arthritis | 21 | 7.7% |
| (a) Seropositive | 9 | 3.3% |
| (b) Seronegative | 12 | 4.4% |
| (i) Monoarthritis | 2 | 0.7% |
| 3. Crystal arthritis (pseudogout) | 1 | 0.4% |
| 4. Remitting seronegative symmetrical synovitis with pitting oedema (RS3PE) | 2 | 0.7% |
| 5. Dactylitis | 1 | 0.4% |
| Polymyalgia rheumatica | 21 | 7.7% |
| Adult-onset stills disease | 22 | 8.1% |
| Bechet’s disease | 3 | 1.1% |
| Sarcoidosis | 8 | 3.0% |
| Miscellaneous | 10 | 3.7% |
| (1) Polyarthralgia and myalgia | 6 | 2.2% |
| (2) Tenosynovitis | 2 | 0.7% |
| (3) Localised scleroderma | 1 | 0.4% |
| (4) Inflammatory myositis with myocarditis and pneumonitis | 1 | 0.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nune, A.; Durkowski, V.; Pillay, S.S.; Barman, B.; Elwell, H.; Bora, K.; Bilgrami, S.; Mahmood, S.; Babajan, N.; Venkatachalam, S.; et al. New-Onset Rheumatic Immune-Mediated Inflammatory Diseases Following SARS-CoV-2 Vaccinations until May 2023: A Systematic Review. Vaccines 2023, 11, 1571. https://doi.org/10.3390/vaccines11101571
Nune A, Durkowski V, Pillay SS, Barman B, Elwell H, Bora K, Bilgrami S, Mahmood S, Babajan N, Venkatachalam S, et al. New-Onset Rheumatic Immune-Mediated Inflammatory Diseases Following SARS-CoV-2 Vaccinations until May 2023: A Systematic Review. Vaccines. 2023; 11(10):1571. https://doi.org/10.3390/vaccines11101571
Chicago/Turabian StyleNune, Arvind, Victor Durkowski, S. Sujitha Pillay, Bhupen Barman, Helen Elwell, Kaustubh Bora, Syed Bilgrami, Sajid Mahmood, Nasarulla Babajan, Srinivasan Venkatachalam, and et al. 2023. "New-Onset Rheumatic Immune-Mediated Inflammatory Diseases Following SARS-CoV-2 Vaccinations until May 2023: A Systematic Review" Vaccines 11, no. 10: 1571. https://doi.org/10.3390/vaccines11101571
APA StyleNune, A., Durkowski, V., Pillay, S. S., Barman, B., Elwell, H., Bora, K., Bilgrami, S., Mahmood, S., Babajan, N., Venkatachalam, S., Ottewell, L., & Manzo, C. (2023). New-Onset Rheumatic Immune-Mediated Inflammatory Diseases Following SARS-CoV-2 Vaccinations until May 2023: A Systematic Review. Vaccines, 11(10), 1571. https://doi.org/10.3390/vaccines11101571








