The Role of Vaccination Centers in a National Mass Immunization Campaign—Policymaker Insights from the German COVID-19 Pandemic Vaccine Roll-Out
Abstract
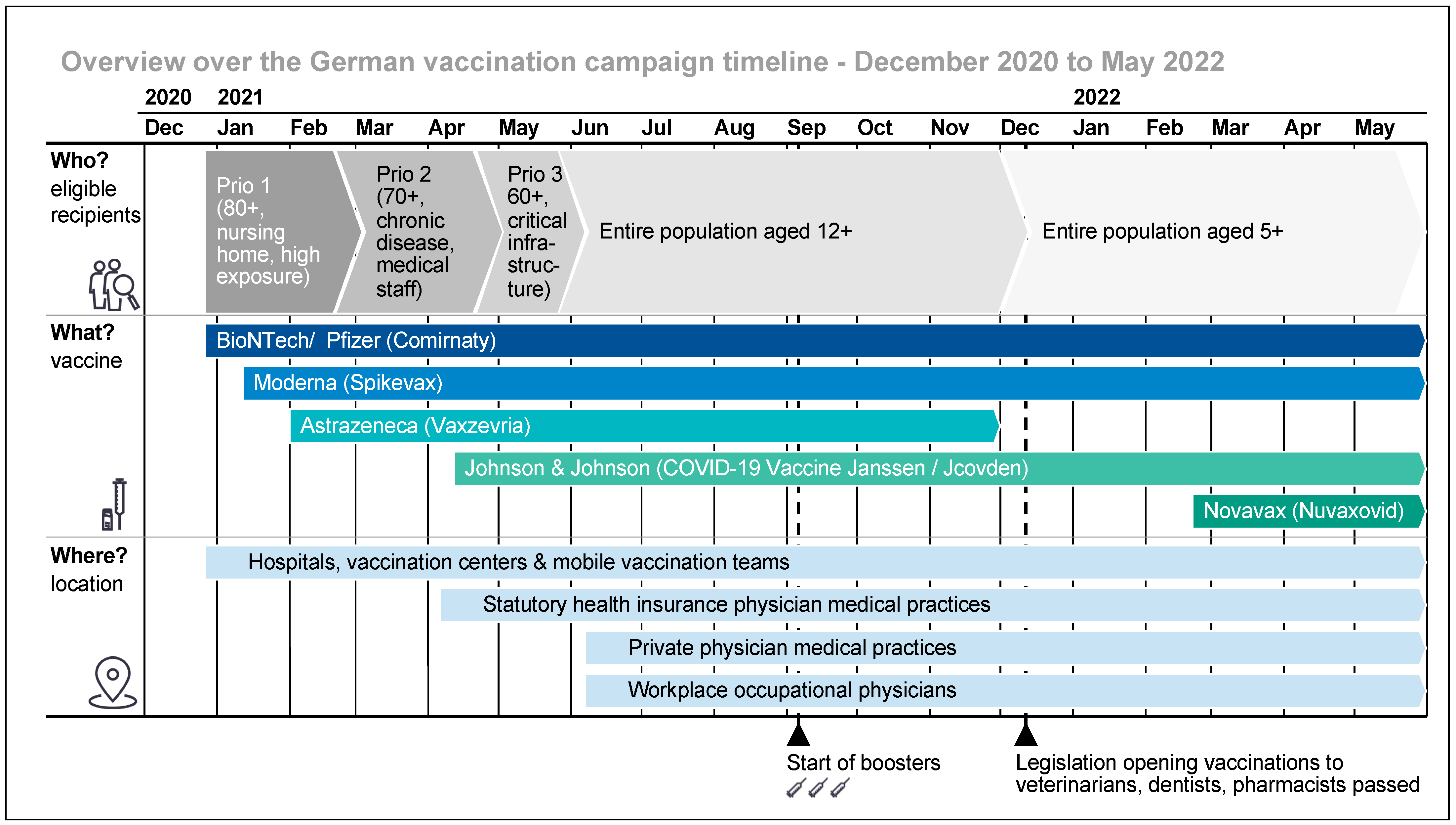
:1. Introduction
2. Materials and Methods
2.1. Questionnaire Development
2.2. Expert Selection and Data Collection
2.3. Data Analysis
3. Results
| Description | Example Expert | Number of Interviews | |||
|---|---|---|---|---|---|
| Federal Level | State Level | Total | Planned | ||
| Coordinator (management, planning, implementation) | 5 | 17 | 22 | 24 | |
| Coordinators defined the general requirements (legislation) for the implementation of the vaccination campaign and/or planned and coordinated the operational implementation of the vaccination campaign in their respective realm |
| ||||
| Key stakeholder representative | 5 | / | 5 | 5 | |
| Representatives of the stakeholders involved in the implementation of the vaccination campaign contributed to the general vaccination campaign roll-out and planning, operation of vaccination centers and coordinated the operation of other vaccination structures. |
| ||||
- Target criteria for a successful national pandemic vaccination campaign;
- Role and optimal deployment of state vaccination centers;
- Additional take-aways for best practice of vaccination center roll-out;
- Post-pandemic: Operational readiness and transfer of the vaccination-center concept.
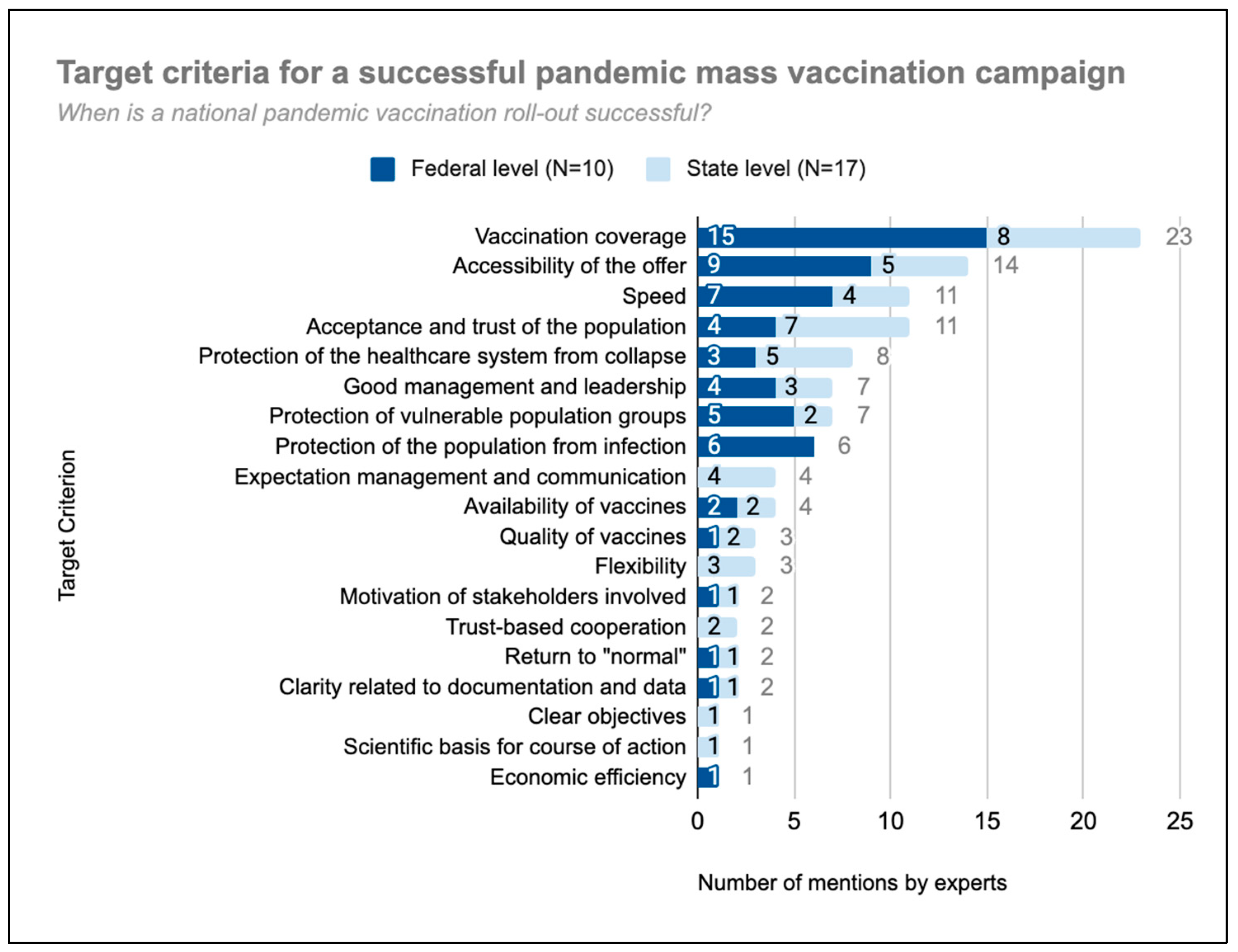
3.1. Target Criteria for a Successful National Pandemic Vaccination Campaign
3.2. Role and Optimal Deployment of State Vaccination Centers
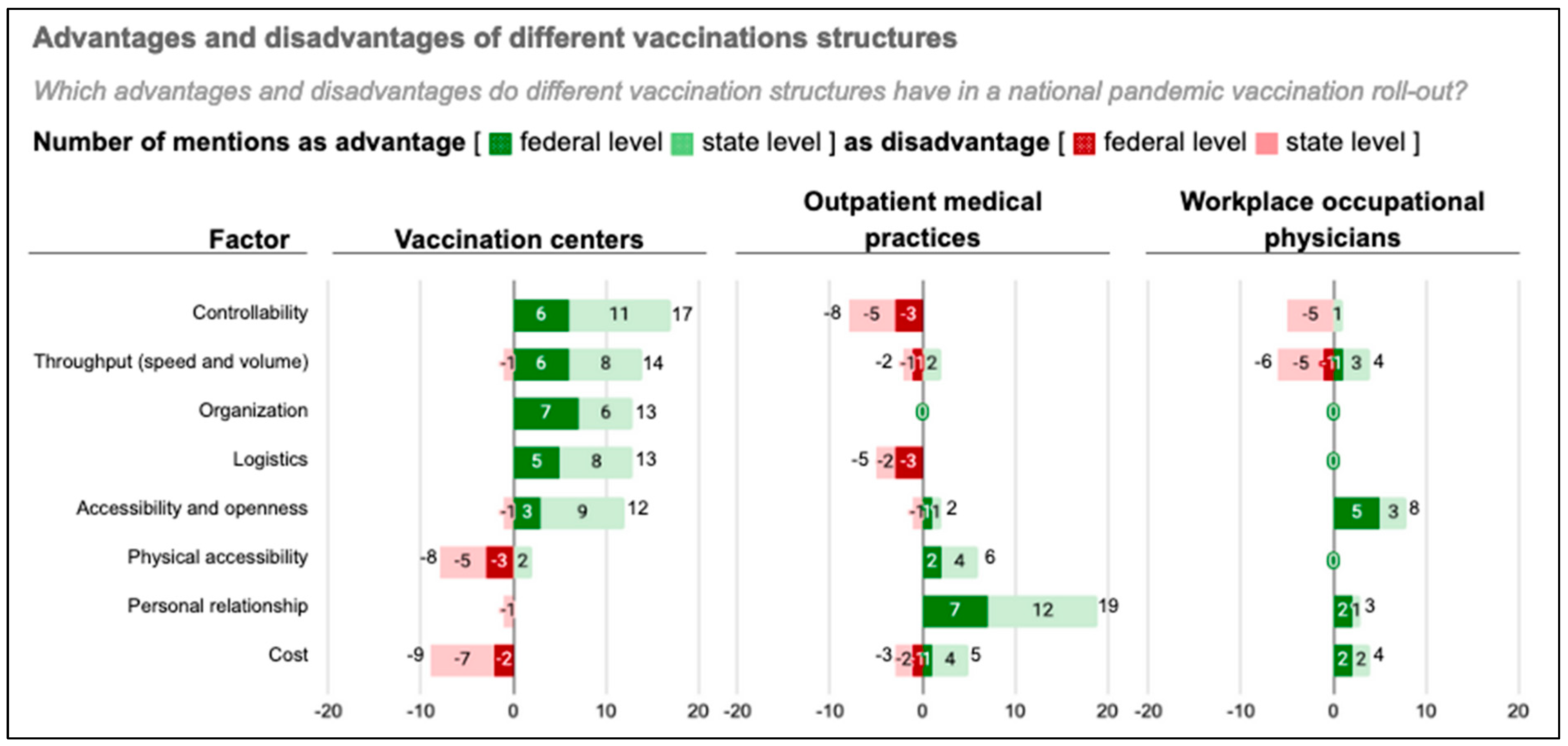
3.2.1. Advantages and Disadvantages of Public Mass Vaccination Centers
Controllability
Throughput (Speed and Volume)
Logistics
Accessibility and Openness
Physical Accessibility
Personal Relationship
Cost
Symbolism
3.2.2. Deployment of Public Vaccination Centers for National Pandemic Vaccination Roll-Out
Deployment Criteria
Transition Timing during the COVID-19 Vaccination Roll-Out
The Changing Role of Government Immunization Services over the Course of a Pandemic
3.3. Additional Take-Aways for Best Practice Vaccination-Center Roll-Out
- The flexibilization of government vaccination structures;
- The better use of available vaccination capacities;
- The broad involvement of stakeholders and expertise.
3.3.1. Flexible Design of Government Vaccination Structures Adapted to Circumstances
3.3.2. Scalability and Optimized Use of Available Vaccination Capacities
3.3.3. Broad Stakeholder Involvement and Use of Domain Specific Expertise
3.4. Post-Pandemic: Operational Readiness and Transfer of the Vaccination Center Concept
4. Discussion
4.1. Principal Results
4.2. Strengths and Limitations
4.3. Future Research Needs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bundeszentrale für gesundheitliche Aufklärung (BZgA). Das Impfsystem in Deutschland. Available online: https://www.impfen-info.de/wissenswertes/impfsystem-in-deutschland/ (accessed on 23 August 2023).
- Vygen-Bonnet, S.; Schlaberg, J.; Rolle, K.J. Arbeitsweise und Empfehlungen der Ständigen Impfkommission (STIKO) im Kontext der COVID-19-Pandemie. Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- German Federal Ministry of Health; Robert Koch-Institut; Bundeszentrale für Gesundheitliche Aufklärung; Paul-Ehrlich-Institut. National COVID-19 Vaccination Strategy—Version 2.0; German Federal Ministry of Health: Berlin, Germany, 2021; pp. 1–24. [Google Scholar]
- Gianfredi, V.; Pennisi, F.; Lume, A.; Ricciardi, G.E.; Minerva, M.; Riccò, M.; Odone, A.; Signorelli, C. Challenges and Opportunities of Mass Vaccination Centers in COVID-19 Times: A Rapid Review of Literature. Vaccines 2021, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- German Infection Protection Act: § 20b IfSG valid until 12 December 2021. Available online: http://www.buzer.de/gesetz/2148/al162447-0.htm (accessed on 23 August 2023).
- Goralnick, E.; Kaufmann, C.; Gawande, A.A. Mass-Vaccination Sites—An Essential Innovation to Curb the COVID-19 Pandemic. N. Engl. J. Med. 2021, 384, e67. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Beardsley, J.; Marais, B.J.; Nguyen, T.A.; Fox, G.J. The Implementation of Mass-Vaccination against SARS-CoV-2: A Systematic Review of Existing Strategies and Guidelines. Vaccines 2021, 9, 326. [Google Scholar] [CrossRef]
- Cadeddu, C.; Rosano, A.; Villani, L.; Coiante, G.B.; Minicucci, I.; Pascucci, D.; de Waure, C. Planning and Organization of the COVID-19 Vaccination Campaign: An Overview of Eight European Countries. Vaccines 2022, 10, 1631. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Rollout of COVID-19 Vaccines in the EU/EEA: Challenges and Good Practice. Stockholm: European Centre for Disease Prevention and Control 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/rollout-covid-19-vaccines-eueea-challenges-and-good-practice (accessed on 23 August 2023).
- European Centre for Disease Prevention and Control. Overview of the Implementation of COVID-19 Vaccination Strategies and Deployment Plans in the EU/EEA. Stockholm: European Centre for Disease Prevention and Control 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-and-deployment-plans (accessed on 23 August 2023).
- Brambilla, A.; Mangili, S.; Macchi, M.; Trucco, P.; Perego, A.; Capolongo, S. Covid-19 massive vaccination center layouts. A modular and scalable model for Lombardy region, Italy. Acta Biomed. 2021, 92, e2021446. [Google Scholar] [CrossRef]
- Neumeier, S. Accessibility of COVID-19 Vaccination Centers in Germany via Different Means of Transport. KN J. Cartogr. Geogr. Inf. 2022, 72, 41–58. [Google Scholar] [CrossRef]
- Leithäuser, N.; Schneider, J.; Johann, S.; Krumke, S.O.; Schmidt, E.; Streicher, M.; Scholz, S. Quantifying Covid19-vaccine location strategies for Germany. BMC Health Serv. Res. 2021, 21, 780. [Google Scholar] [CrossRef]
- Wise, S.; Lanternier, F.; Cotteret, C.; Chasport, C.; Juin-Leonard, V.; Cantat, A.; Scemla, A.; Delage, C.; Mantz, B.; Telion, C.; et al. Setting up of a hospital COVID-19 vaccination center: A descriptive study. Health Sci. Rep. 2023, 6, e968. [Google Scholar] [CrossRef]
- Liu, L.; Luo, M.; Chan, Y.S.; Chan, H.H.W. The Planning and Implementation of COVID-19 Mass Vaccination Center in a Megacity in China. Asia Pac. J. Public Health 2022, 34, 836–839. [Google Scholar] [CrossRef]
- Goldberg, S.A.; Callaway, D.; Resnick-Ault, D.; Mandavia, S.; Martinez, R.; Bass, M.; Goralnick, E. Critical Concepts for COVID-19 Mass Vaccination Site Operations. Disaster Med. Public Health Prep. 2021, 17, e60. [Google Scholar] [CrossRef] [PubMed]
- Capolongo, S.; Brambilla, A.; Girardi, A.; Signorelli, C. Validation Checklist for Massive Vaccination Centers. Ann. Ig. Med. Prev. E Comunita 2021, 33, 513–517. [Google Scholar] [CrossRef]
- Heinrich, D.; Scheidel, H.-P. Deutschlands größtes Impfzentrum. Dtsch. Ärzteb.l 2021, 118, A-1858–A-1862. [Google Scholar]
- Fischl, B.; Patterson, A.T.; Baxter, J.; Watson, J.; Hemsworth, J.; Valentine, D.; Wessler, J.; Wong, D. Planning Considerations and Lessons Learned From a COVID-19 Mass Community Vaccination Center. Mil. Med. 2022, 187, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rowh, M.; Rowh, A.; Lambert, S.; Nickerson, H.; Webb, C. Drive-Through Mass Vaccination Center Operations in a Rural, Medically Underserved Area Using Military Civilian Partnership During the COVID-19 Pandemic. Disaster Med. Public Health Prep. 2023, 17, e354. [Google Scholar] [CrossRef]
- Meyer, F.; Schwenzer, C.; Spelmeyer, D. Umsetzung der COVID-19-Impfkampagne: Erfahrungen und Perspektiven aus Sicht der Kassenärztlichen Vereinigung Westfalen-Lippe (KVWL). Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 1262–1271. [Google Scholar] [CrossRef]
- Rice, K.; Backer, H.; McFadden, H.; Bode, J.; Metzner, M.; Noste, E.; Devereaux, A.V. Mass Immunizations: A Pocket Guide. Disaster Med. Public Health Prep. 2023, 17, e379. [Google Scholar] [CrossRef]
- Houze-Cerfon, V.; Viault, B.; Zerdoud, L.; Ged, M.; Vergé, S.; Metz, F.; Ciottone, G.; Hart, A.; Bounes, V. Setting up the Largest Mass Vaccination Center in Europe: The One-Physician One-Nurse Protocol. Vaccines 2023, 11, 643. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, L.; Wu, X.; Luo, C.; Huang, A.; Wang, W. COVID-19 vaccination in the mass vaccination center: Clinical practice and effectiveness analysis. Front. Public Health 2023, 11, 1072883. [Google Scholar] [CrossRef]
- Merriam Webster. Policymaker. Merriam-Webster.com Dictionary. 2023. Available online: https://www.merriam-webster.com/dictionary/policymaker (accessed on 10 September 2023).
- Van Audenhove, L.; Donders, K. Talking to People III: Expert Interviews and Elite Interviews. In The Palgrave Handbook of Methods for Media Policy Research; Van den Bulck, H., Puppis, M., Donders, K., Van Audenhove, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 179–197. [Google Scholar] [CrossRef]
- Meuser, M.; Nagel, U. The Expert Interview and Changes in Knowledge Production. In Interviewing Experts; Bogner, A., Littig, B., Menz, W., Eds.; Palgrave Macmillan: London, UK, 2009; pp. 17–42. [Google Scholar] [CrossRef]
- Bogner, A.; Littig, B.; Menz, W. Interviews Mit Experten: Eine Praxisorientierte Einführung; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Kaiser, R. Qualitative Experteninterviews: Konzeptionelle Grundlagen und Praktische Durchführung; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2021. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Creswell, J.W.; Poth, C.N. Qualitative Inquiry & Research Design: Choosing among Five Approaches, 4th ed.; SAGE: Los Angeles, CA, USA, 2018. [Google Scholar]
- German Federal Ministry of Health. Empfehlungen für die Organisation und Durchführung von Impfungen Gegen SARS-CoV-2 in Impfzentren und Mit Mobilen Teams; German Federal Ministry of Health: Berlin, Germany, 2020. [Google Scholar]
- WHO. Framework for Decision-Making: Implementation of Mass Vaccination Campaigns in the Context of COVID-19. In WHO Guidelines; WHO: Geneva, Switzerland, 2020; Volume 1, pp. 1–8. [Google Scholar]
- Rodemers, L. Impfstoffversorgung für Deutschland aus Quakenbrück. Bundeswehr Aktuelles. 2022. Available online: https://www.bundeswehr.de/de/organisation/sanitaetsdienst/aktuelles-im-sanitaetsdienst/impfstoffversorgung-fuer-deutschland-aus-quakenbrueck-5441090 (accessed on 10 September 2023).
- ABDA. Ohne Bundeswehrapotheker geht bei der Corona-Impfung nichts. 2021. Available online: https://www.abda.de/aktuelles-und-presse/newsroom/detail/ohne-bundeswehrapotheker-geht-bei-der-corona-impfung-nichts/ (accessed on 10 September 2023).
- Siedler, A.; Schönfeld, V.; Peine, C.; Perumal, N.; Friedsam, A.; Stoliaroff-Pépin, A. Evaluation der COVID-19-Impfung nach breiter Anwendung-ein Zwischenfazit für Deutschland im Juli 2022. Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Trint Limited. Trint. Available online: https://trint.com/ (accessed on 5 December 2022).
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Lochmiller, C. Conducting Thematic Analysis with Qualitative Data. Qual. Rep. 2021, 26, 2029–2044. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Thematic Analysis: A Practical Guide; SAGE: Los Angeles, CA, USA; London, UK; New Delhi, India; Singapore; Washington, DC, USA; Melbourne, Australia, 2022. [Google Scholar]
- Berger, R. Now I see it, now I don’t: Researcher’s position and reflexivity in qualitative research. Qual. Res. 2015, 15, 219–234. [Google Scholar] [CrossRef]
- Gaudet, S.; Robert, D. A Journey through Qualitative Research: From Design to Reporting; Sage: Los Angeles, CA, USA, 2018. [Google Scholar]
- Kordes, H.; Just, V.; Müller, M. Corona-Impfungen: Gutes Geschäft für Ärzte. Erste—Monit. 2022. Available online: https://www1.wdr.de/daserste/monitor/sendungen/corona-impfungen-108.html (accessed on 20 August 2023).
- Tutt, C. Impftempo: Impfzentrum oder Hausarzt? Das ist nicht nur Eine Frage des Geldes. WirtschaftsWoche. 2021. Available online: https://www.wiwo.de/politik/deutschland/impftempo-impfzentrum-oder-hausarzt-das-ist-nicht-nur-eine-frage-des-geldes/27167584.html (accessed on 20 August 2023).
- Lohner, C. Das Verdienen Ärzte, die Gegen Corona Impfen. Capital. 2021. Available online: https://www.capital.de/allgemein/das-verdienen-aerzte-die-gegen-corona-impfen (accessed on 20 August 2023).
- Müller, M. For Some Doctors, COVID Vaccinations Are a Money-Maker—DW—01/25/2022. Deutsche Welle. 2022. Available online: https://www.dw.com/en/opinion-for-germanys-private-practice-doctors-vaccinations-are-business-boosters/a-60549464 (accessed on 20 August 2023).
- DPA. Schweinegrippe: Volle Praxen, zu wenig Impfstoff. Frankfurter Allgemeine Zeitung. 2009. Available online: https://www.faz.net/aktuell/gesellschaft/gesundheit/schweinegrippe-volle-praxen-zu-wenig-impfstoff-1879009.html (accessed on 23 August 2023).
- Götz, G.; Herold, D.; Klotz, P.-A.; Schäfer, J.T. Efficiency in COVID-19 Vaccination Campaigns—A Comparison across Germany’s Federal States. Vaccines 2021, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Danek, S.; Büttner, M.; Krois, J.; Schwendicke, F. How Do Users Respond to Mass Vaccination Centers? A Cross-Sectional Study Using Natural Language Processing on Online Reviews to Explore User Experience and Satisfaction with COVID-19 Vaccination Centers. Vaccines 2023, 11, 144. [Google Scholar] [CrossRef]
- Jentzsch, A.; Geier, A.-K.; Bleckwenn, M.; Schrimpf, A. Differences in Demographics of Vaccinees, Access to, and Satisfaction with SARS-CoV-2 Vaccination Procedures between German General Practices and Mass Vaccination Centers. Vaccines 2022, 10, 1823. [Google Scholar] [CrossRef]
- Grimm, V.; Lembcke, F.K.; Schwarz, M. Impffortschritt in Deutschland und der Welt: Chancen und Risiken. Wirtschaftsdienst 2021, 101, 266–275. [Google Scholar] [CrossRef]
- Landenburger, K.; Neugebauber, T.; Joggerst, B. Konzertierte Impfangebote—Kooperation von Ärzteschaft, Kliniken und ÖGD. In Das Gesundheitswesen; Georg Thieme Verlag: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Garcia, G.; Crenner, C. Comparing International Experiences With Electronic Health Records Among Emergency Medicine Physicians in the United States and Norway: Semistructured Interview Study. JMIR Hum. Factors 2022, 9, e28762. [Google Scholar] [CrossRef]
- Simkhada, P.; Tamang, P.; Timilsina, L.; Simkhada, B.; Bissell, P.; van Teijlingen, E.; Sah, S.K.; Wasti, S.P. Factors Influencing COVID-19 Vaccine Uptake among Nepali People in the UK: A Qualitative Study. Vaccines 2022, 10, 780. [Google Scholar] [CrossRef]
- Bourreau, C.; Baron, A.; Schwarzinger, M.; Alla, F.; Cambon, L.; Donzel Godinot, L.; CoVaMax Study Group. Determinants of COVID-19 Vaccination Intention among Health Care Workers in France: A Qualitative Study. Vaccines 2022, 10, 1661. [Google Scholar] [CrossRef] [PubMed]
- Anger, M.; Wendelborn , C.; Winkler, E.C.; Schickhardt, C. Neither carrots nor sticks? Challenges surrounding data sharing from the perspective of research funding agencies—A qualitative expert interview study. PLoS ONE 2022, 17, e0273259. [Google Scholar] [CrossRef] [PubMed]
- Amponsa-Achiano, K.; Frimpong, J.A.; Barradas, D.; Bandoh, D.A.; Kenu, E. Leveraging Lessons Learned from Yellow Fever and Polio Immunization Campaigns during COVID-19 Pandemic, Ghana, 2021. Emerg. Infect. Dis. 2022, 28, 232–237. [Google Scholar] [CrossRef]
- Walldorf, J.A.; Date, K.A.; Sreenivasan, N.; Harris, J.B.; Hyde, T.B. Lessons Learned from Emergency Response Vaccination Efforts for Cholera, Typhoid, Yellow Fever, and Ebola. Emerg. Infect. Dis. 2017, 23, S210. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Westerveld, R.; Nelson, K.A.; Rohan, H.; Bower, H.; Lazenby, S.; Ikilezi, G.; Bartlein, R.; Bausch, D.; Kennedy, D. ‘Learn from the lessons and don’t forget them’: Identifying transferable lessons for COVID-19 from meningitis A, yellow fever and Ebola virus disease vaccination campaigns. BMJ Glob. Health 2021, 6, e006951. [Google Scholar] [CrossRef]
- Becker, M.H.; Maiman, L.A. Sociobehavioral determinants of compliance with health and medical care recommendations. Med. Care 1975, 13, 10–24. [Google Scholar] [CrossRef]
- Kasai, T. COVID-19 Vaccines Offer Hope, Other Prevention Measures Must Continue. WHO West. Pac. 2021. Available online: https://www.who.int/westernpacific/news-room/commentaries/detail-hq/covid-19-vaccines-offer-hope-but-other-prevention-measures-must-continue (accessed on 12 April 2022).
- Infektionsschutzgesetz (IfSG)—Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten Beim Menschen. Available online: https://www.gesetze-im-internet.de/ifsg/index.html (accessed on 24 August 2023).
- Robert Koch-Institut (RKI). STIKO-Empfehlung zur COVID-19-Impfung. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/COVID-19/Impfempfehlung-Zusfassung.html (accessed on 24 August 2023).
- European Medicines Agency (EMA). First COVID-19 Vaccine Approved for Children Aged 12 to 15 in EU. European Medicines Agency. 2021. Available online: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu (accessed on 24 August 2023).
- European Medicines Agency (EMA). Comirnaty COVID-19 Vaccine: EMA Recommends Approval for Children Aged 5 to 11. European Medicines Agency. 2021. Available online: https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11 (accessed on 24 August 2023).
- European Medicines Agency (EMA). COVID-19 Medicines. European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 24 August 2023).
- Bundesregierung. Impf-Priorisierung für Corona-Impfung entfällt. 07 June 2021. Available online: https://www.bundesregierung.de/breg-de/themen/coronavirus/corona-impfung-priorisierung-entfaellt-1914756 (accessed on 29 September 2022).
- Kassenzahnärztliche Bundesvereinigung; Bundeszahnärztekammer. FAQs—Impfen Durch Zahnärztinnen und Zahnärzte; Kassenzahnärztliche Bundesvereinigung and Bundeszahnärztekammer: Berlin, Germany, 2022. [Google Scholar]
- Kassenärztliche Bundesvereinigung (KBV). Impfungen mit AstraZeneca nur noch bis Ende November Möglich. PraxisNachrichten. 2021. Available online: https://www.kbv.de/html/1150_55025.php (accessed on 12 September 2023).
- Bundesministerium für Gesundheit. Informationen zur COVID-19 Impfung. Meldungen Zum Coronavirus. 2023. Available online: https://www.bundesgesundheitsministerium.de/coronavirus/faq-covid-19-impfung.html (accessed on 12 September 2023).
| Factors Increasing the Need to Establish Centrally Managed Government Vaccination Structures beyond Regular Primary Care Infrastructure | |
|---|---|
| Pandemic conditions (N = 23) * |
|
| Product properties and logistics (N = 15) * |
|
| Local circumstances and capacities (N = 16) * |
|
| Operational Readiness Measures for the Rapid Establishment of Crisis Response Structures | |
|---|---|
| Processes |
|
| Locations/properties |
|
| Staff |
|
| Materials/ technology |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danek, S.; Achelrod, D.; Wichmann, O.; Schwendicke, F. The Role of Vaccination Centers in a National Mass Immunization Campaign—Policymaker Insights from the German COVID-19 Pandemic Vaccine Roll-Out. Vaccines 2023, 11, 1552. https://doi.org/10.3390/vaccines11101552
Danek S, Achelrod D, Wichmann O, Schwendicke F. The Role of Vaccination Centers in a National Mass Immunization Campaign—Policymaker Insights from the German COVID-19 Pandemic Vaccine Roll-Out. Vaccines. 2023; 11(10):1552. https://doi.org/10.3390/vaccines11101552
Chicago/Turabian StyleDanek, Stella, Dmitrij Achelrod, Ole Wichmann, and Falk Schwendicke. 2023. "The Role of Vaccination Centers in a National Mass Immunization Campaign—Policymaker Insights from the German COVID-19 Pandemic Vaccine Roll-Out" Vaccines 11, no. 10: 1552. https://doi.org/10.3390/vaccines11101552
APA StyleDanek, S., Achelrod, D., Wichmann, O., & Schwendicke, F. (2023). The Role of Vaccination Centers in a National Mass Immunization Campaign—Policymaker Insights from the German COVID-19 Pandemic Vaccine Roll-Out. Vaccines, 11(10), 1552. https://doi.org/10.3390/vaccines11101552







