Conserved Candidate Antigens and Nanoparticles to Develop Vaccine against Giardia intestinalis
Abstract
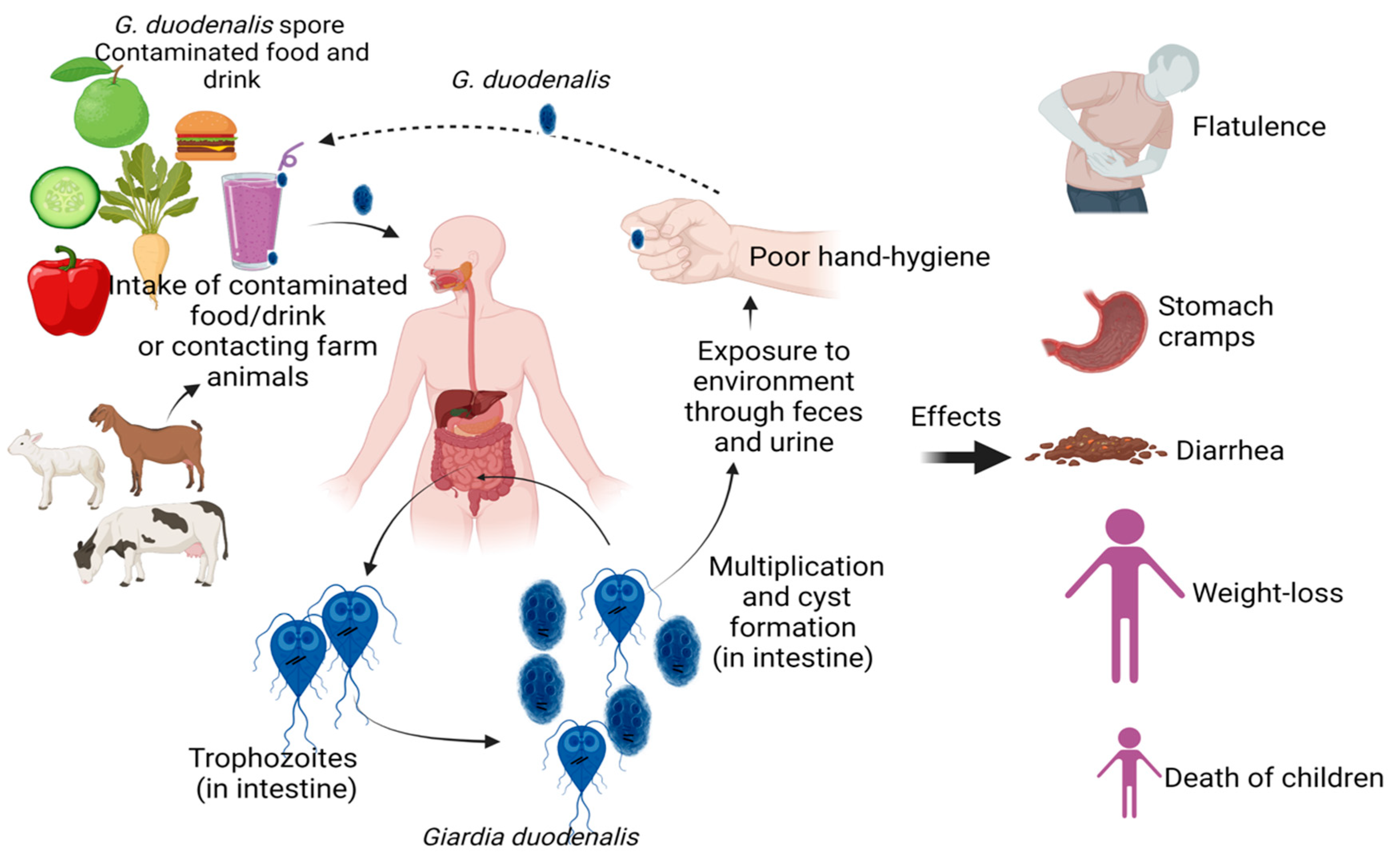
1. Introduction
2. Methods
3. Antigenic Protein Candidates in Giardia
| Protein | Antigen Template/Challenge Strain | Outcome | Model | Ref. |
|---|---|---|---|---|
| VSP H7 | G. intestinalis clone GS/M-83-H7 | Increased serum IgG, IgM | Mouse | [54] |
| VSPs1267 whole protein | Template, G. intestinalis WB strain ATCC 50803 | Developed local (intestinal secretory IgA (S-IgA)) and systemic (serum IgG) | Mouse | [55] |
| CWP2 whole protein | Template, G. intestinalis WB strain ATCC 30957 | Increased fecal IgA, increased serum IgG, cyst shedding reduction 80% | Mouse | [44] |
| CWP2 (M6-CWP2 fusion protein) | Template, G. intestinalis WB strain ATCC 30957 | Increased fecal IgA, cyst shedding reduction 63% | Mouse | [56] |
| CWP2 (M6-CWP2 fusion protein) | Template, G. intestinalis WB strain ATCC 30957 | Increased fecal IgA, increased serum IgG, cyst shedding reduction 70% | Mouse | [57] |
| CWP2 whole protein | Template, G. intestinalis WB strain ATCC 30957 | Increased fecal IgA, increased serum IgG, cyst shedding reduction 60% | Mouse | [58] |
| α1-g whole protein | Template, G. intestinalis WB strain ATCC 50803 Challenge, G. intestinalis GSM strain ATCC 50581 | Increased fecal IgA, increased serum IgG, reduced trophozoite load by 80–90% | Mouse | [17] |
| 5G8 protein (fusion protein) | Template, G. intestinalis strain ATCC 50581 | Increased serum IgG2b, increased agglutination of trophozoites >70–90% | Mouse | [59] |
| CWP2 aa 248–363 α1-g whole protein | Template, synthesized sequence based on GL50803_5435 (WB) Challenge, G. intestinalis C2 Template, G. intestinalis C2 Challenge, G. intestinalis C2 | Increased fecal IgA, increased serum IgG, cyst shedding reduction 93%, reduced trophozoite load by 79% | Mouse | [60] |
4. Micro- and Nanoparticles and Lipid-Based Delivery Systems
5. Immune Responses against Giardia
5.1. Innate Immune Responses to Giardia
5.2. Adaptive Immune Response to Giardia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Messa, A., Jr.; Köster, P.C.; Garrine, M.; Gilchrist, C.; Bartelt, L.A.; Nhampossa, T.; Massora, S.; Kotloff, K.; Levine, M.M.; Alonso, P.L.; et al. Molecular diversity of Giardia duodenalis in children under 5 years from the Manhiça district, Southern Mozambique enrolled in a matched case-control study on the aetiology of diarrhea. PLoS Negl. Trop. Dis. 2021, 15, e0008987. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, E.; Ma’ayeh, S.; Svärd, S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef]
- Leung, A.K. Giardiasis. In Common Problems in Ambulatory Pediatrics: Specific Clinical Problems; Leung, A.K., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; Volume 2, pp. 39–42. [Google Scholar]
- Adam, E.A.; Yoder, J.S.; Gould, L.H.; Hlavsa, M.C.; Gargano, J.W. Giardiasis outbreaks in the United States, 1971–2011. Epidemiol. Infect. 2016, 144, 2790–2801. [Google Scholar] [CrossRef]
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kam, J.K.M. Giardiasis: An overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Transmission|Giardia|Parasites—CDC. Available online: https://www.cdc.gov/parasites/giardia/infection-sources.html (accessed on 3 December 2022).
- Krakovka, S.; Ribacke, U.; Miyamoto, Y.; Eckmann, L.; Svärd, S. Characterization of metronidazole-resistant Giardia intestinalis lines by comparative transcriptomics and proteomics. Front. Microbiol. 2022, 13, 834008. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Abdul-Wahid, A.; Faubert, G. Vaccination against Giardia. In Giardia; Luján, H.D., Svärd, S., Eds.; Springer: Vienna, Austria, 2011. [Google Scholar] [CrossRef]
- Olson, M.E.; Morck, D.W.; Ceri, H. The efficacy of a Giardia lamblia vaccine in kittens. Can. J. Vet Res. 1996, 60, 249–256. [Google Scholar]
- Olson, M.E.; Ceri, H.; Morck, D.W. Giardia vaccination. Parasitol. Today 2000, 16, 213–217. [Google Scholar] [CrossRef]
- Davids, B.J.; Liu, C.M.; Hanson, E.M.; Le, C.H.Y.; Ang, J.; Hanevik, K.; Fischer, M.; Radunovic, M.; Langeland, N.; Ferella, M.; et al. Identification of conserved candidate vaccine antigens in the surface proteome of Giardia lamblia. Infect. Immun. 2019, 87, e00219-e19. [Google Scholar] [CrossRef]
- Zoetis (n.d.) Giardia Vax. Available online: https://ar.zoetis.com/products/caninos/Giardia-vax.aspx (accessed on 3 December 2022).
- Díaz, V.M.M. Pharmacological treatment of giardiasis. In Current Topics in Giardiasis; Rodriguez, A.J., Ed.; IntechOpen: London, UK, 2017; pp. 133–145. [Google Scholar] [CrossRef]
- Vets and pets to reap benefits from new drug to treat common infection. Available online: https://www.unisa.edu.au/media-centre/Releases/2022/vets-and-pets-to-reap-benefits-from-new-drug-to-treat-common-infection/ (accessed on 3 December 2022).
- Jenikova, G.; Hruz, P.; Andersson, M.K.; Tejman-Yarden, N.; Ferreira, P.C.; Andersen, Y.S.; Davids, B.J.; Gillin, F.D.; Svard, S.G.; Curtiss, R., 3rd; et al. Alpha1-giardin based live heterologous vaccine protects against Giardia lamblia infection in a murine model. Vaccine 2011, 29, 9529–9537. [Google Scholar] [CrossRef] [PubMed]
- Radunovic, M.; Klotz, C.; Saghaug, C.S.; Brattbakk, H.R.; Aebischer, T.; Langeland, N.; Hanevik, K. Genetic variation in potential Giardia vaccine candidates cyst wall protein 2 and α1-giardin. Parasitol. Res. 2017, 116, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Garzon, T.; Ortega-Tirado, D.; Lopez-Romero, G.; Alday, E.; Robles-Zepeda, R.E.; Garibay-Escobar, A.; Velazquez, C. Immunoinformatic identification of T-Cell and B-Cell epitopes from Giardia lamblia immunogenic proteins as candidates to develop peptide-based vaccines against giardiasis. Front. Cell Infect. Microbiol. 2021, 11, 769446. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N. Biosynthesis of nanoparticles: Technological concepts and future applications biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell Infect. Microbiol. 2013, 25, 13. [Google Scholar] [CrossRef]
- Sun, Z.; Li, W.; Lenzo, J.C.; Holden, J.A.; McCullough, M.J.; O’Connor, A.J.; O’Brien-Simpson, N.M. The potential of calcium phosphate nanoparticles as adjuvants and vaccine delivery vehicles. Front. Mater. 2021, 8, 788373. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, X. Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development. Cancer Biol. Med. 2021, 18, 352–371. [Google Scholar] [CrossRef]
- Abbas, B.M.; AL-Saqur, I.M.; Majeed, H.A. Detection and genotyping of Giardia lamblia in clinical and environmental samples in some regions of Baghdad city. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 459–468. [Google Scholar] [CrossRef]
- Gasparinho, C.; Ferreira, F.S.; Mayer, A.C.; Mirante, M.C.; Vaz Nery, S.; Santos-Reis, A.; Portugal-Calisto, D.; Brito, M. Molecular characterization of Giardia lamblia in children < 5 years of age with diarrhea attending the Bengo General Hospital, Angola. Trans. R Soc. Trop. Med. Hyg. 2017, 111, 497–503. [Google Scholar] [PubMed]
- Nash, T.E.; Mowatt, M.R. Characterization of a Giardia lamblia variant-specific surface protein (VSP) gene from isolate GS/M and estimation of the VSP gene repertoire size. Mol. Biochem. Parasitol. 1992, 51, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Ryan, U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008, 160, 75–80. [Google Scholar] [CrossRef]
- Itagaki, T.; Kinoshita, S.; Aoki, M.; Itoh, N.; Saeki, H.; Sato, N.; Uetsuki, J.; Izumiyama, S.; Yagita, K.; Endo, T. Genotyping of Giardia intestinalis from domestic and wild animals in Japan using glutamete dehydrogenase gene sequencing. Vet. Parasitol. 2005, 133, 283–287. [Google Scholar] [CrossRef]
- Prucca, C.G.; Lujan, H.D. Antigenic variation in Giardia lamblia. Cell Microbiol. 2009, 11, 1706–1715. [Google Scholar] [CrossRef]
- Faubert, G. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 2000, 13, 35–54. [Google Scholar] [CrossRef]
- Adam, R.D.; Nigam, A.; Seshadri, V.; Martens, C.A.; Farneth, G.A.; Morrison, H.G.; Nash, T.E.; Porcella, S.F.; Patel, R. The Giardia lamblia vsp gene repertoire: Characteristics, genomic organization, and evolution. BMC Genom. 2010, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.D.; Nash, T.E.; Sterling, C.R.; Adam, R.D. (Eds.) The Pathogenic Enteric Protozoa: Giardia, Entamoeba, Cryptosporidium and Cyclospora; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 59–73. [Google Scholar]
- Luján, H.D.; Mowatt, M.R.; Wu, J.J.; Lu, Y.; Lees, A.; Chance, M.R.; Nash, T.E. Purification of a variant-specific surface protein of Giardia lamblia and characterization of its metal-binding properties. J. Biol. Chem. 1995, 270, 13807–13813. [Google Scholar] [CrossRef]
- Nash, T.E. Antigenic variation in Giardia lamblia and the host’s immune response. Philos. Trans. R Soc. Lond. B Biol. Sci. 1997, 352, 1369–1375. [Google Scholar] [CrossRef]
- Rodríguez-Walker, M.; Molina, C.R.; Luján, L.A.; Saura, A.; Jerlström-Hultqvist, J.; Svärd, S.G.; Fernández, E.A.; Luján, H.D. Comprehensive characterization of Cysteine-rich protein-coding genes of Giardia lamblia and their role during antigenic variation. Genomics 2022, 114, 110462. [Google Scholar] [CrossRef]
- Nash, T. Surface antigen variability and variation in Giardia lamblia. Parasitol. Today 1992, 8, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; McCutchan, T.; Keister, D.; Dame, J.B.; Conrad, J.D.; Gillin, F.D. Restriction endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J. Infect. Dis. 1985, 152, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Deplazes, P.; Tanner, I. In vitro synthesized immunoglobulin A from nu/+ and reconstituted nu/nu mice against a dominant surface antigen of Giardia lamblia. Parasitol. Res. 1993, 79, 644–648. [Google Scholar] [CrossRef]
- Gottstein, B.; Nash, T.E. Antigenic variation in Giardia lamblia: Infection of congenitally athymic nude and scid mice. Parasite Immunol. 1991, 13, 649–659. [Google Scholar] [CrossRef]
- Müller, N.; Stäger, S.; Gottstein, B. Serological analysis of antigenic heterogeneity of Giardia lamblia variant surface proteins. Infect. Immun. 1996, 64, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Rivero, F.D.; Saura, A.; Prucca, C.G.; Carranza, P.G.; Torri, A.; Lujan, H.D. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat. Med. 2010, 16, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Larocque, R.; Nakagaki, K.; Lee, P.; Abdul-Wahid, A.; Faubert, G.M. Oral immunization of BALB/c mice with Giardia duodenalis recombinant cyst wall protein inhibits shedding of cysts. Infect. Immun. 2003, 71, 5662–5669. [Google Scholar] [CrossRef]
- Ebneter, J.A.; Heusser, S.D.; Schraner, E.M.; Hehl, A.B.; Faso, C. Cyst-Wall-Protein-1 is fundamental for Golgi-like organelle neogenesis and cyst-wall biosynthesis in Giardia lamblia. Nat. Commun. 2016, 7, 13859. [Google Scholar] [CrossRef]
- Hehl, A.B.; Marti, M.; Kohler, P. Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol. Biol. Cell 2000, 11, 1789–1800. [Google Scholar] [CrossRef]
- Davis-Hayman, S.R.; Hayman, J.R.; Nash, T.E. Encystation-specific regulation of the cyst wall protein 2 gene in Giardia lamblia by multiple cis-acting elements. Int. J. Parasitol. 2003, 33, 1005–1012. [Google Scholar] [CrossRef]
- Elmendorf, H.G.; Dawson, S.C.; McCaffery, J.M. The cytoskeleton of Giardia lamblia. Int. J. Parasitol. 2003, 33, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Alonso, R.A.; Peattie, D.A. Identification and characterization of gamma-giardin and the gamma-giardin gene from Giardia lamblia. Mol. Biochem. Parasitol. 1992, 56, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Steuart, R.F.; O’Handley, R.; Lipscombe, R.J.; Lock, R.A.; Thompson, R.C. Alpha 2 giardin is an assemblage A-specific protein of human infective Giardia duodenalis. Parasitology 2008, 135, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Franzen, O.; Jerlstrom-Hultqvist, J.; Castro, E.; Sherwood, E.; Ankarklev, J.; Reiner, D.S.; Palm, D.; Andersson, J.O.; Andersson, B.; Svard, S.G. Draft genome sequencing of Giardia Intestinalis assemblage B isolate GS: Is human giardiasis caused by two different species? PLoS Pathog. 2009, 5, e1000560. [Google Scholar] [CrossRef]
- Palm, J.E.; Weiland, M.E.; Griffiths, W.J.; Ljungstrom, I.; Svard, S.G. Identification of immunoreactive proteins during acute human giardiasis. J. Infect. Dis. 2003, 187, 1849–1859. [Google Scholar] [CrossRef]
- Weiland, M.E.; Palm, J.E.; Griffiths, W.J.; McCaffery, J.M.; Svard, S.G. Characterisation of alpha-1giardin: An immunodominant Giardia lamblia annexin with glycosamino-glycan-binding activity. Int. J. Parasitol. 2003, 33, 1341–1351. [Google Scholar] [CrossRef]
- Gottstein, B.; Harriman, G.R.; Conrad, J.T.; Nash, T.E. Antigenic variation in Giardia lamblia: Cellular and humoral immune response in a mouse model. Parasite Immunol. 1990, 12, 659–673. [Google Scholar] [CrossRef]
- Serradell, M.C.; Gargantini, P.R.; Saura, A.; Oms, S.R.; Rupil, L.L.; Berod, L.; Sparwasser, T.; Lujáncorresponding, H.D. Cytokines, antibodies, and histopathological profiles during Giardia infection and variant-specific surface protein-based vaccination. Infect Immun. 2018, 86, e00773-17. [Google Scholar] [CrossRef]
- Lee, P.; Faubert, G.M. Expression of the Giardia lamblia cyst wall protein 2 in Lactococcus lactis. Microbiology 2006, 152, 1981–1990. [Google Scholar] [CrossRef]
- Lee, P.; Faubert, G.M. Oral immunization of BALB/c mice by intragastric delivery of Streptococcus gordonii-expressing Giardia cyst wall protein 2 decreases cyst shedding in challenged mice. FEMS Microbiol. Lett. 2006, 265, 225–236. [Google Scholar] [CrossRef]
- Abdul-Wahid, A.; Faubert, G. Mucosal delivery of a transmission blocking DNA vaccine encoding Giardia lamblia CWP2 by Salmonella typhimurium bactofection vehicle. Vaccine 2007, 25, 8372–8383. [Google Scholar] [CrossRef] [PubMed]
- Quintero, J.; Figueroa, D.C.; Barcelo, R.; Breci, L.; Astiazaran-Garcia, H.; Rascon, L.; Robles-Zepeda, R.; Garibay-Escobar, A.; Velazquez-Contreras, E.; Avila, G.L.; et al. Identification of an immunogenic protein of Giardia lamblia using monoclonal antibodies generated from infected mice. Mem. Inst. Oswaldo Cruz. 2013, 108, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Zheng, W.Y.; Zhang, H.M.; Shi, W.Y.; Li, Y.; Cui, B.J.; Wang, H.Y. Vaccination with bivalent DNA vaccine of alpha1-giardin and CWP2 delivered by attenuated Salmonella typhimurium reduces trophozoites and cysts in the feces of mice infected with Giardia lamblia. PLoS ONE 2016, 11, e0157872. [Google Scholar] [CrossRef]
- Van der Weken, H.; Cox, E.; Devriendt, B. Advances in oral subunit vaccine design. Vaccines 2021, 9, 1. [Google Scholar] [CrossRef]
- Thiele, L.; Rothen-Rutishauser, B.; Jilek, S.; Wunderli-Allenspach, H.; Merkle, H.P.; Walter, E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J. Control. Release 2001, 76, 59–71. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Singh, M.; Ulmer, J.B. Microparticle-based technologies for vaccines. Methods 2006, 40, 10–19. [Google Scholar] [CrossRef]
- Deng, S.; Bai, L.; Reboulet, R.; Matthew, R.; Engler, D.A.; Teyton, L.; Bendelac, A.; Savage, P.B. A peptide-free, liposome-based oligosaccharide vaccine, adjuvanted with a natural killer T cell antigen, generates robust antibody responses in vivo. Chem. Sci. 2014, 5, 1437–1441. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Li, A.V.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 2012, 109, 1080–1085. [Google Scholar] [CrossRef]
- Zhou, J.; Miyamoto, Y.; Ihara, S.; Kroll, A.V.; Nieskens, N.; Tran, V.N.; Hanson, E.M.; Fang, R.H.; Zhang, L.; Eckmann, L. Codelivery of antigens and adjuvant in polymeric nanoparticles coated with native parasite membranes induces protective mucosal immunity against Giardia lamblia. J. Infect. Dis. 2022, 226, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Lee, K.; Choi, J.-N.; Hwang, Y.-K.; Yun, M.-Y.; Kim, H.-J.; Won, Y.S.; Kim, S.-J.; Kwon, H.; Huh, S. Intracellular protein delivery by hollow mesoporous silica capsules with a large surface hole. Nanotechnology 2012, 23, 085101. [Google Scholar] [CrossRef] [PubMed]
- Pusic, K.; Aguilar, Z.; McLoughlin, J.; Kobuch, S.; Xu, H.; Tsang, M.; Wang, A.; Hui, G. Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. FASEB J. 2013, 27, 1153–1166. [Google Scholar] [CrossRef]
- Richards, R.L.; Rao, M.; Wassef, N.M.; Glenn, G.M.; Rothwell, S.W.; Alving, C.R. Liposomes containing lipid a serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS, S malaria antigen. Infect. Immun. 1998, 66, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Paolicelli, P.; Diaz, B.; Vicente, S.; Sanchez, A.; Gonzalez-Fernandez, A.; Jose Alonso, M. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine 2010, 28, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ackerman, A.L.; Cody, V.; Giodini, A.; Hinson, E.R.; Cresswell, P.; Edelson, R.L.; Saltzman, W.M.; Hanlon, D.J. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 2006, 117, 78–88. [Google Scholar] [CrossRef]
- Diwan, M.; Tafaghodi, M.; Samuel, J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J. Control. Release 2002, 85, 247–262. [Google Scholar] [CrossRef]
- Mintern, J.D.; Percival, C.; Kamphuis, M.M.J.; Chin, W.J.; Caruso, F.; Johnston, A.P.R. Targeting dendritic cells: The role of specific receptors in the internalization of polymer capsules. Adv. Healthc. Mater. 2013, 2, 940–944. [Google Scholar] [CrossRef]
- Wang, X.; Uto, T.; Akagi, T.; Akashi, M.; Baba, M. Induction of potent CD8+ T-Cell responses by novel biodegradable nanoparticles carrying human immunodeficiency virus type 1 gp120. J. Virol. 2007, 81, 10009–10016. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Aley, S.B.; Zimmerman, M.; Hetsko, M.; Selsted, M.E.; Gillin, F.D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 1994, 62, 5397–5403. [Google Scholar] [CrossRef] [PubMed]
- Turchany, J.M.; Aley, S.B.; Gillin, F.D. Giardicidal activity of lactoferrin and N-terminal peptides. Infect. Immun. 1995, 63, 4550–4552. [Google Scholar] [CrossRef]
- Eckmann, L.; Laurent, F.; Langford, T.D.; Hetsko, M.L.; Smith, J.R.; Kagnoff, M.F.; Gillin, F.D. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 2000, 164, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Rockett, K.A. Nitric oxide and parasitic disease. Adv. Parasitol. 1996, 37, 1. [Google Scholar] [PubMed]
- Alican, I.; Kubes, P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am. J. Physiol. 1996, 270, G225. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.L.; Allen, C.L.; Stevens, D.P. Phagocytosis of Giardia muris by macrophages in Peyer’s patch epithelium in mice. Infect. Immun. 1981, 33, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.R.; Pearson, R.D. Ingestion of Giardia lamblia trophozoites by human mononuclear phagocytes. Infect. Immun. 1987, 55, 3155–3161. [Google Scholar] [CrossRef]
- McDermott, J.R.; Bartram, R.E.; Knight, P.A.; Miller, H.R.; Garrod, D.R.; Grencis, R.K. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 2003, 100, 7761–7766. [Google Scholar] [CrossRef]
- Li, E.; Zhou, P.; Petrin, Z.; Singer, S.M. Mast cell-dependent control of Giardia lamblia infections in mice. Infect. Immun. 2004, 72, 6642–6649. [Google Scholar] [CrossRef]
- Li, E.; Zhao, A.; Shea-Donohue, T.; Singer, S.M. Mast cell mediated changes in smooth muscle contractility during mouse giardiasis. Infect. Immun. 2007, 75, 4514–4518. [Google Scholar] [CrossRef]
- Singer, S.M. Control of giardiasis by interleukin-17 in humans and mice are the questions all answered? Clin. Vaccine Immunol. 2015, 23, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Casanova, J.L.; Puel, A. Mucocutaneous IL-17 immunity in mice and humans: Host defense vs. excessive inflammation. Mucosal. Immunol. 2018, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Solaymani-Mohammadi, S.; Singer, S.M. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J. Immunol. 2011, 187, 3769–3775. [Google Scholar] [CrossRef]
- Dreesen, L.; De Bosscher, K.; Grit, G.; Staels, B.; Lubberts, E.; Bauge, E.; Geldhof, P. Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infect Immun. 2014, 82, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.M.; Manthey, C.F.; Le, C.; Miyamoto, Y.; Gima, L.; Abrahim, A.; Cao, A.T.; Hanson, E.M.; Kolls, J.K.; Raz, E.; et al. IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp. Parasitol. 2015, 156, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, E.A.; Lee, K.J.; Lee, K.H.; Park, S.J. Increased innate lymphoid cell 3 and IL-17 production in mouse lamina propria stimulated with Giardia lamblia. Korean J. Parasitol. 2019, 57, 225–232. [Google Scholar] [CrossRef]
- Saghaug, C.S.; Sørnes, S.; Peirasmaki, D.; Svärd, S.; Langeland, N.; Hanevik, K. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin. Vaccine Immunol. 2016, 23, 11–18. [Google Scholar] [CrossRef]
- Rodríguez, O.L.; Hagel, I.; González, Y.; Roque, M.E.; Vásquez, N.; López, E.; Di Prisco, M.C. Secretory IgA antibody responses in Venezuelan children infected with Giardia duodenalis. J. Trop. Pediatr. 2004, 50, 68–72. [Google Scholar] [CrossRef][Green Version]
- Jiménez, J.C.; Fontaine, J.; Creusy, C.; Fleurisse, L.; Grzych, J.M.; Capron, M.; Dei-Cas, E. Antibody and cytokine responses to Giardia excretory/secretory proteins in Giardia intestinalis-infected BALB/c mice. Parasitol. Res. 2014, 113, 2709–2718. [Google Scholar] [CrossRef]
- Hjøllo, T.; Bratland, E.; Steinsland, H.; Radunovic, M.; Langeland, N.; Hanevik, K. Longitudinal cohort study of serum antibody responses towards Giardia lamblia variant-specific surface proteins in a non-endemic area. Exp. Parasitol. 2018, 191, 66–72. [Google Scholar] [CrossRef]
| Type of Drug Delivery System | Nanoparticle Material | Size | Antigen (Pathogen) | Ref. |
|---|---|---|---|---|
| Inorganic (non-degradable) | Iron silica | 20–300 nm | MSP1 (Plasmodium falciparum) BSA | [69] [70] |
| Liposome (non-viral lipids particle) | Cholesterol lipid lipid | 200 nm | Polysaccharides (Streptococcus pneumoniae serotype 14) VMP001 (Plasmodium vivax) RTS,S/AS01B (Plasmodium falciparum CSP + hepatitis B protein hybrid) | [66] [67] [71] |
| Polymeric | Chitosan PLGA PLGA PVPONAlk γ-PGA PLGA | 160–1000 nm | Hepatitis B Ovalbumin Tetanus toxoid Ovalbumin gp120 (HIV-1) Membrane vesicles (G. intestinalis WB ATCC 50803 and G. intestinalis GS/M ATCC 50581) | [72] [73] [74] [75] [76] [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangkanu, S.; Paul, A.K.; Chuprom, J.; Mitsuwan, W.; Boonhok, R.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Wilairatana, P.; Rahmatullah, M.; Wiart, C.; et al. Conserved Candidate Antigens and Nanoparticles to Develop Vaccine against Giardia intestinalis. Vaccines 2023, 11, 96. https://doi.org/10.3390/vaccines11010096
Sangkanu S, Paul AK, Chuprom J, Mitsuwan W, Boonhok R, de Lourdes Pereira M, Oliveira SMR, Wilairatana P, Rahmatullah M, Wiart C, et al. Conserved Candidate Antigens and Nanoparticles to Develop Vaccine against Giardia intestinalis. Vaccines. 2023; 11(1):96. https://doi.org/10.3390/vaccines11010096
Chicago/Turabian StyleSangkanu, Suthinee, Alok K. Paul, Julalak Chuprom, Watcharapong Mitsuwan, Rachasak Boonhok, Maria de Lourdes Pereira, Sonia Marlene Rodrigues Oliveira, Polrat Wilairatana, Mohammed Rahmatullah, Christophe Wiart, and et al. 2023. "Conserved Candidate Antigens and Nanoparticles to Develop Vaccine against Giardia intestinalis" Vaccines 11, no. 1: 96. https://doi.org/10.3390/vaccines11010096
APA StyleSangkanu, S., Paul, A. K., Chuprom, J., Mitsuwan, W., Boonhok, R., de Lourdes Pereira, M., Oliveira, S. M. R., Wilairatana, P., Rahmatullah, M., Wiart, C., Nawaz, M., Sin, C., Kayesth, S., & Nissapatorn, V. (2023). Conserved Candidate Antigens and Nanoparticles to Develop Vaccine against Giardia intestinalis. Vaccines, 11(1), 96. https://doi.org/10.3390/vaccines11010096












