A Delta–Omicron Bivalent Subunit Vaccine Elicited Antibody Responses in Mice against Both Ancestral and Variant Strains of SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Yeast Strains, Plasmids, and Other Materials
2.2. RBD Gene Cloning and Protein Level of SARS-CoV-2 Variants
2.3. Strain Fermentation and the Purification Procedure for the RBDs
2.4. Feature of Recombinant SARS-CoV-2 RBD Proteins
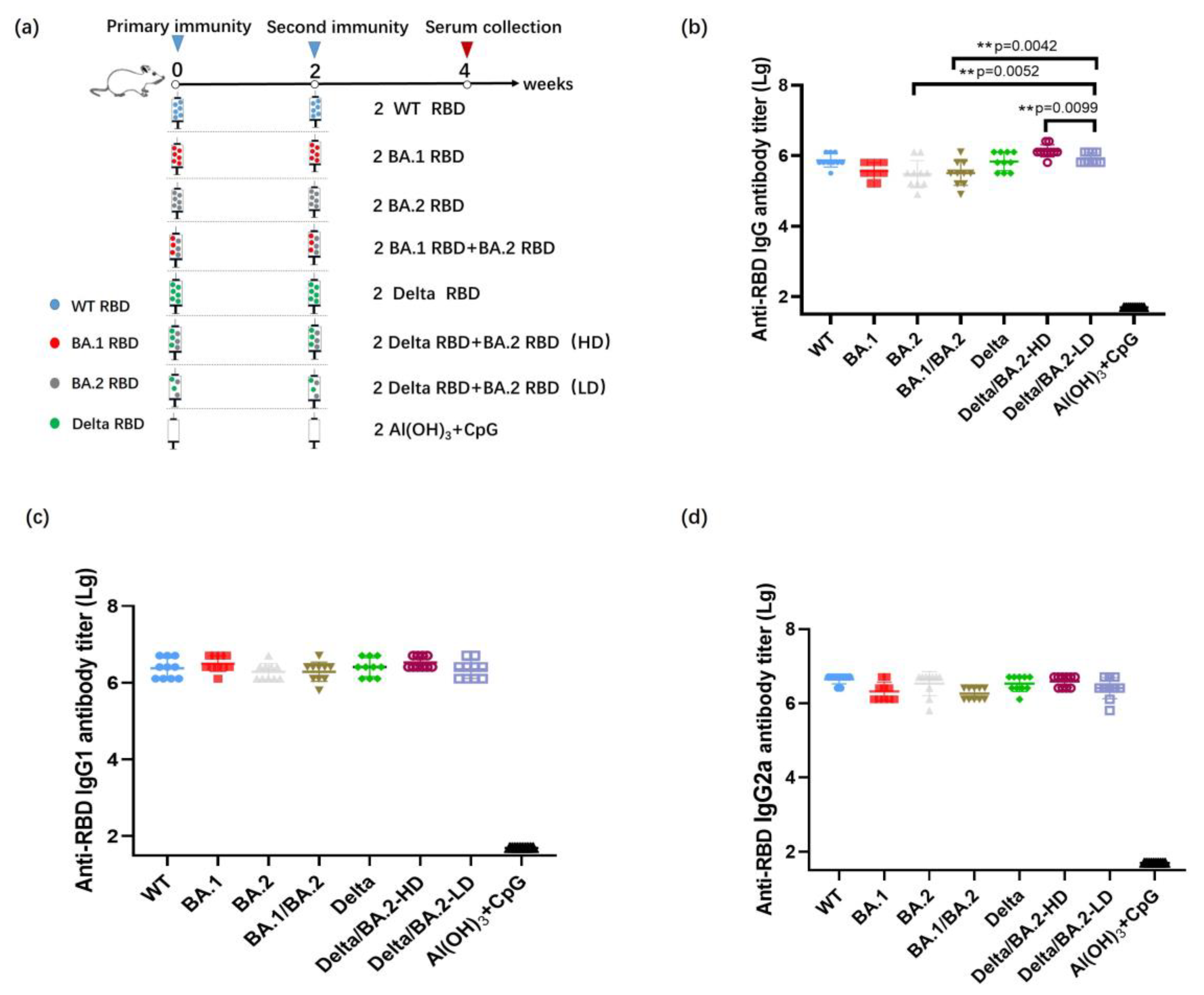
2.5. Vaccination of Mice and Identification of Antibodies against Recombinant RBD in Mice Serum
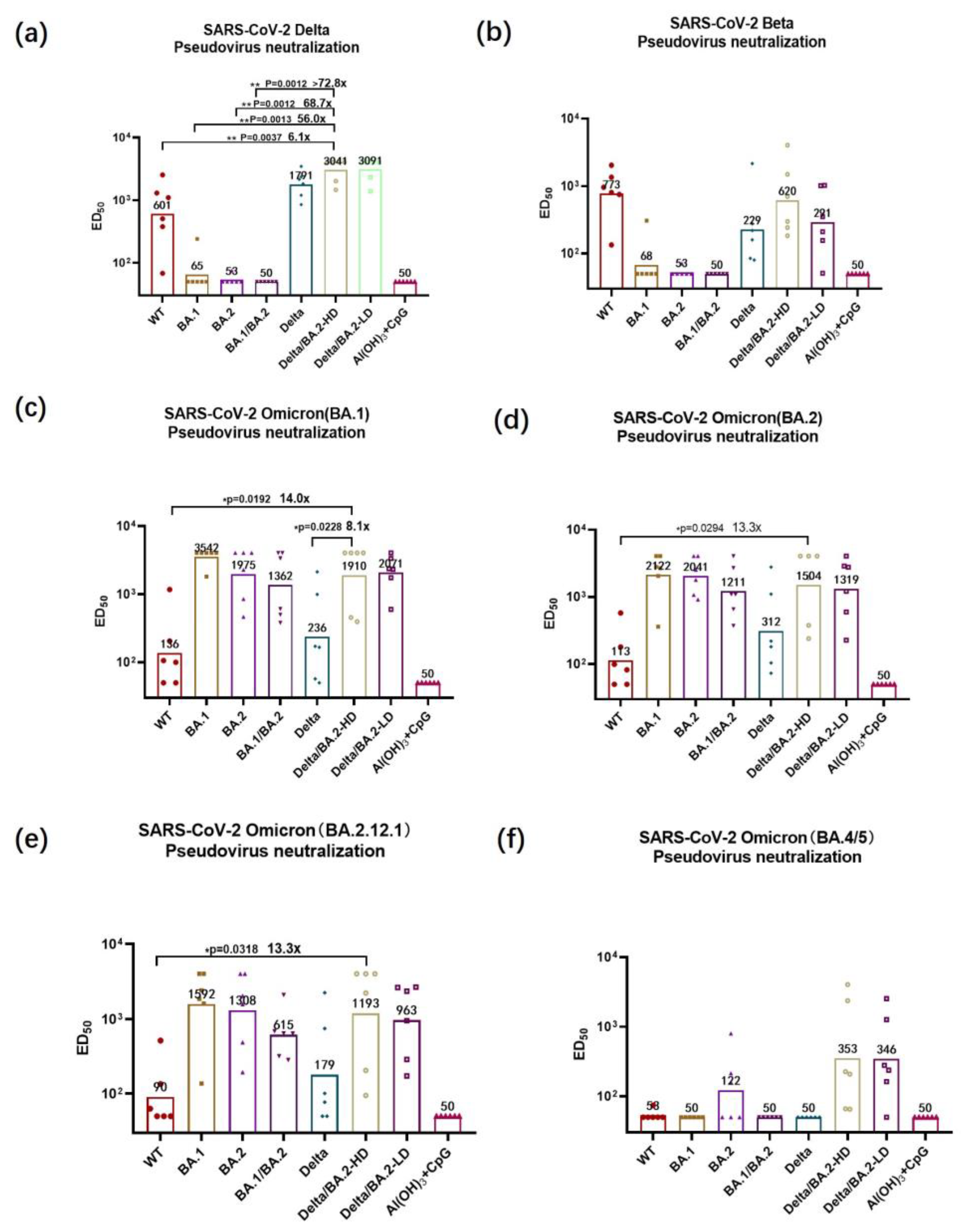
2.6. Pseudovirus Neutralization Assay
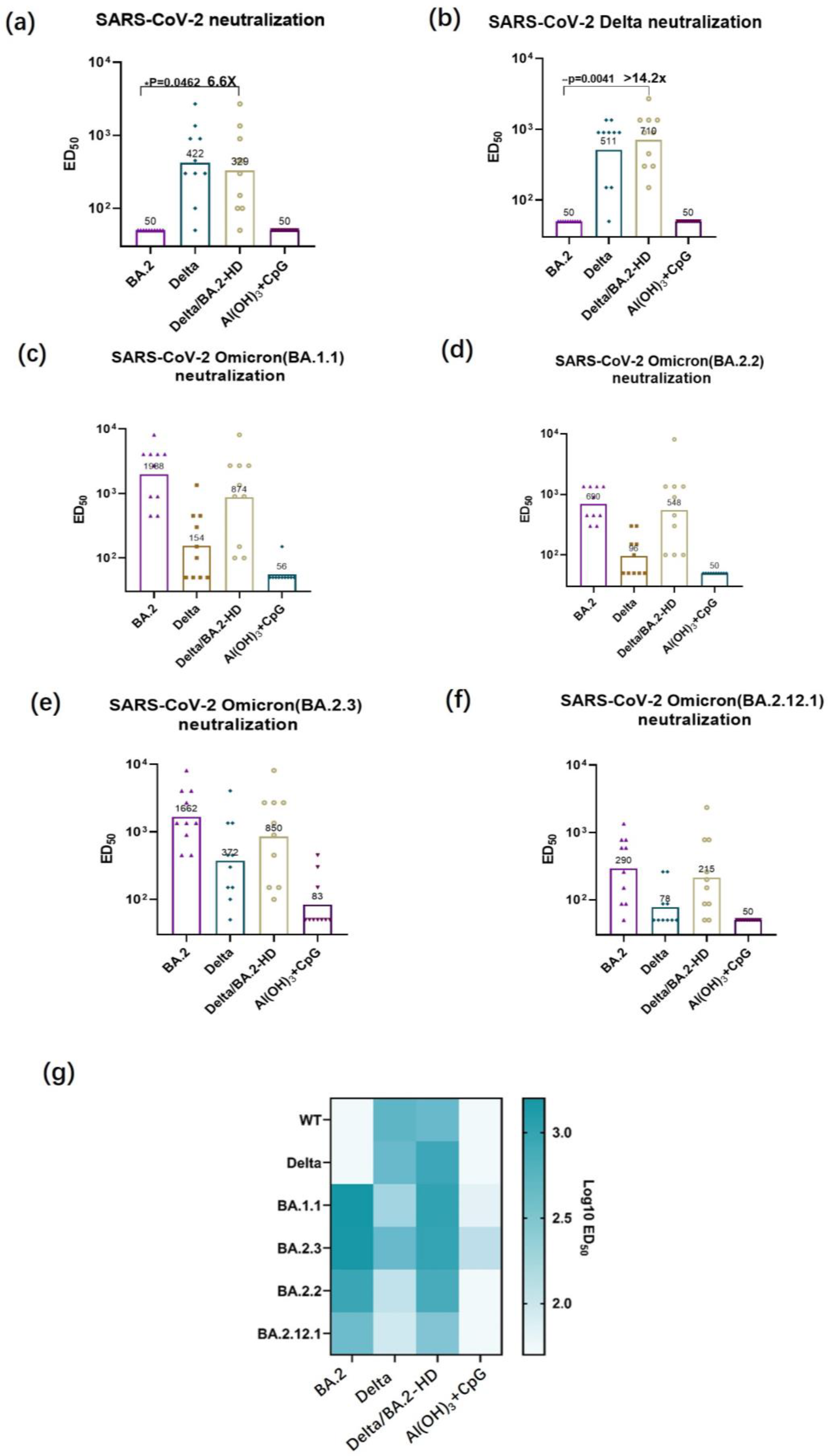
2.7. Authentic Virus-Neutralizing Assay
3. Results
3.1. Expression of the RBD Proteins of SARS-CoV-2 Variants in Glycoengineered P. pastoris
3.2. Antibody Titers after Two Immunizations with the Bivalent RBD Vaccine
3.3. Pseudovirus-Neutralizing Antibody Titers
3.4. Authentic Virus-Neutralizing Antibody Titer Determination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- Zhang, N.N.; Zhang, R.R.; Zhang, Y.F.; Ji, K.; Xiong, X.C.; Qin, Q.S.; Gao, P.; Lu, X.S.; Zhou, H.Y.; Song, H.F.; et al. Rapid development of an updated mRNA vaccine against the SARS-CoV-2 Omicron variant. Cell Res. 2022, 32, 401–403. [Google Scholar] [CrossRef]
- Tai, W.; Zhang, X.; Drelich, A.; Shi, J.; Hsu, J.C.; Luchsinger, L.; Hillyer, C.D.; Tseng, C.T.; Jiang, S.; Du, L. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020, 30, 932–935. [Google Scholar] [CrossRef]
- Afkhami, S.; D’Agostino, M.R.; Zhang, A.; Stacey, H.D.; Marzok, A.; Kang, A.; Singh, R.; Bavananthasivam, J.; Ye, G.; Luo, X.; et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 2022, 185, 896–915.e19. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- Moyo-Gwete, T.; Madzivhandila, M.; Makhado, Z.; Ayres, F.; Mhlanga, D.; Oosthuysen, B.; Lambson, B.E.; Kgagudi, P.; Tegally, H.; Iranzadeh, A.; et al. SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies. bioRxiv 2021. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, J.; Yuan, R.Y.; Wang, M.Y.; He, P.; Su, J.G.; Han, Z.B.; Jin, Y.Q.; Hou, J.W.; Zhang, H.; et al. Design of a mutation-integrated trimeric RBD with broad protection against SARS-CoV-2. Cell Discov. 2022, 8, 17. [Google Scholar] [CrossRef]
- Du, P.; Li, N.; Xiong, X.; Tang, S.; Dai, Q.; Liu, Z.; Wang, T.; Gu, X.; Zhou, Z. A bivalent vaccine containing D614G and BA.1 spike trimer proteins or a BA.1 spike trimer protein booster shows broad neutralizing immunity. J. Med. Virol. 2022, 94, 4287–4293. [Google Scholar] [CrossRef]
- Quandt, J.; Muik, A.; Salisch, N.; Lui, B.G.; Lutz, S.; Krüger, K.; Wallisch, A.K.; Adams-Quack, P.; Bacher, M.; Finlayson, A.; et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci. Immunol. 2022, 7, eabq2427. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Werner, A.P.; Leist, S.R.; Stevens, L.J.; Falconer, E.; Goldsmith, J.A.; Chou, C.W.; Abiona, O.M.; West, A.; Westendorf, K.; et al. Stabilized coronavirus spike stem elicits a broadly protective antibody. Cell Rep. 2021, 37, 109929. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, X.; Yuan, L.; Wang, S.; Zhang, Y.; Xiong, H.; Chen, R.; Ma, J.; Qi, R.; Nie, M.; et al. A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamsters. Sci. Transl. Med. 2021, 13, eabg1143. [Google Scholar] [CrossRef]
- Liu, B.; Yin, Y.; Liu, Y.; Wang, T.; Sun, P.; Ou, Y.; Gong, X.; Hou, X.; Zhang, J.; Ren, H.; et al. A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses. Engineering 2022, 13, 107–115. [Google Scholar] [CrossRef]
- Liu, B.; Shi, P.; Wang, T.; Zhao, Y.; Lu, S.; Li, X.; Luo, S.; Chang, S.; Wang, S.; Sun, P.; et al. Recombinant H7 hemagglutinin expressed in glycoengineered Pichia pastoris forms nanoparticles that protect mice from challenge with H7N9 influenza virus. Vaccine 2020, 38, 7938–7948. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef]
- Xu, H.; Wang, T.; Sun, P.; Hou, X.; Gong, X.; Zhang, B.; Wu, J.; Liu, B. A bivalent subunit vaccine efficiently produced in Pichia pastoris against SARS-CoV-2 and emerging variants. Front. Microbiol. 2022, 13, 1093080. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Abid, T.; Pereira Ribeiro, S.; Edara, V.V.; Floyd, K.; Smith, J.C.; Latif, M.B.; Pacheco-Sanchez, G.; Dutta, D.; Wang, S.; et al. A yeast expressed RBD-based SARS-CoV-2 vaccine formulated with 3M-052-alum adjuvant promotes protective efficacy in non-human primates. Sci. Immunol. 2021, 6, eabh3634. [Google Scholar] [CrossRef]
- Huang, H.Y.; Liao, H.Y.; Chen, X.; Wang, S.W.; Cheng, C.W.; Shahed-Al-Mahmud, M.; Liu, Y.M.; Mohapatra, A.; Chen, T.H.; Lo, J.M.; et al. Vaccination with SARS-CoV-2 spike protein lacking glycan shields elicits enhanced protective responses in animal models. Sci. Transl. Med. 2022, 14, eabm0899. [Google Scholar] [CrossRef]
- Tai, W.; Chai, B.; Feng, S.; Zhuang, X.; Ma, J.; Pang, M.; Pan, L.; Yang, Z.; Tian, M.; Cheng, G. Development of a ferritin-based nanoparticle vaccine against the SARS-CoV-2 Omicron variant. Signal Transduct. Target. Ther. 2022, 7, 173. [Google Scholar] [CrossRef]
- Li, T.; Han, X.; Gu, C.; Guo, H.; Zhang, H.; Wang, Y.; Hu, C.; Wang, K.; Liu, F.; Luo, F.; et al. Potent SARS-CoV-2 neutralizing antibodies with protective efficacy against newly emerged mutational variants. Nat. Commun. 2021, 12, 6304. [Google Scholar] [CrossRef]
- Napodano, C.; Marino, M.; Stefanile, A.; Pocino, K.; Scatena, R.; Gulli, F.; Rapaccini, G.L.; Delli Noci, S.; Capozio, G.; Rigante, D.; et al. Immunological Role of IgG Subclasses. Immunol. Investig. 2021, 50, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Scheaffer, S.M.; Lee, D.; Whitener, B.; Ying, B.; Wu, K.; Liang, C.Y.; Jani, H.; Martin, P.; Amato, N.J.; Avena, L.E.; et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat. Med. 2023, 29, 247–257. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zheng, J.; Xu, H.; Wang, Z.; Sun, P.; Hou, X.; Gong, X.; Zhang, B.; Wu, J.; Liu, B. A Delta–Omicron Bivalent Subunit Vaccine Elicited Antibody Responses in Mice against Both Ancestral and Variant Strains of SARS-CoV-2. Vaccines 2023, 11, 1539. https://doi.org/10.3390/vaccines11101539
Wang T, Zheng J, Xu H, Wang Z, Sun P, Hou X, Gong X, Zhang B, Wu J, Liu B. A Delta–Omicron Bivalent Subunit Vaccine Elicited Antibody Responses in Mice against Both Ancestral and Variant Strains of SARS-CoV-2. Vaccines. 2023; 11(10):1539. https://doi.org/10.3390/vaccines11101539
Chicago/Turabian StyleWang, Tiantian, Jing Zheng, Huifang Xu, Zhongyi Wang, Peng Sun, Xuchen Hou, Xin Gong, Bin Zhang, Jun Wu, and Bo Liu. 2023. "A Delta–Omicron Bivalent Subunit Vaccine Elicited Antibody Responses in Mice against Both Ancestral and Variant Strains of SARS-CoV-2" Vaccines 11, no. 10: 1539. https://doi.org/10.3390/vaccines11101539
APA StyleWang, T., Zheng, J., Xu, H., Wang, Z., Sun, P., Hou, X., Gong, X., Zhang, B., Wu, J., & Liu, B. (2023). A Delta–Omicron Bivalent Subunit Vaccine Elicited Antibody Responses in Mice against Both Ancestral and Variant Strains of SARS-CoV-2. Vaccines, 11(10), 1539. https://doi.org/10.3390/vaccines11101539





