Impact of Improper Storage of ChAdOx1-S (AstraZeneca) Vaccine on Its Efficacy and Safety
Abstract
1. Introduction
2. Patients and Methods
2.1. Questionnaires
2.2. Measurement of Antibodies against SARS-CoV-2 Phosphorylated Nucleocapsid Protein
2.3. Measurement of Antibodies against SARS-CoV-2 Subunit S1 Spike Protein
2.4. Statistics
3. Results
3.1. Characteristics of the Samples
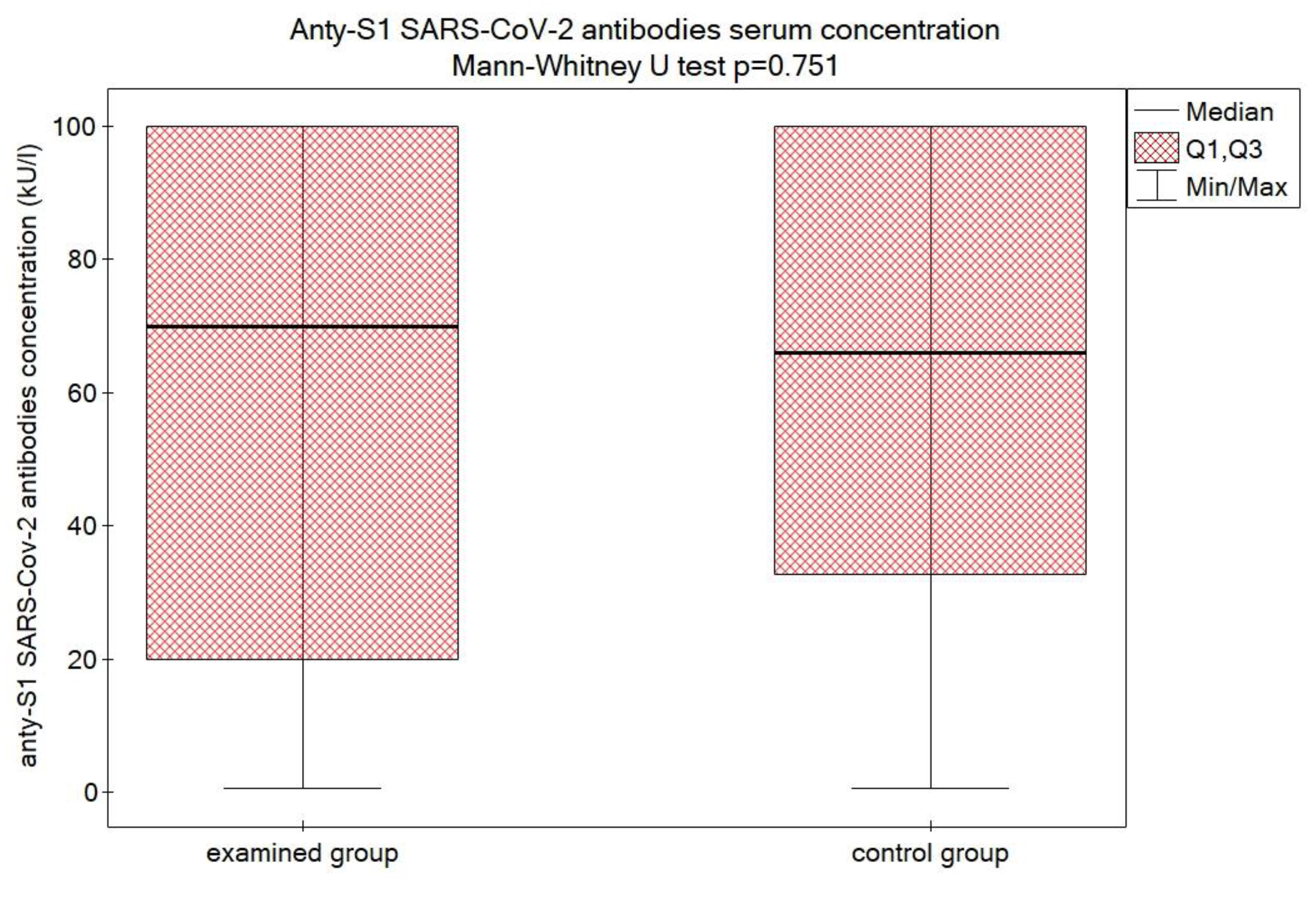
3.2. Efficacy
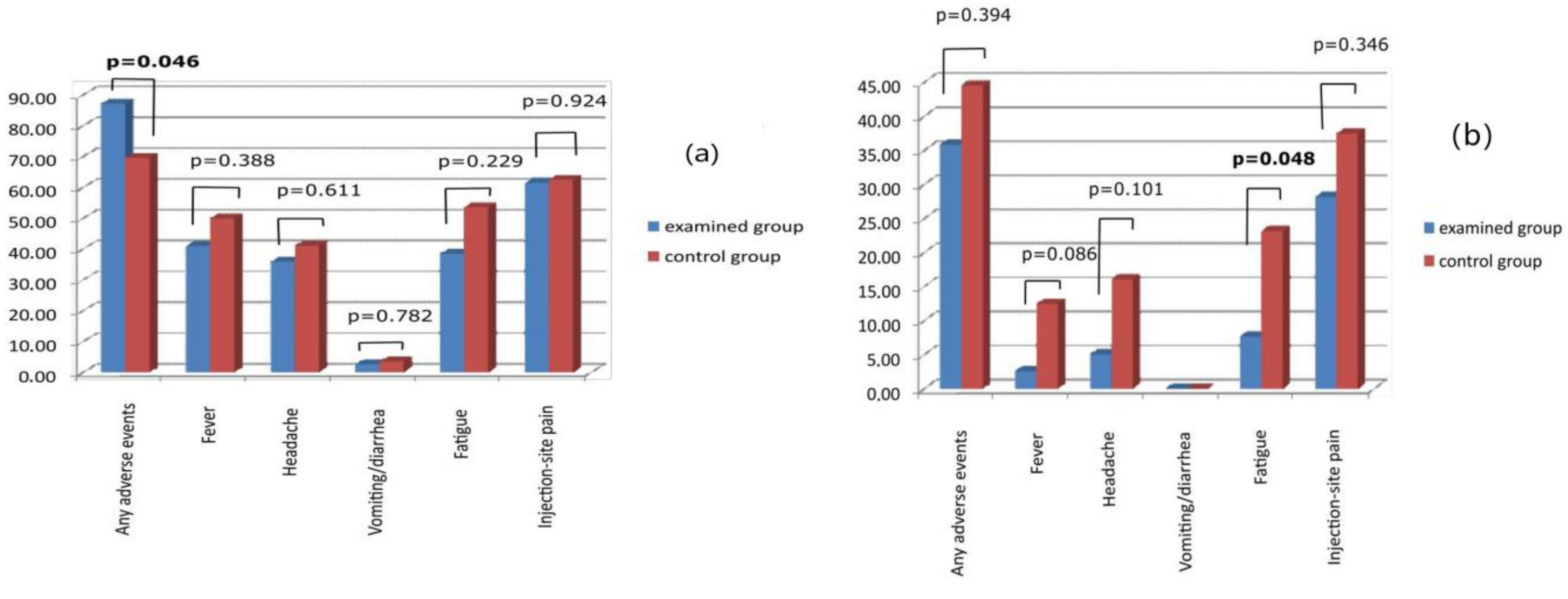
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1577–1578. [Google Scholar] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 Vaccine Efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 680–684. [Google Scholar]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side Effects and Perceptions Following Sinopharm COVID-19 Vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Pambudi, N.A.; Sarifudin, A.; Gandidi, I.M.; Romadhon, R. Vaccine Cold Chain Management and Cold Storage Technology to Address the Challenges of Vaccination Programs. Energy Rep. 2022, 8, 955–972. [Google Scholar] [CrossRef]
- Omole, T.M.; Sanni, F.O.; Olaiya, P.A.; Aturaka, O.; Abdulsalam, M.; Gwa, Z.T.; Ajani, O.F.; Aiden, P.J.; Njemanze, C.G. The Challenges of Nigeria Vaccine Supply Chain, a Community of Practice Perspective. Int. J. Res. Sci. Innov. 2019, 6, 151–157. [Google Scholar]
- Lee, B.Y.; Haidari, L.A. The Importance of Vaccine Supply Chains to Everyone in the Vaccine World. Vaccine 2017, 35, 4475–4479. [Google Scholar] [CrossRef]
- Hanson, C.M.; George, A.M.; Sawadogo, A.; Schreiber, B. Is Freezing in the Vaccine Cold Chain an Ongoing Issue? A Literature Review. Vaccine 2017, 35, 2127–2133. [Google Scholar] [CrossRef]
- Toback, S.L.; Susla, G.M.; Darling, A.J.; Ambrose, C.S. Clinical Guidance on the Use of Live Attenuated Influenza Vaccine After Inadvertent Freezing and Warming. J. Pediatr. Nurs. 2012, 27, 163–167. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of MRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Holm, M.R.; Poland, G.A. Critical Aspects of Packaging, Storage, Preparation, and Administration of MRNA and Adenovirus-Vectored COVID-19 Vaccines for Optimal Efficacy. Vaccine 2021, 39, 457–459. [Google Scholar] [CrossRef]
- Kumru, O.S.; Joshi, S.B.; Smith, D.E.; Middaugh, C.R.; Prusik, T.; Volkin, D.B. Vaccine Instability in the Cold Chain: Mechanisms, Analysis and Formulation Strategies. Biologicals 2014, 42, 237–259. [Google Scholar] [CrossRef]
- AboulFotouh, K.; Cui, Z.; Williams, R.O. Next-Generation COVID-19 Vaccines Should Take Efficiency of Distribution into Consideration. AAPS PharmSciTech 2021, 22, 126. [Google Scholar] [CrossRef]
- Dulal, P.; Wright, D.; Ashfield, R.; Hill, A.V.S.; Charleston, B.; Warimwe, G.M. Potency of a Thermostabilised Chimpanzee Adenovirus Rift Valley Fever Vaccine in Cattle. Vaccine 2016, 34, 2296–2298. [Google Scholar] [CrossRef]
- Witberg, G.; Magen, O.; Hoss, S.; Talmor-Barkan, Y.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Shiyovich, A.; Schamroth-Pravda, N.; et al. Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. N. Engl. J. Med. 2022, 387, 1816–1817. [Google Scholar] [CrossRef]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious Adverse Events of Special Interest Following MRNA COVID-19 Vaccination in Randomized Trials in Adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 NCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 NCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.-D.; et al. Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding after Vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population Based Cohort Study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of Thrombocytopenia and Thromboembolism after COVID-19 Vaccination and SARS-CoV-2 Positive Testing: Self-Controlled Case Series Study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef] [PubMed]
- Introna, A.; Caputo, F.; Santoro, C.; Guerra, T.; Ucci, M.; Mezzapesa, D.M.; Trojano, M. Guillain-Barré Syndrome after AstraZeneca COVID-19-Vaccination: A Causal or Casual Association? Clin. Neurol. Neurosurg. 2021, 208, 106887. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.G.; Ju, W.; Ha, Y.-E.; Ban, J.-J.; Lee, S.A.; Sung, J.-J.; Shin, J.-Y. Sensory Guillain-Barre Syndrome Following the ChAdOx1 NCov-19 Vaccine: Report of Two Cases and Review of Literature. J. Neuroimmunol. 2021, 359, 577691. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 NCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 NCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 Trial of SARS-CoV-2 Vaccine ChAdOx1 NCoV-19 with a Booster Dose Induces Multifunctional Antibody Responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef]
| Variables | Examined Group | Control Group | p-Value | |
|---|---|---|---|---|
| Sample Size | 39 | 56 | ||
| Gender | Male (%) | 23 (55%) | 31 (59%) | 0.726 |
| Female (%) | 16 (45%) | 25 (41%) | ||
| Age (years, mean ± SD) | 44.38 ± 18.10 | 45.77 ± 17.32 | 0.706 | |
| Chronic diseases | 12 | 22 | 0.394 | |
| Confirmed COVID-19 in the past | 10 | 12 | 0.632 | |
| Anti-N antibodies concentration (kU/L) (median (25; 75 pc)) | 0.54 (0.3; 1.65) | 0.33 (0.27; 0.91) | 0.078 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikołajczyk, M.; Lewandowski, R.A.; Goncharuk, A.G. Impact of Improper Storage of ChAdOx1-S (AstraZeneca) Vaccine on Its Efficacy and Safety. Vaccines 2023, 11, 93. https://doi.org/10.3390/vaccines11010093
Mikołajczyk M, Lewandowski RA, Goncharuk AG. Impact of Improper Storage of ChAdOx1-S (AstraZeneca) Vaccine on Its Efficacy and Safety. Vaccines. 2023; 11(1):93. https://doi.org/10.3390/vaccines11010093
Chicago/Turabian StyleMikołajczyk, Marek, Roman A. Lewandowski, and Anatoliy G. Goncharuk. 2023. "Impact of Improper Storage of ChAdOx1-S (AstraZeneca) Vaccine on Its Efficacy and Safety" Vaccines 11, no. 1: 93. https://doi.org/10.3390/vaccines11010093
APA StyleMikołajczyk, M., Lewandowski, R. A., & Goncharuk, A. G. (2023). Impact of Improper Storage of ChAdOx1-S (AstraZeneca) Vaccine on Its Efficacy and Safety. Vaccines, 11(1), 93. https://doi.org/10.3390/vaccines11010093







