Successful SARS-CoV-2 mRNA Vaccination Program in Allogeneic Hematopoietic Stem Cell Transplant Recipients—A Retrospective Single-Center Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busca, A.; Salmanton-García, J.; Marchesi, F.; Farina, F.; Seval, G.C.; Van Doesum, J.; De Jonge, N.; Bahr, N.C.; Maertens, J.; Meletiadis, J.; et al. Outcome of COVID-19 in allogeneic stem cell transplant recipients: Results from the EPICOVIDEHA registry. Front. Immunol. 2023, 14, 1125030. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stahel, V.P. Review the safety of COVID-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. COVE Study Group Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Tan, J.Y.; Wee, L.E.; Tan, Y.H.; Conceicao, E.P.; Lim, F.W.I.; Chen, Y.; Than, H.; Quek, J.K.S.; Nagarajan, C.; Goh, Y.T.; et al. Favorable outcomes of COVID-19 in vaccinated hematopoietic stem cell transplant recipients: A single-center experience. Transpl. Infect. Dis. 2023, 25, e14024. [Google Scholar] [CrossRef]
- Maillard, A.; Redjoul, R.; Klemencie, M.; Labussière Wallet, H.; Le Bourgeois, A.; D’Aveni, M.; Huynh, A.; Berceanu, A.; Marchand, T.; Chantepie, S.; et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood 2022, 139, 134–137. [Google Scholar] [CrossRef]
- Mori, Y.; Uchida, N.; Harada, T.; Katayama, Y.; Wake, A.; Iwasaki, H.; Eto, T.; Morishige, S.; Fujisaki, T.; Ito, Y.; et al. Predictors of impaired antibody response after SARS-CoV-2 mRNA vaccination in hematopoietic cell transplant recipients: A Japanese multicenter observational study. Am. J. Hematol. 2023, 98, 102–111. [Google Scholar] [CrossRef]
- Abbott AdviseDx SARS-CoV-2 IgG II Assay. Available online: https://www.fda.gov/media/146371/download (accessed on 17 September 2023).
- Mattiuzzo, G.; Bentley, E.M.; Hassall, M.; Routley, S. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody. Available online: https://www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed on 17 September 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 26 September 2023).
- Beerlage, A.; Leuzinger, K.; Valore, L.; Mathew, R.; Junker, T.; Drexler, B.; Passweg, J.R.; Hirsch, H.H.; Halter, J. Antibody response to mRNA SARS-CoV-2 vaccination in 182 patients after allogeneic hematopoietic cell transplantation. Transpl. Infect. Dis. 2022, 24, e13828. [Google Scholar] [CrossRef]
- Jullien, M.; Le Bourgeois, A.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Rimbert, M.; Imbert, B.-M.; Le Gouill, S.; Moreau, P.; Mahe, B.; et al. B Cell Aplasia Is the Most Powerful Predictive Marker for Poor Humoral Response after BNT162b2 mRNA SARS-CoV-2 Vaccination in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Transplant. Cell. Ther. 2022, 28, 279.e1–279.e4. [Google Scholar] [CrossRef] [PubMed]
- Tamari, R.; Politikos, I.; Knorr, D.A.; Vardhana, S.A.; Young, J.C.; Marcello, L.T.; Doddi, S.; Devlin, S.M.; Ramanathan, L.V.; Pessin, M.S.; et al. Predictors of Humoral Response to SARS-CoV-2 Vaccination after Hematopoietic Cell Transplantation and CAR T-cell Therapy. Blood Cancer Discov. 2021, 2, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Xu, L.-P.; Wang, Y.; Zhang, X.-H.; Chen, H.; Chen, Y.-H.; Wang, F.-R.; Han, W.; Sun, Y.-Q.; Yan, C.-H.; et al. Controlled, Randomized, Open-Label Trial of Risk-Stratified Corticosteroid Prevention of Acute Graft-Versus-Host Disease After Haploidentical Transplantation. J. Clin. Oncol. 2016, 34, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.F.; Labopin, M.; Bader, P.; Basak, G.W.; Bonini, C.; Chabannon, C.; Corbacioglu, S.; Dreger, P.; Dufour, C.; Gennery, A.R.; et al. European Society for Blood and Marrow Transplantation (EBMT) Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transplant. 2019, 54, 1525–1552. [Google Scholar] [CrossRef]
- Shem-Tov, N.; Yerushalmi, R.; Danylesko, I.; Litachevsky, V.; Levy, I.; Olmer, L.; Lusitg, Y.; Avigdor, A.; Nagler, A.; Shimoni, A.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in haematopoietic stem cell transplantation recipients. Br. J. Haematol. 2022, 196, 884–891. [Google Scholar] [CrossRef]
- Haller, M.C.; Kaiser, R.A.; Langthaler, S.; Brandstetter, C.; Apfalter, P.; Kerschner, H.; Cejka, D. Comparison of mRNA-1273 and BNT162b2 SARS-CoV-2 mRNA Vaccine Immunogenicity in Kidney Transplant Recipients. Transpl. Int. 2021, 35, 10026. [Google Scholar] [CrossRef]
- Chiarucci, M.; Paolasini, S.; Isidori, A.; Guiducci, B.; Loscocco, F.; Capalbo, M.; Visani, G. Immunological Response Against SARS-COV-2 After BNT162b2 Vaccine Administration Is Impaired in Allogeneic but Not in Autologous Stem Cell Transplant Recipients. Front. Oncol. 2021, 11, 737300. [Google Scholar] [CrossRef]
- Schulz, E.; Hodl, I.; Forstner, P.; Hatzl, S.; Sareban, N.; Moritz, M.; Fessler, J.; Dreo, B.; Uhl, B.; Url, C.; et al. CD19+IgD+CD27- Naïve B Cells as Predictors of Humoral Response to COVID 19 mRNA Vaccination in Immunocompromised Patients. Front. Immunol. 2021, 12, 803742. [Google Scholar] [CrossRef]
- Hill, J.A.; Martens, M.J.; Young, J.-A.H.; Bhavsar, K.; Kou, J.; Chen, M.; Lee, L.W.; Baluch, A.; Dhodapkar, M.V.; Nakamura, R.; et al. SARS-CoV-2 vaccination in the first year after allogeneic hematopoietic cell transplant: A prospective, multicentre, observational study. EClinicalMedicine 2023, 59, 101983. [Google Scholar] [CrossRef]
- Huang, A.; Cicin-Sain, C.; Pasin, C.; Epp, S.; Audigé, A.; Müller, N.J.; Nilsson, J.; Bankova, A.; Wolfensberger, N.; Vilinovszki, O.; et al. Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2022, 28, 214.e1–214.e11. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Müller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2022, 22, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Caocci, G.; Mulas, O.; Mantovani, D.; Costa, A.; Galizia, A.; Barabino, L.; Greco, M.; Murru, R.; La Nasa, G. Ruxolitinib does not impair humoral immune response to COVID-19 vaccination with BNT162b2 mRNA COVID-19 vaccine in patients with myelofibrosis. Ann. Hematol. 2022, 101, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.A.; Cambria, D.; La Spina, E.; Duminuco, A.; Laneri, A.; Longo, A.; Vetro, C.; Giallongo, S.; Romano, A.; Di Raimondo, F.; et al. Ruxolitinib treatment in myelofibrosis and polycythemia vera causes suboptimal humoral immune response following standard and booster vaccination with BNT162b2 mRNA COVID-19 vaccine. Front. Oncol. 2023, 13, 1117815. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yakushijin, K.; Funakoshi, Y.; Ohji, G.; Ichikawa, H.; Sakai, H.; Hojo, W.; Saeki, M.; Hirakawa, Y.; Matsumoto, S.; et al. A Third Dose COVID-19 Vaccination in Allogeneic Hematopoietic Stem Cell Transplantation Patients. Vaccines 2022, 10, 1830. [Google Scholar] [CrossRef] [PubMed]
- Toya, T.; Sadato, D.; Sanada, T.; Honda, T.; Atsuta, Y.; Sekiya, N.; Shimizu, H.; Najima, Y.; Kobayashi, T.; Harada, Y.; et al. A third dose of COVID-19 mRNA vaccine induces limited humoral response in stem cell transplant recipients who got two vaccine doses before transplant. eJHaem 2022, 4, 309–311. [Google Scholar] [CrossRef]
- Henig, I.; Isenberg, J.; Yehudai-Ofir, D.; Leiba, R.; Ringelstein-Harlev, S.; Ram, R.; Avni, B.; Amit, O.; Grisariu, S.; Azoulay, T.; et al. Third BNT162b2 mRNA SARS-CoV-2 Vaccine Dose Significantly Enhances Immunogenicity in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Vaccines 2023, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.M.; Bruns, A.H.W.; Verduyn Lunel, F.M.; Raijmakers, R.A.P.; de Weijer, R.J.; Nanlohy, N.M.; Smits, G.P.; van Baarle, D.; Kuball, J. Predictive factors for vaccine failure to guide vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021, 56, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Wherry, E.J.; Barouch, D.H. T cell immunity to COVID-19 vaccines. Science 2022, 377, 821–822. [Google Scholar] [CrossRef]
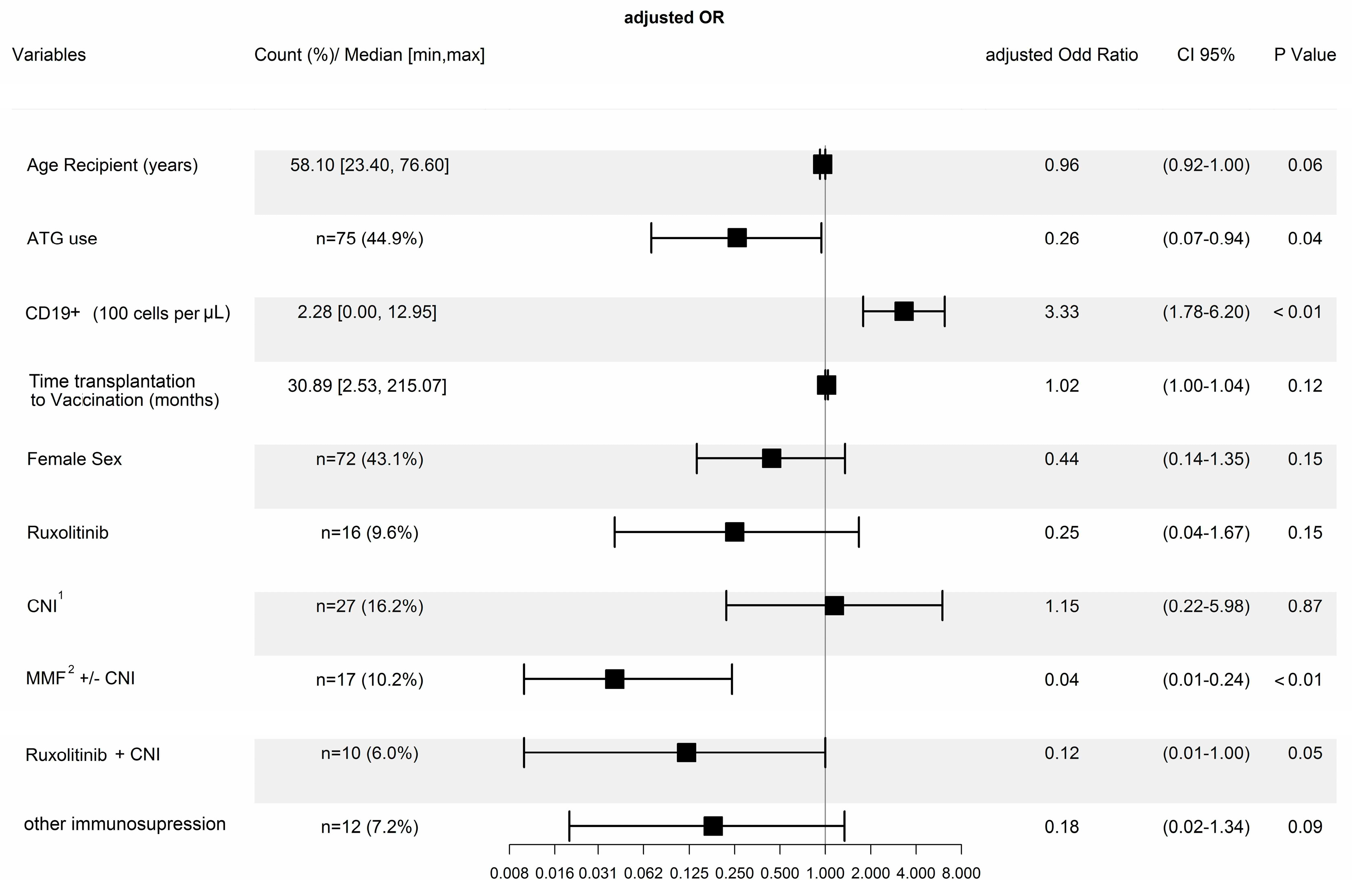

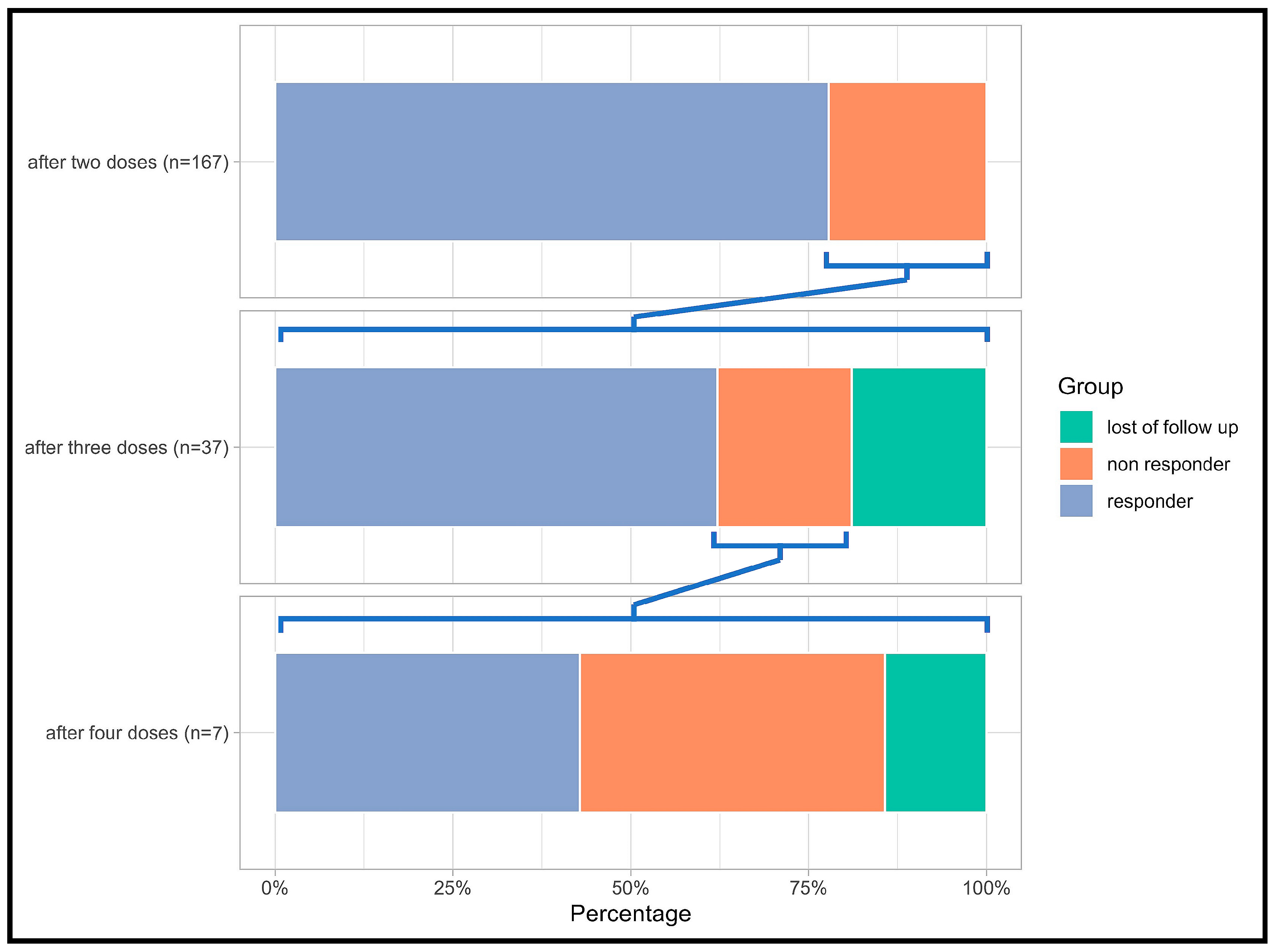
| All | Responders | Non-Responders | p-Value | ||
|---|---|---|---|---|---|
| (n = 167) | (n = 130) | (n = 37) | |||
| Baseline characteristics | Unit | ||||
| Age (median [min, max]) | Years | 58.10 [23.40, 76.60] | 57.20 [23.40, 76.50] | 64.30 [23.50, 76.60] | 0.03 |
| Sex (%) | |||||
| Male | 95 (56.9) | 79 (60.8) | 16 (43.2) | 0.06 | |
| Female | 72 (43.1) | 51 (39.2) | 21 (56.8) | ||
| Vaccine (%) | |||||
| BNT162b2 | 147 (88.0) | 32 (86.5) | 115 (88.5) | 0.78 | |
| mRNA-1273 | 20 (12.0) | 5 (13.5) | 15 (11.5) | ||
| Time from transplantation to vaccination (median [min, max]) | Months | 30.89 [2.53, 215.07] | 35.26 [2.99, 215.07] | 10.20 [2.53, 88.88] | <0.001 |
| Unrelated donor (%) | |||||
| Related | 112 (67.1) | 90 (69.2) | 22 (59.5) | 0.32 | |
| Unrelated | 55 (32.9) | 40 (30.8) | 15 (40.5) | ||
| Antineoplastic maintenance treatment (%) | |||||
| No | 141 (84.4) | 110 (84.6) | 31 (83.8) | 1.00 | |
| Yes | 26 (15.6) | 20 (15.4) | 6 (16.2) | ||
| Ongoing steroid treatment (%) | |||||
| No | 113 (67.7) | 100 (76.9) | 13 (35.1) | <0.001 | |
| Yes | 54 (32.3) | 30 (23.1) | 24 (64.9) | ||
| Immunosuppressives (%) | |||||
| Off immunosuppression | 85 (50.9) | 78 (60.0) | 7 (18.9) | <0.001 | |
| Ruxolitinib | 16 (9.6) | 12 (9.2) | 4 (10.8) | ||
| CNI 1 (CSA 2 or TAC 3) | 27 (16.2) | 22 (16.9) | 5 (13.5) | ||
| MMF 4 +/− CNI | 17 (10.2) | 4 (3.1) | 13 (35.1) | ||
| Ruxolitinib + CNI | 10 (6.0) | 7 (5.4) | 3 (8.1) | ||
| Other IST 5 | 12 (7.2) | 7 (5.4) | 5 (13.5) | ||
| Lymphocyte counts (median [min, max]) | (Cells/μL) | ||||
| CD4+ | 354 [7, 1629] | 386 [79, 1629] | 154 [7, 1007] | <0.001 | |
| CD8+ | 459 [33, 4654] | 488 [33, 4654] | 295 [44, 2473] | 0.01 | |
| CD19+ | 228 [0, 1295] | 295 [0, 1295] | 17 [0, 530] | <0.001 | |
| NK | 239 [14, 1979] | 250 [15, 1979] | 218 [14, 646] | 0.05 | |
| Immunoglobulin levels (median [min, max]) | (mg/dL) | ||||
| IgA | 79.00 [1.00, 1299.00] | 92.00 [5.00, 454.00] | 51.00 [1.00, 1299.00] | 0.001 | |
| IgG | 760.00 [72.00, 2137.00] | 828.50 [72.00, 2137.00] | 436.00 [161.00, 2079.00] | <0.001 | |
| IgM | 55.00 [1.00, 299.00] | 62.50 [5.00, 299.00] | 33.00 [1.00, 142.00] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoloudis, A.; Neumann, I.J.; Buxhofer-Ausch, V.; Machherndl-Spandl, S.; Binder, M.; Kaynak, E.; Milanov, R.; Nocker, S.; Stiefel, O.; Strassl, I.; et al. Successful SARS-CoV-2 mRNA Vaccination Program in Allogeneic Hematopoietic Stem Cell Transplant Recipients—A Retrospective Single-Center Analysis. Vaccines 2023, 11, 1534. https://doi.org/10.3390/vaccines11101534
Nikoloudis A, Neumann IJ, Buxhofer-Ausch V, Machherndl-Spandl S, Binder M, Kaynak E, Milanov R, Nocker S, Stiefel O, Strassl I, et al. Successful SARS-CoV-2 mRNA Vaccination Program in Allogeneic Hematopoietic Stem Cell Transplant Recipients—A Retrospective Single-Center Analysis. Vaccines. 2023; 11(10):1534. https://doi.org/10.3390/vaccines11101534
Chicago/Turabian StyleNikoloudis, Alexander, Ines Julia Neumann, Veronika Buxhofer-Ausch, Sigrid Machherndl-Spandl, Michaela Binder, Emine Kaynak, Robert Milanov, Stefanie Nocker, Olga Stiefel, Irene Strassl, and et al. 2023. "Successful SARS-CoV-2 mRNA Vaccination Program in Allogeneic Hematopoietic Stem Cell Transplant Recipients—A Retrospective Single-Center Analysis" Vaccines 11, no. 10: 1534. https://doi.org/10.3390/vaccines11101534
APA StyleNikoloudis, A., Neumann, I. J., Buxhofer-Ausch, V., Machherndl-Spandl, S., Binder, M., Kaynak, E., Milanov, R., Nocker, S., Stiefel, O., Strassl, I., Wipplinger, D., Moyses, M., Kerschner, H., Apfalter, P., Girschikofsky, M., Petzer, A., Weltermann, A., & Clausen, J. (2023). Successful SARS-CoV-2 mRNA Vaccination Program in Allogeneic Hematopoietic Stem Cell Transplant Recipients—A Retrospective Single-Center Analysis. Vaccines, 11(10), 1534. https://doi.org/10.3390/vaccines11101534






