Vaccination against SARS-CoV-2 Does Not Protect against the Development of Anosmia in a Hamster Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Vaccines
2.2. Animal Experiments
2.3. Anosmia Testing
2.4. Viral Titrations
2.5. Statistical Analysis
3. Results
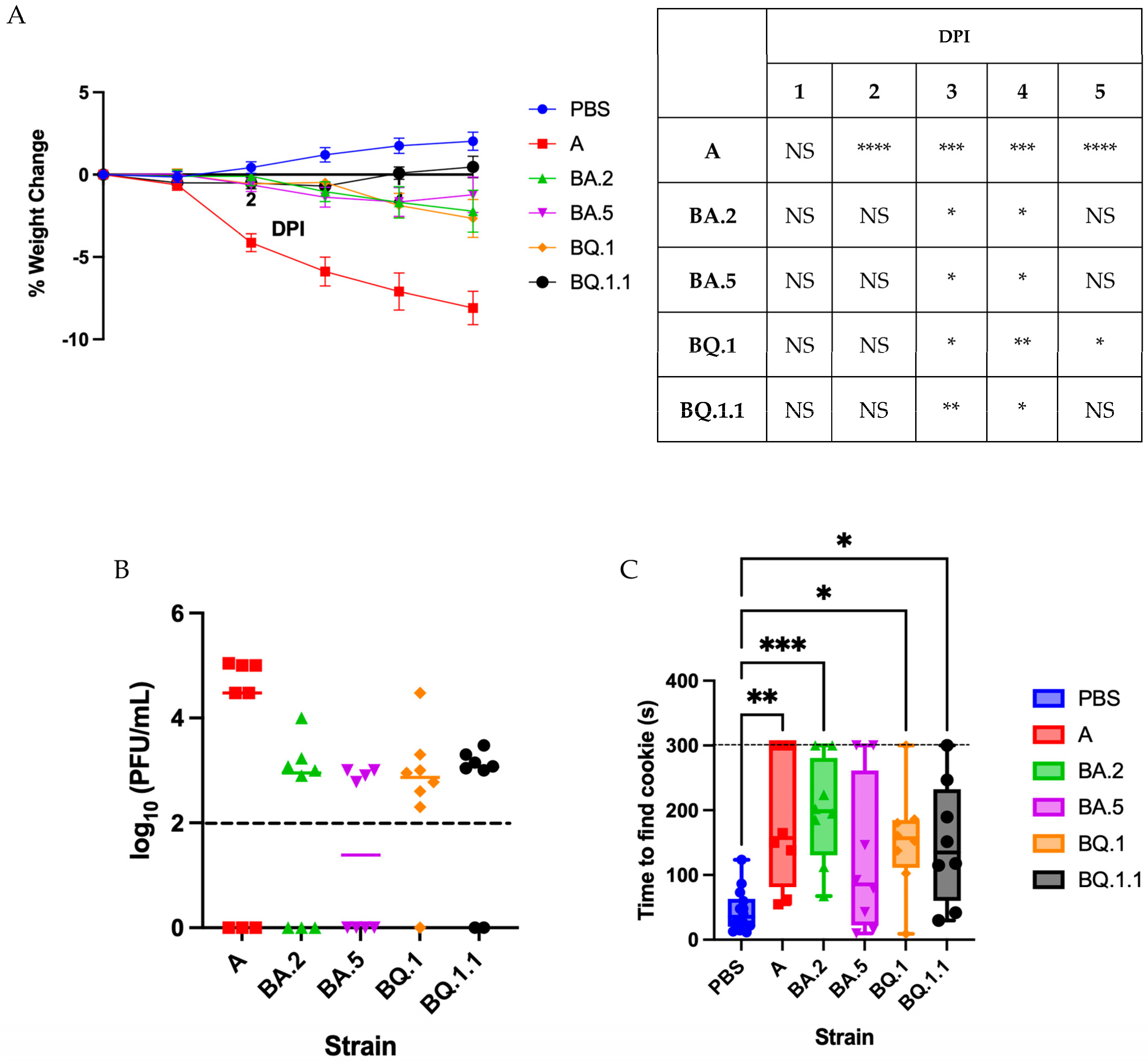
3.1. Omicron Variants Induce a Less Severe Disease in Golden Syrian Hamsters
3.2. Characterizing Anosmia with Recent Variants
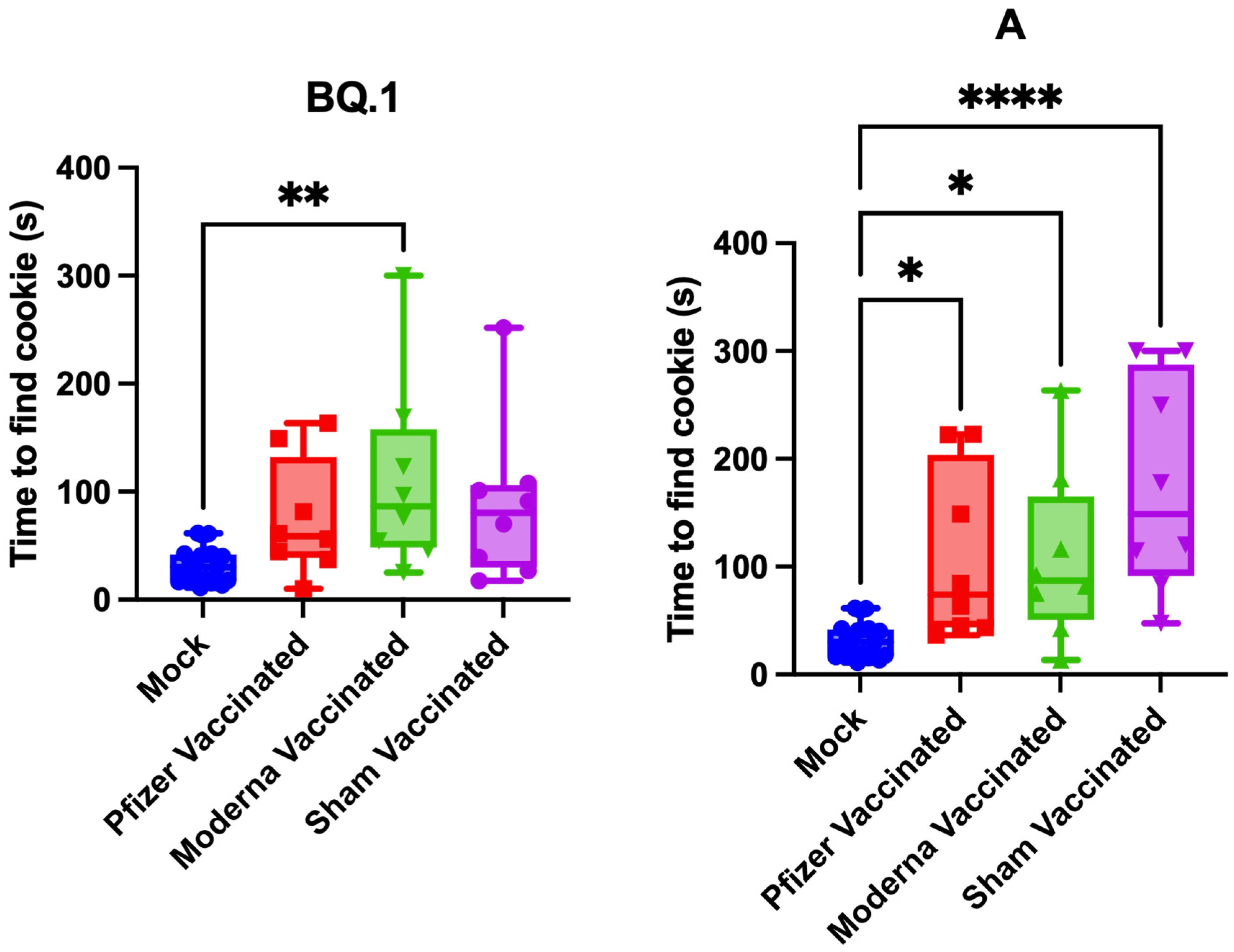
3.3. Impact of Vaccination on Pathogenesis and Anosmia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). COVID-19 Dashboard. Available online: https://coronavirus.jhu.edu/map.html (accessed on 14 March 2023).
- Butowt, R.; Bilinska, K.; Von Bartheld, C.S. Chemosensory Dysfunction in COVID-19: Integration of Genetic and Epidemiological Data Points to D614G Spike Protein Variant as a Contributing Factor. ACS Chem. Neurosci. 2020, 11, 3180–3184. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Karimi, E.; Firouzifar, M.; Khamushian, P.; Ansari, R.; Mohammadi Ardehali, M.; Heidari, F. Anosmia as a prominent symptom of COVID-19 infection. Rhinology 2020, 58, 302–303. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Meng, X.; Deng, Y.; Dai, Z.; Meng, Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am. J. Otolaryngol. 2020, 41, 102581. [Google Scholar] [CrossRef] [PubMed]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Research 2021, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-Analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020, 11, 2944–2961. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Marquardt, B.; Kardashi, R.; de With, K.; Rossler, S.; Landis, B.N.; Welge-Luessen, A.; Hummel, T. SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses. Life 2022, 12, 461. [Google Scholar] [CrossRef]
- Mariani, F.; Morello, R.; Traini, D.O.; La Rocca, A.; De Rose, C.; Valentini, P.; Buonsenso, D. Risk Factors for Persistent Anosmia and Dysgeusia in Children with SARS-CoV-2 Infection: A Retrospective Study. Children 2023, 10, 597. [Google Scholar] [CrossRef]
- Mendes Paranhos, A.C.; Nazareth Dias, A.R.; Machado da Silva, L.C.; Vieira Hennemann Koury, G.; de Jesus Sousa, E.; Cerasi, A.J., Jr.; Souza, G.S.; Simoes Quaresma, J.A.; Magno Falcao, L.F. Sociodemographic Characteristics and Comorbidities of Patients with Long COVID and Persistent Olfactory Dysfunction. JAMA Netw. Open 2022, 5, e2230637. [Google Scholar] [CrossRef]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Charani, E.; Ariyanayagam, D.; Abdulaal, A.; Denny, S.J.; Mughal, N.; Moore, L.S.P. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020, 26, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Yom-Tov, E.; Lekkas, D.; Jacobson, N.C. Association of COVID-19-induced anosmia and ageusia with depression and suicidal ideation. J. Affect. Disord. Rep. 2021, 5, 100156. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Zhang, A.J.; Yuan, S.; Poon, V.K.; Chan, C.C.; Lee, A.C.; Chan, W.M.; Fan, Z.; Tsoi, H.W.; Wen, L.; et al. Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin. Infect. Dis. 2020, 71, 2428–2446. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Reyna, R.A.; Kishimoto-Urata, M.; Urata, S.; Makishima, T.; Paessler, S.; Maruyama, J. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci. Rep. 2022, 12, 628. [Google Scholar] [CrossRef]
- Bryche, B.; St Albin, A.; Murri, S.; Lacote, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef]
- Urata, S.; Maruyama, J.; Kishimoto-Urata, M.; Sattler, R.A.; Cook, R.; Lin, N.; Yamasoba, T.; Makishima, T.; Paessler, S. Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters. ACS Chem. Neurosci. 2021, 12, 589–595. [Google Scholar] [CrossRef]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2020, 27, 582–603. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Brann, D.H.; Datta, S.R. Mechanisms of SARS-CoV-2-associated anosmia. Physiol. Rev. 2023, 103, 2759–2766. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2022, 602, 294–299. [Google Scholar] [CrossRef]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2021, 592, 116–121. [Google Scholar] [CrossRef]
- Potts, K.G.; Noyce, R.S.; Gafuik, C.; John, C.M.; Todesco, H.M.; de Heuvel, E.; Favis, N.; Kelly, M.M.; Evans, D.H.; Mahoney, D.J. Booster vaccines protect hamsters with waning immunity from Delta VOC infection, disease, and transmission. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ying, B.; Scheaffer, S.M.; Whitener, B.; Liang, C.Y.; Dmytrenko, O.; Mackin, S.; Wu, K.; Lee, D.; Avena, L.E.; Chong, Z.; et al. Boosting with variant-matched or historical mRNA vaccines protects against Omicron infection in mice. Cell 2022, 185, 1572–1587.e11. [Google Scholar] [CrossRef]
- McLean, G.; Kamil, J.; Lee, B.; Moore, P.; Schulz, T.F.; Muik, A.; Sahin, U.; Tureci, O.; Pather, S. The Impact of Evolving SARS-CoV-2 Mutations and Variants on COVID-19 Vaccines. mBio 2022, 13, e0297921. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target. Ther. 2022, 7, 141. [Google Scholar] [CrossRef]
- Machado, R.R.G.; Walker, J.L.; Scharton, D.; Rafael, G.H.; Mitchell, B.M.; Reyna, R.A.; de Souza, W.M.; Liu, J.; Walker, D.H.; Plante, J.A.; et al. Immunogenicity and efficacy of vaccine boosters against SARS-CoV-2 Omicron subvariant BA.5 in male Syrian hamsters. Nat. Commun. 2023, 14, 4260. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mule, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.K.; Kashour, T.; Hamid, Q.; Halwani, R.; Tleyjeh, I.M. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front. Immunol. 2021, 12, 686029. [Google Scholar] [CrossRef]
- Kishimoto-Urata, M.; Urata, S.; Kagoya, R.; Imamura, F.; Nagayama, S.; Reyna, R.A.; Maruyama, J.; Yamasoba, T.; Kondo, K.; Hasegawa-Ishii, S.; et al. Prolonged and extended impacts of SARS-CoV-2 on the olfactory neurocircuit. Sci. Rep. 2022, 12, 5728. [Google Scholar] [CrossRef] [PubMed]
- Frere, J.J.; Serafini, R.A.; Pryce, K.D.; Zazhytska, M.; Oishi, K.; Golynker, I.; Panis, M.; Zimering, J.; Horiuchi, S.; Hoagland, D.A.; et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci. Transl. Med. 2022, 14, eabq3059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Alwani, M.; Yassin, A.; Al-Zoubi, R.M.; Aboumarzouk, O.M.; Nettleship, J.; Kelly, D.; Al-Qudimat, A.R.; Shabsigh, R. Sex-based differences in severity and mortality in COVID-19. Rev. Med. Virol. 2021, 31, e2223. [Google Scholar] [CrossRef]

| Percentage Weight Loss and Anosmia | Nasal Wash Titer and Anosmia | |
|---|---|---|
| Pearson’s r | −0.389 | 0.235 |
| p value | 0.004 | 0.144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyna, R.A.; Walker, J.; Mitchell, B.; Shinde, D.P.; Plante, J.A.; Weaver, S.C.; Plante, K.S. Vaccination against SARS-CoV-2 Does Not Protect against the Development of Anosmia in a Hamster Model. Vaccines 2023, 11, 1564. https://doi.org/10.3390/vaccines11101564
Reyna RA, Walker J, Mitchell B, Shinde DP, Plante JA, Weaver SC, Plante KS. Vaccination against SARS-CoV-2 Does Not Protect against the Development of Anosmia in a Hamster Model. Vaccines. 2023; 11(10):1564. https://doi.org/10.3390/vaccines11101564
Chicago/Turabian StyleReyna, Rachel A., Jordyn Walker, Brooke Mitchell, Divya P. Shinde, Jessica A. Plante, Scott C. Weaver, and Kenneth S. Plante. 2023. "Vaccination against SARS-CoV-2 Does Not Protect against the Development of Anosmia in a Hamster Model" Vaccines 11, no. 10: 1564. https://doi.org/10.3390/vaccines11101564
APA StyleReyna, R. A., Walker, J., Mitchell, B., Shinde, D. P., Plante, J. A., Weaver, S. C., & Plante, K. S. (2023). Vaccination against SARS-CoV-2 Does Not Protect against the Development of Anosmia in a Hamster Model. Vaccines, 11(10), 1564. https://doi.org/10.3390/vaccines11101564





