Abstract
We aimed to explore the influence of comorbid asthma on the risk for mortality among patients with coronavirus disease 2019 (COVID-19) in Asia by using a meta-analysis. Electronic databases were systematically searched for eligible studies. The pooled odds ratio (OR) with 95% confidence interval (CI) was estimated by using a random-effect model. An inconsistency index (I2) was utilized to assess the statistical heterogeneity. A total of 103 eligible studies with 198,078 COVID-19 patients were enrolled in the meta-analysis; our results demonstrated that comorbid asthma was significantly related to an increased risk for COVID-19 mortality in Asia (pooled OR = 1.42, 95% CI: 1.20–1.68; I2 = 70%, p < 0.01). Subgroup analyses by the proportion of males, setting, and sample sizes generated consistent findings. Meta-regression indicated that male proportion might be the possible sources of heterogeneity. A sensitivity analysis exhibited the reliability and stability of the overall results. Both Begg’s analysis (p = 0.835) and Egger’s analysis (p = 0.847) revealed that publication bias might not exist. In conclusion, COVID-19 patients with comorbid asthma might bear a higher risk for mortality in Asia, at least among non-elderly individuals.
1. Introduction
Coronavirus disease 2019 (COVID-19), which is brought on by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has ravaged the world. As of 26 October 2022, 624 million patients have been confirmed with the COVID-19 diagnosis globally of which 6.5 million patients have died [1]. Vaccines have shown to be very effective against severe COVID-19 disease and mortality [2,3,4,5,6,7]; it is also important to understand risk factors (e.g., to decide whom to prioritize for vaccination). Until now, several variables (age, sex, and certain past medical history) have been identified as risk factors for COVID-19 mortality [8,9,10,11,12,13]. Although there have been several meta-analyses exploring the relationship of comorbid asthma with the risk for COVID-19 mortality in the full regions [14,15,16,17,18], the findings from previous meta-analyses were still inconclusive, which might suffer limitations from considerable variability in the prevalence of asthma across different regions [14,19,20]. Therefore, it is an urgent requirement to investigate the relationship of comorbid asthma with the risk for COVID-19 mortality based on specific regions.
To the best of our knowledge, three meta-analyses have explored this relationship in Asia [15,18,21]. However, the number of included studies (all are less than twelve) and the sample sizes are limited. Moreover, the conclusions drawn from these articles are inconsistent or even contradictory. Additionally, a substantial number of articles on this topic in Asia have emerged since then. Taken together, we conducted this updated meta-analysis to ascertain the relationship between comorbid asthma and COVID-19 mortality in Asia on the basis of the latest data.
2. Methods
2.1. Search Strategy and Literature Management
This quantitative meta-analysis was performed according to the statement of PRISMA (preferred reporting items for systematic reviews and meta-analyses). A systematic literature search was undertaken among electronic databases containing PubMed, Scopus, EMBASE, Springer, Web of Science, and Wiley to recognize eligible studies from inception to 22 October 2022. Searching strategies were as follows: (“COVID-19” OR “coronavirus disease 2019” OR “SARS-CoV-2” OR “2019-nCoV” OR “novel coronavirus”) and (“asthma” OR “bronchial asthma”) and (“mortality” OR “non-survivor” OR “fatality” OR “deceased” OR “death”). Additionally, to achieve extensive searches, relevant references of included studies and reviews were also taken into consideration.
2.2. Selection Criteria
Studies were selected if they were amenable to the following criteria: (1) Adult COVID-19 patients should be diagnosed in line with the World Health Organization (WHO) guidance. (2) Studies were conducted in Asia and explicitly reported the number of COVID-19 patients with comorbid asthma and outcome of interest (alive or dead) or the effect size with 95% confidence interval (CI) concerning the relationship of asthma with COVID-19 mortality. (3) Articles should be written in English. Studies based on criteria as follows must be cast off accordingly: (1) preprints, comments, errata, reviews, and repeated articles. (2) articles without available data concerning the incidence of asthma and death among patients with COVID-19 in Asia.
2.3. Data Extraction
Two researchers respectively inspected all the literature depending on the criteria of inclusion and exclusion and then extracted the relevant information, including author, male proportion, country, cases, study design, setting, mean age with standard deviation or median age with interquartile range, incidence of non-survivors and survivors among patients with COVID-19 and comorbid asthma and those without, or the effect size with corresponding 95% CI. If two or more publications are sourced with the same author or the same institute, we then reviewed the time period of participant enrollment among the studies. If the time period of participant enrollment was the same or the study start and end times were crossed among the studies, we regarded these studies as having the same participants or overlapping participants; otherwise, we regarded these studies as different. For these studies based on the same data source, we included only the articles with the most complete data. If there was any disagreement, it was settled through a third investigator or by discussion to reach a consensus.
2.4. Statistical Analysis
All the statistical analyses were implemented on STATA (Version 16) and R software (Version 4.2.1) with attached “meta” package (Version 5.5-0). Pooled OR and 95% CI were computed by a random-effect model to describe the relationship of asthma with COVID-19 mortality in Asia. Two tailed p-value less than 0.05 was considered as statistical significance. An inconsistency index (I2) was applied to evaluate the statistical heterogeneity among studies [22]. Meta-regression and subgroup analyses were undertaken to find possible sources of heterogeneity. To test the stability of our study, a sensitivity analysis by omitting one single study at a time was carried out. Both Begg’s analysis and Egger’s analysis were implemented to test the potential publication bias [23,24].
3. Results
3.1. Study Selection
Online literature searches yielded 22,361 citations from electronic databases, and an additional 50 records were found from the references of cited lists. After removing 19,168 duplicates, 3243 articles were initially identified. Next, 2943 articles were excluded after reading the abstracts. After that, 300 articles were evaluated for full-text eligibility, and 197 articles with available data but not Asian were excluded. Ultimately, 103 studies conducted in Asia were enrolled in this meta-analysis [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127]. The flow chart of the literature search and selection process is illustrated in Figure 1.
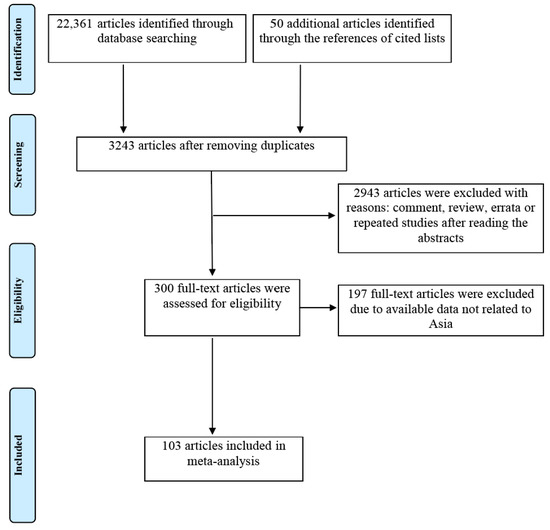
Figure 1.
Flow chart of study search and selection process.
3.2. Descriptive Characteristics
Summary characteristics of the enrolled studies are tabulated in Table 1. This meta-analysis was based on a total of 103 eligible studies with 198,078 COVID-19 patients. In terms of study design, there were eighty-five retrospective studies, ten prospective studies, six cross-sectional studies, one case series study, and one clinical trial study. From the point of view of geographical settings, we characterized the country’s levels of social and economic development based on the 4-tier Human Development Index (HDI) from the United Nation’s 2022 Human Development Report. Additionally, countries were categorized as very high HDI country (Korea, Singapore, Israel, Japan, Turkey, Kuwait, Saudi Arabia, and United Arab Emirates), high HDI country (China, Iran, and Indonesia), medium HDI country (India, Philippines, and Bangladesh), and low HDI country (Pakistan). Among these studies, ninety-eight studies reported the exact numbers of non-survivors and survivors of COVID-19 patients with asthma, while five studies reported OR with 95% CI to reflect the effect of comorbid asthma on Asian COVID-19 mortality.

Table 1.
Main characteristics of the included studies.
3.3. Asthma and COVID-19 Mortality in Asia
Overall, combining the data from 103 studies, our meta-analysis indicated there was a significant association between comorbid asthma and increased risk for mortality of COVID-19 patients (pooled OR = 1.42, 95% CI: 1.20–1.68; I2 = 70%, p < 0.01, Figure 2). Consistent results were observed in the subgroup analyses stratified by sample sizes (pooled OR = 1.41, 95% CI: 1.10–1.82 for <1000 cases and 1.43, 95% CI: 1.14–1.79 for ≥1000 cases), setting (pooled OR = 1.37, 95% CI: 1.14–1.64 for hospitalized patients and 1.91, 95% CI: 1.36–2.68 for all patients) and male proportion (pooled OR = 2.08, 95% CI: 1.78–2.44 for <50% and 1.24, 95% CI: 1.00–1.55 for ≥50%). When the subgroup analysis was performed by age, the significant relationship existed in the subgroup of mean/median age <60 years old (pooled OR = 1.44, 95% CI: 1.18–1.76) but did not exist in the subgroup of mean/median age ≥60 years old (pooled OR = 1.36, 95% CI: 0.95–1.94). The significant association existed among studies with the prevalence of obesity ≥20% (pooled OR = 2.27, 95% CI: 1.65–3.12) but did not exist among studies with the prevalence of obesity <20% (pooled OR = 1.21, 95% CI: 0.60–2.46). The subgroup analysis according to ICU and non-ICU patients suggested COVID-19 patients with asthma had a significantly increased risk of mortality among studies with non-ICU patients (pooled OR = 1.45, 95% CI: 1.22–1.72) but not among studies with ICU patients (pooled OR = 1.40, 95% CI: 0.58–3.38). A further subgroup analysis by country characterized by homogenous socioeconomic features revealed COVID-19 patients with asthma had a significantly increased risk for mortality compared with patients without asthma among very high HDI countries (pooled OR = 1.55, 95% CI: 1.29–1.87) but not among high HDI countries (pooled OR = 1.38, 95% CI: 0.91–2.11), medium HDI countries (pooled OR = 0.88, 95% CI: 0.50–1.56), and low HDI countries (pooled OR = 1.60, 95% CI: 0.77–3.33) (as shown in Figure 2). The meta-regression displayed male proportion (p = 0.034) might be the potential sources of heterogeneity, while country (p = 0.301), sample sizes (p = 0.966), setting (p = 0.254), age (p = 0.961), and obesity (p = 0.103) might not.
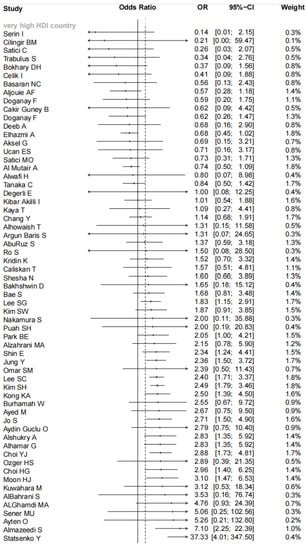
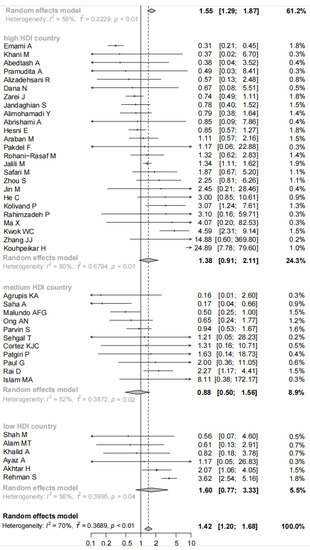
Figure 2.
Forest plot indicated there was a significant association between comorbid asthma and the increased risk for mortality of COVID-19 patients in Asia.
Considering comorbidities could lead to additional ”noise” and measurement error, we subsequently calculated the pooled OR on the basis of adjusted effect estimates. The results indicated asthma was significantly associated with the increased risk of mortality among Asian COVID-19 patients on the basis of 19 studies (adjusted OR = 1.22, 95% CI: 1.05–1.42), which supported the findings based on crude effects.
3.4. Sensitivity Analysis and Publication Bias
The sensitivity analysis showed the effect estimate was not unduly impacted by any single study, indicating the stability and robustness of our results. Begg’s analysis (p = 0.835, Figure 3A) and Egger’s analysis (p = 0.847, Figure 3B) demonstrated publication bias might not exist in this study.
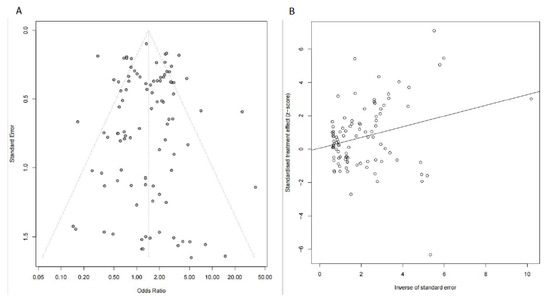
Figure 3.
Publication bias was evaluated by Begg’s analysis (A) and Egger’s analysis (B).
4. Discussion
Our findings based on 103 eligible studies indicated comorbid asthma was significantly associated with an increased risk for mortality in COVID-19 patients compared with those without in Asia. The subgroup analyses by male proportion, sample sizes, and setting yielded consistent results, but the subgroup analysis by age indicated comorbid asthma was significantly associated with higher risk for COVID-19 mortality in Asia among studies with mean/median age <60 years old and not among studies with mean/median age ≥60 years old. Previous studies have shown advanced age and asthmatic patients are prone to other comorbidities, such as hypertension and diabetes mellitus, which are closely related to the severity and mortality of COVID-19 patients [128]. We also investigated the proportion of hypertension and diabetes mellitus in enrolled studies among age <60 years old and ≥60 years old and found the proportions of hypertension and diabetes mellitus were relatively higher in groups ≥60 (44.39% and 30.64%, respectively) than in groups <60 years old (32.03% and 25.8%, respectively). Thus, we speculated the existence of other comorbidities (such as hypertension and diabetes mellitus) might mask the relationship between asthma and COVID-19 mortality.
Asthma is a heterogeneous disease, and some phenotypes are related to obesity, which has continued to attract respiratory experts’ attention since the pandemic of COVID-19 [129]. Furthermore, high body mass index has been identified as a risk factor for COVID-19 mortality. In our subgroup analyses stratified according to the proportion of males, the odds ratio increased to 2.08 in the group with males being less than 50% and was reduced to 1.24 in the subgroup where males dominated, while Wenzel et al. showed females usually dominate in obesity-related asthma [129]. This suggested part of the conflicting results on asthma and COVID-19 mortality could be due to the differences in handling of obesity in different studies. Our further analysis stratified by obesity prevalence supported this opinion. The subgroup results regarding ICU versus non-ICU deaths may be explained by the facts that ICU patients may rely either on a better economic status (theirs or for their countries) than non-ICU patients (whose access to intensive care may be impaired by economic status).
At present, research has explored the potential mechanism of the association between COVID-19 and asthma from the standpoints of pathophysiology. Viral infections, including SARS-CoV-2 and Middle East respiratory syndrome coronavirus (MERS) could directly result in the exacerbation of asthma and thus lead to serious airway inflammation, which might be linked to critical unfavorable outcomes [130,131]. Additionally, several researchers proposed host antiviral immunity was decreased due to asthma associated type II inflammatory response [132,133]. In addition, interferon responses as a crucial step of antiviral immune reaction were shown to be lacking in asthmatic patients attributed to decreased production [134,135]. Additionally, asthma resulted in mucus plugging in the lower respiratory tract, limiting the airflow and worsening the hypoxemia from diffuse alveolar damage by SARS-CoV-2 infection [136]. However, these theoretical relationships remain to be observed. Further studies focusing on the molecular mechanisms underlying the association between comorbid asthma and the increased risk for COVID-19 mortality are warranted to verify our results.
The strengths of this study were the number of eligible studies included (103 eligible studies), and the sample sizes (198,078 cases) were large, and subgroup analyses were conducted. However, several limitations need to be acknowledged in this study. First, although the investigators tried to avoid duplicates in the process of article selection, several studies collected clinical records of COVID-19 patients retrospectively from large national public databases. This inevitably led to repeated populations and selective bias. Second, most of the included articles were observational retrospective studies, which resulted in lack of proof for casual links between asthma and mortality risk of COVID-19. Third, the medical history of enrolled patients could not be clearly analyzed, especially for the use of theophylline, inhaled corticosteroids, leukotriene receptor antagonists, and other drugs. Length of in-hospital treatment, the severity of asthma, types of asthma, and other comorbidities could not be extracted either, which should be a focus in the future study. Fourth, the existence or history of other comorbidities, such as coronary artery disease, chronic obstructive pulmonary disease, and so on was not addressed presently, which still restricted the generalization of our findings.
5. Conclusions
Our findings demonstrated comorbid asthma significantly increased the risk for mortality among patients with COVID-19 in Asia, at least among non-elderly individuals.
Author Contributions
F.L. and H.Y. conceptualized the study. L.S., J.R. and H.F. performed the literature search. L.S., J.R. and Y.W. performed data extraction. L.S. and J.R. analyzed the data. L.S. and J.R. wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from Henan Young and Middle-aged Health Science and Technology Innovation Talent Project (No. YXKC2021021). The funder has no role in the data collection, data analysis, preparation of manuscript and decision to submission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are included in this article and are available from the corresponding authors upon reasonable requests.
Conflicts of Interest
The authors declare they have no potential conflicts of interest regarding this submitted manuscript.
References
- World Health Organization. COVID-19 Weekly Epidemiological Update. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---26-october-2022 (accessed on 26 October 2022).
- Sabu, J.M.; Zahid, I.; Jacob, N.; Alele, F.O.; Malau-Aduli, B.S. Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1880. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Purja, S.; Shin, H.; Kim, M.S.; Park, S.; Kronbichler, A.; Smith, L.; Eisenhut, M.; Shin, J.I.; Kim, E. Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis. Vaccines 2022, 10, 1572. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, R.; Yuan, Z.; Xu, Z.; Suo, L.; Wang, Q.; Li, Y.; Gao, Y.; Li, X.; Chen, X.; et al. Protective Effect of Inactivated COVID-19 Vaccines against Progression of SARS-CoV-2 Omicron and Delta Variant Infections to Pneumonia in Beijing, China, in 2022. Vaccines 2022, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zheng, D.; Yu, B.; Tan, X.; Chen, Q.; Wang, L.; Zhang, J.; Liu, Y.; Weng, H.; Cai, Y.; et al. Effectiveness of Inactivated COVID-19 Vaccines against COVID-19 Caused by the SARS-CoV-2 Delta and Omicron Variants: A Retrospective Cohort Study. Vaccines 2022, 10, 1753. [Google Scholar] [CrossRef] [PubMed]
- Hardgrave, H.; Wells, A.; Nigh, J.; Klutts, G.; Krinock, D.; Osborn, T.; Bhusal, S.; Rude, M.K.; Burdine, L.; Giorgakis, E. COVID-19 Mortality in Vaccinated vs. Unvaccinated Liver & Kidney Transplant Recipients: A Single-Center United States Propensity Score Matching Study on Historical Data. Vaccines 2022, 10, 1921. [Google Scholar]
- Arbel, R.; Pliskin, J. Vaccinations versus Lockdowns to Prevent COVID-19 Mortality. Vaccines 2022, 10, 1347. [Google Scholar] [CrossRef]
- Nasiri, M.J.; Haddadi, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Hasanzadeh, S.; Jamshidi, P.; Murthi, M.; Mirsaeidi, M. COVID-19 Clinical Characteristics, and Sex-Specific Risk of Mortality: Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 459. [Google Scholar] [CrossRef]
- Yang, H.; Xu, J.; Liang, X.; Shi, L.; Wang, Y. Autoimmune diseases are independently associated with COVID-19 severity: Evidence based on adjusted effect estimates. J. Infect. 2021, 82, e23–e26. [Google Scholar] [CrossRef]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Castagna, F.; Kataria, R.; Madan, S.; Ali, S.Z.; Diab, K.; Leyton, C.; Arfaras-Melainis, A.; Kim, P.; Giorgi, F.M.; Vukelic, S.; et al. A History of Heart Failure Is an Independent Risk Factor for Death in Patients Admitted with Coronavirus 19 Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 77. [Google Scholar] [CrossRef]
- Bajči, M.P.; Lendak, D.F.; Ristić, M.; Drljača, M.M.; Brkić, S.; Turkulov, V.; Petrović, V. COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview. Vaccines 2022, 10, 1818. [Google Scholar]
- Nasrullah, A.; Gangu, K.; Shumway, N.B.; Cannon, H.R.; Garg, I.; Shuja, H.; Bobba, A.; Chourasia, P.; Sheikh, A.B.; Shekhar, R. COVID-19 and Pulmonary Embolism Outcomes among Hospitalized Patients in the United States: A Propensity-Matched Analysis of National Inpatient Sample. Vaccines 2022, 10, 2104. [Google Scholar] [CrossRef]
- Terry, P.D.; Heidel, R.E.; Dhand, R. Asthma in Adult Patients with COVID-19. Prevalence and Risk of Severe Disease. Am. J. Respir. Crit. Care Med. 2021, 203, 893–905. [Google Scholar] [CrossRef]
- Hou, H.; Xu, J.; Li, Y.; Wang, Y.; Yang, H. The Association of Asthma With COVID-19 Mortality: An Updated Meta-Analysis Based on Adjusted Effect Estimates. J. Allergy Clin. Immunol. Pract. 2021, 9, 3944–3968.e5. [Google Scholar] [CrossRef]
- Hussein, M.H.; Elshazli, R.M.; Attia, A.S.; Nguyen, T.P.; Aboueisha, M.; Munshi, R.; Toraih, E.A.; Fawzy, M.S.; Kandil, E. Asthma and COVID-19; different entities, same outcome: A meta-analysis of 107,983 patients. J. Asthma 2021, 59, 851–858. [Google Scholar] [CrossRef]
- Sitek, A.N.; Ade, J.M.; Chiarella, S.E.; Divekar, R.D.; Pitlick, M.M.; Iyer, V.N.; Wang, Z.; Joshi, A.Y. Outcomes among patients with COVID-19 and asthma: A systematic review and meta-analysis. Allergy Asthma Proc. 2021, 42, 267–273. [Google Scholar] [CrossRef]
- Sunjaya, A.P.; Allida, S.M.; Di Tanna, G.L.; Jenkins, C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: Systematic review and meta-analysis. J. Asthma 2021, 59, 866–879. [Google Scholar] [CrossRef]
- Liu, S.; Cao, Y.; Du, T.; Zhi, Y. Prevalence of Comorbid Asthma and Related Outcomes in COVID-19: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2021, 9, 693–701. [Google Scholar] [CrossRef]
- Shi, L.; Xu, J.; Xiao, W.; Wang, Y.; Jin, Y.; Chen, S.; Duan, G.; Yang, H.; Wang, Y. Asthma in patients with coronavirus disease 2019: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2021, 126, 524–534. [Google Scholar] [CrossRef]
- Reyes, F.M.; Hache-Marliere, M.; Karamanis, D.; Berto, C.G.; Estrada, R.; Langston, M.; Ntaios, G.; Gulani, P.; Shah, C.D.; Palaiodimos, L. Assessment of the Association of COPD and Asthma with In-Hospital Mortality in Patients with COVID-19. A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. J. Clin. Med. 2021, 10, 2087. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Abedtash, A.; Taherkhani, M.; Shokrishakib, S.; Nikpour, S.; Taherkhani, A. Association between Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers and Mortality in Patients with Hypertension Hospitalized with COVID-19. J. Tehran Heart Cent. 2021, 16, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Eslami, V.; Arab-Ahmadi, M.; Alahyari, S.; Azhideh, A.; Sanei-Taheri, M. Prognostic value of inflammatory biomarkers for predicting the extent of lung involvement and final clinical outcome in patients with COVID-19. J. Res. Med. Sci. 2021, 26, 115. [Google Scholar] [PubMed]
- AbuRuz, S.; Al-Azayzih, A.; ZainAlAbdin, S.; Beiram, R.; Al Hajjar, M. Clinical characteristics and risk factors for mortality among COVID-19 hospitalized patients in UAE: Does ethnic origin have an impact. PLoS ONE 2022, 17, e0264547. [Google Scholar] [CrossRef]
- Agrupis, K.A.; Smith, C.; Suzuki, S.; Villanueva, A.M.; Ariyoshi, K.; Solante, R.; Telan, E.F.; Estrada, K.A.; Uichanco, A.C.; Sagurit, J.; et al. Epidemiological and clinical characteristics of the first 500 confirmed COVID-19 inpatients in a tertiary infectious disease referral hospital in Manila, Philippines. Trop. Med. Health 2021, 49, 48. [Google Scholar] [CrossRef]
- Akhtar, H.; Khalid, S.; Rahman, F.U.; Ali, S.; Afridi, M.; Khader, Y.S.; Hassan, F.; Akhtar, N.; Khan, M.M.; Ikram, A. Delayed admissions and efficacy of steroid use in patients with critical and severe COVID-19: An apprehensive approach. J. Public Health 2021, 43 (Suppl. 3), iii43–iii48. [Google Scholar] [CrossRef]
- Aksel, G.; Islam, M.M.; Algin, A.; Eroglu, S.E.; Yasar, G.B.; Ademoglu, E.; Dolek, U.C. Early predictors of mortality for moderate to severely ill patients with Covid-19. Am. J. Emerg. Med. 2021, 45, 290–296. [Google Scholar] [CrossRef]
- Al Mutair, A.; Elhazmi, A.; Alhumaid, S.; Ahmad, G.Y.; Rabaan, A.A.; Alghdeer, M.A.; Chagla, H.; Tirupathi, R.; Sharma, A.; Dhama, K.; et al. Examining the Clinical Prognosis of Critically Ill Patients with COVID-19 Admitted to Intensive Care Units: A Nationwide Saudi Study. Medicina 2021, 57, 878. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.A.; Al-Raddadi, R.M.; Ramadan, I.K.; Mirza, A.A.; Alsaab, H.A.; Alobaidi, H.F.; Hayd, M.Y.B. Survival, mortality, and related comorbidities among COVID-19 patients in Saudi Arabia: A hospital-based retrospective cohort study. Saudi Med. J. 2022, 43, 915–926. [Google Scholar] [CrossRef]
- Alam, M.T.; Mehdi, A.; Timsaal, Y.; Rehan, M.; Kumar, A.; Shaikh, I.S.; Yasmin, F.; Memon, G.M.; Ahmed, N.; Asghar, M.S. The clinical course, biochemical markers, and clinical outcomes of COVID-19 positive patients from the third wave in Pakistan: A retrospective cohort study. Ann. Med. Surg. 2022, 77, 103599. [Google Scholar] [CrossRef]
- AlBahrani, S.; Al-Tawfiq, J.A.; Jebakumar, A.Z.; Alghamdi, M.; Zakary, N.; Seria, M.; Alrowis, A. Clinical Features and Outcome of Low and High Corticosteroids in Admitted COVID-19 Patients. J. Epidemiol. Glob. Health 2021, 11, 316–319. [Google Scholar] [CrossRef]
- Alhamar, G.; Maddaloni, E.; Al Shukry, A.; Al-Sabah, S.; Al-Haddad, M.; Al-Youha, S.; Jamal, M.; Almazeedi, S.; Al-Shammari, A.A.; Abu-Farha, M.; et al. Development of a clinical risk score to predict death in patients with COVID-19. Diabetes Metab. Res. Rev. 2022, 38, e3526. [Google Scholar] [CrossRef]
- Alhowaish, T.S.; Alhamadh, M.S.; Alhabeeb, A.Y.; Aldosari, S.F.; Masuadi, E.; Alrashid, A. Outcomes of COVID-19 in Inflammatory Rheumatic Diseases: A Retrospective Cohort Study. Cureus 2022, 14, e26343. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Sepandi, M.; Rashti, R.; Sedighinezhad, H.; Afrashteh, S. COVID-19: Clinical features, case fatality, and the effect of symptoms on mortality in hospitalized cases in Iran. J. Taibah Univ. Med. Sci. 2022, 17, 725–731. [Google Scholar] [CrossRef]
- Alizadehsani, R.; Eskandarian, R.; Behjati, M.; Zahmatkesh, M.; Roshanzamir, M.; Izadi, N.H.; Shoeibi, A.; Haddadi, A.; Khozeimeh, F.; Sani, F.A.; et al. Factors associated with mortality in hospitalized cardiovascular disease patients infected with COVID-19. Immun. Inflamm. Dis. 2022, 10, e561. [Google Scholar] [CrossRef]
- Aljouie, A.F.; Almazroa, A.; Bokhari, Y.; Alawad, M.; Mahmoud, E.; Alawad, E.; Alsehawi, A.; Rashid, M.; Alomair, L.; Almozaai, S.; et al. Early Prediction of COVID-19 Ventilation Requirement and Mortality from Routinely Collected Baseline Chest Radiographs, Laboratory, and Clinical Data with Machine Learning. J. Multidiscip. Healthc. 2021, 14, 2017–2033. [Google Scholar] [CrossRef]
- Almazeedi, S.; Al-Youha, S.; Jamal, M.H.; Al-Haddad, M.; Al-Muhaini, A.; Al-Ghimlas, F.; Al-Sabah, S. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine 2020, 24, 100448. [Google Scholar] [CrossRef]
- Alshukry, A.; Ali, H.; Ali, Y.; Al-Taweel, T.; Abu-Farha, M.; AbuBaker, J.; Devarajan, S.; Dashti, A.A.; Bandar, A.; Taleb, H.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients in Kuwait. PLoS ONE 2020, 15, e0242768. [Google Scholar] [CrossRef]
- Alwafi, H.; Naser, A.Y.; Qanash, S.; Brinji, A.S.; Ghazawi, M.A.; Alotaibi, B.; Alghamdi, A.; Alrhmani, A.; Fatehaldin, R.; Alelyani, A.; et al. Predictors of Length of Hospital Stay, Mortality, and Outcomes Among Hospitalised COVID-19 Patients in Saudi Arabia: A Cross-Sectional Study. J. Multidiscip. Healthc. 2021, 14, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.A.; Almalki, F.; Aljohani, A.; Alharbi, B.; Alsulami, B.; Alhaddad, A.; Althubaiti, A.; Khawaji, B.; Farahat, F. The Association Between Vitamin D Serum Level and COVID-19 Patients’ Outcomes in a Tertiary Center in Saudi Arabia: A Retrospective Cohort Study. Cureus 2022, 14, e26266. [Google Scholar] [CrossRef]
- Araban, M.; Karimy, M.; Koohestani, H.; Montazeri, A.; Delaney, D. Epidemiological and clinical characteristics of patients with COVID-19 in Islamic Republic of Iran. East Mediterr. Health J. 2022, 28, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Argun Baris, S.; Boyaci, H.; Akhan, S.; Mutlu, B.; Deniz, M.; Basyigit, I. Charlson Comorbidity Index in Predicting Poor Clinical Outcomes and Mortality in Patients with COVID-19. Turk. Thorac. J. 2022, 23, 145–153. [Google Scholar] [CrossRef]
- Ayaz, A.; Arshad, A.; Malik, H.; Ali, H.; Hussain, E.; Jamil, B. Risk factors for intensive care unit admission and mortality in hospitalized COVID-19 patients. Acute Crit. Care 2020, 35, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Aydin Guclu, O.; Goktas, S.S.; Gorek Dilektasli, A.; Acet Ozturk, N.A.; Demirdogen, E.; Coskun, F.; Ediger, D.; Ursavas, A.; Uzaslan, E.; Erol, H.A.; et al. Pilot study for immunoglobulin E as a prognostic biomarker in coronavirus disease 2019. Intern. Med. J. 2022, 52, 1495–1504. [Google Scholar] [CrossRef]
- Ayed, M.; Borahmah, A.A.; Yazdani, A.; Sultan, A.; Mossad, A.; Rawdhan, H. Assessment of Clinical Characteristics and Mortality-Associated Factors in COVID-19 Critical Cases in Kuwait. Med. Princ. Pract. 2021, 30, 185–192. [Google Scholar] [CrossRef]
- Ayten, O.; Saylan, B. Retrospective analysis of severe COVID-19 pneumonia patients treated with lopinavir/ritonavir: A comparison with survivor and non-survivor patients. S. Afr. J. Infect. Dis. 2020, 35, 233. [Google Scholar] [CrossRef]
- Bae, S.; Kim, Y.; Hwang, S.; Kwon, K.T.; Chang, H.H.; Kim, S.W. New Scoring System for Predicting Mortality in Patients with COVID-19. Yonsei Med. J. 2021, 62, 806–813. [Google Scholar] [CrossRef]
- Bakhshwin, D.; Alotaibi, M.; Ali, A.S.; Althomali, A.; Alsuwat, A.; Alhamyani, A.; Alwathnani, A.; Alsaggaf, S.; Alrafiah, A. Mortality Predictors Among COVID-19 Elderly in Taif, Saudi Arabia. Infect. Drug Resist. 2022, 15, 3213–3223. [Google Scholar] [CrossRef]
- Basaran, N.C.; Ozdede, M.; Uyaroglu, O.A.; Sahin, T.K.; Ozcan, B.; Oral, H.; Ozisik, L.; Guven, G.S.; Tanriover, M.D. Independent predictors of in-hospital mortality and the need for intensive care in hospitalized non-critical COVID-19 patients: A prospective cohort study. Intern. Emerg. Med. 2022, 17, 1413–1424. [Google Scholar] [CrossRef]
- Bokhary, D.H.; Bokhary, N.H.; Seadawi, L.E.; Moafa, A.M.; Khairallah, H.H.; Bakhsh, A. The Role of Demographic, Clinical, and Laboratory Characteristics in Predicting the In-Hospital Outcomes of Patients With COVID-19. Cureus 2022, 14, e23418. [Google Scholar] [CrossRef]
- Burhamah, W.; Qahi, I.; Oroszlanyova, M.; Shuaibi, S.; Alhunaidi, R.; Alduwailah, M.; Alhenaidi, M.; Mohammad, Z. Prognostic Factors and Predictors of In-Hospital Mortality Among COVID-19 Patients Admitted to the Intensive Care Unit: An Aid for Triage, Counseling, and Resource Allocation. Cureus 2021, 13, e16577. [Google Scholar] [CrossRef]
- Cakir Guney, B.; Hayiroglu, M.; Senocak, D.; Cicek, V.; Cinar, T.; Kaplan, M. Evaluation of N/LP Ratio as a Predictor of Disease Progression and Mortality in COVID-19 Patients Admitted to the Intensive Care Unit. Medeni Med. J. 2021, 36, 241–248. [Google Scholar]
- Caliskan, T.; Saylan, B. Smoking and comorbidities are associated with COVID-19 severity and mortality in 565 patients treated in Turkey: A retrospective observational study. Rev. Assoc. Med. Bras. (1992) 2020, 66, 1679–1684. [Google Scholar] [CrossRef]
- Celik, I.; Eryilmaz-Eren, E.; Kilinc-Toker, A.; Eren, D.; Yildiz, M.; Kanat, A.; Topaloglu, U.S.; Guzeldag, S.; Kara, M.; Ulu-Kilic, A. Low-dose tocilizumab is associated with improved outcome and a low risk of secondary infection in severe COVID-19 pneumonia. Int. J. Clin. Pract. 2021, 75, e14997. [Google Scholar] [CrossRef]
- Chang, Y.; Jeon, J.; Song, T.J.; Kim, J. Association between the fatty liver index and the risk of severe complications in COVID-19 patients: A nationwide retrospective cohort study. BMC Infect. Dis. 2022, 22, 384. [Google Scholar] [CrossRef]
- Choi, H.G.; Wee, J.H.; Kim, S.Y.; Kim, J.H.; Il Kim, H.; Park, J.Y.; Park, S.; Il Hwang, Y.; Jang, S.H.; Jung, K.S. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean Disease Control and Prevention. Allergy 2021, 76, 921–924. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, J.Y.; Lee, H.S.; Suh, J.; Song, J.Y.; Byun, M.K.; Cho, J.H.; Kim, H.J.; Lee, J.H.; Park, J.W.; et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur. Respir. J. 2021, 57, 2002226. [Google Scholar] [CrossRef]
- Cilingir, B.M.; Askar, S.; Meral, A.; Askar, M. Can B-Type Natriuretic Peptide (BNP) Levels Serve as an Early Predictor of Clinical Severity in Patients with COVID-19 Pneumonia? Clin. Lab. 2022, 68, 207–213. [Google Scholar] [CrossRef]
- Cortez, K.J.C.; Demot, B.A.; Bartolo, S.S.; Feliciano, D.D.; Ciriaco, V.M.P.; Labi, I.I.E.; Viray, D.D.M.; Casuga, J.C.M.; Camonayan-Flor, K.A.B.; Gomez, P.M.A.; et al. Clinical characteristics and outcomes of COVID-19 patients in a tertiary hospital in Baguio City, Philippines. West. Pac. Surveill. Response J. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dana, N.; Nasirian, M.; Vaseghi, G.; Heshmat-Ghahdarijani, K.; Ataei, B.; Mosayebi, A.; Manteghinejad, A.; Javanmard, S.H. Vitamin D Level in Laboratory Confirmed COVID-19 and Disease Progression. Eurasian J. Med. 2022, 54, 206–212. [Google Scholar] [CrossRef]
- Deeb, A.; Khawaja, K.; Sakrani, N.; AlAkhras, A.; Al Mesabi, A.; Trehan, R.; Kumar, P.C.; Babiker, Z.; Nagelkerke, N.; Fru-Nsutebu, E. Impact of Ethnicity and Underlying Comorbidity on COVID-19 Inhospital Mortality: An Observational Study in Abu Dhabi, UAE. BioMed. Res. Int. 2021, 2021, 6695707. [Google Scholar] [CrossRef] [PubMed]
- Degerli, E.; Derin, S.; Oruc, K.; Sengul Samanci, N.; Bedir, S.; Celik, E.; Senturk Oztas, N.; Alkan, G.; Demirelli, F.H.; Demirci, N.S. The demographic characteristics, prognosis, and relationship with cancer subtypes of hospitalized COVID-19 patients with malignancy: A single-center experience. J. Med. Virol. 2021, 93, 5839–5845. [Google Scholar] [CrossRef] [PubMed]
- Doganay, F.; Ak, R. Performance of the CURB-65, ISARIC-4C and COVID-GRAM scores in terms of severity for COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14759. [Google Scholar] [CrossRef] [PubMed]
- Doganay, F.; Elkonca, F.; Seyhan, A.U.; Yilmaz, E.; Batirel, A.; Ak, R. Shock index as a predictor of mortality among the Covid-19 patients. Am. J. Emerg. Med. 2021, 40, 106–109. [Google Scholar] [CrossRef]
- Elhazmi, A.; Al-Omari, A.; Sallam, H.; Mufti, H.N.; Rabie, A.A.; Alshahrani, M.; Mady, A.; Alghamdi, A.; Altalaq, A.; Azzam, M.H.; et al. Machine learning decision tree algorithm role for predicting mortality in critically ill adult COVID-19 patients admitted to the ICU. J. Infect. Public Health 2022, 15, 826–834. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Akbari, A.; Yeganeh, B.S.; Rezaei, T.; Bakhtiari, H.; Pirbonyeh, N. Liver Biomarkers Assay in COVID-19 Cases: A Comparison Study between Alive and Dead Patients. Iran J. Public Health 2022, 51, 172–177. [Google Scholar] [CrossRef]
- He, C.; Liu, C.; Yang, J.; Tan, H.; Ding, X.; Gao, X.; Yang, Y.; Shen, Y.; Xiang, H.; Ke, J.; et al. Prognostic significance of day-by-day in-hospital blood pressure variability in COVID-19 patients with hypertension. J. Clin. Hypertens 2022, 24, 224–233. [Google Scholar] [CrossRef]
- Hesni, E.; Sayad, B.; Khosravi Shadmani, F.; Najafi, F.; Khodarahmi, R.; Rahimi, Z.; Bozorgomid, A.; Sayad, N. Demographics, clinical characteristics, and outcomes of 27,256 hospitalized COVID-19 patients in Kermanshah Province, Iran: A retrospective one-year cohort study. BMC Infect. Dis. 2022, 22, 319. [Google Scholar] [CrossRef]
- Islam, M.A.; Mazumder, M.A.; Akhter, N.; Huq, A.F.; Al-Mahtab, M.; Khan, M.S.I.; Akbar, S.M. Extraordinary Survival Benefits of Severe and Critical Patients with COVID-19 by Immune Modulators: The Outcome of a Clinical Trial in Bangladesh. Euroasian J. Hepatogastroenterol. 2020, 10, 68–75. [Google Scholar]
- Jalili, M.; Payandemehr, P.; Saghaei, A.; Sari, H.N.; Safikhani, H.; Kolivand, P. Characteristics and Mortality of Hospitalized Patients With COVID-19 in Iran: A National Retrospective Cohort Study. Ann. Intern. Med. 2021, 174, 125–127. [Google Scholar] [CrossRef]
- Jandaghian, S.; Vaezi, A.; Manteghinejad, A.; Nasirian, M.; Vaseghi, G.; Haghjooy Javanmard, S. Red Blood Cell Distribution Width (RDW) as a Predictor of In-Hospital Mortality in COVID-19 Patients; a Cross Sectional Study. Arch. Acad. Emerg. Med. 2021, 9, e67. [Google Scholar]
- Jin, M.; Chen, C.; Huang, J.; Zhang, F.; Dong, T.; Zhang, M.; Yue, H.; Liu, K.; Li, G.; Hu, K.; et al. Clinical characteristics of COVID-19 patients with asthma in Wuhan, China: A retrospective cohort study. J. Asthma 2020, 59, 230–238. [Google Scholar] [CrossRef]
- Jo, S.; Nam, H.K.; Kang, H.; Cho, S.I. Associations of symptom combinations with in-hospital mortality of coronavirus disease-2019 patients using South Korean National data. PLoS ONE 2022, 17, e0273654. [Google Scholar] [CrossRef]
- Jung, Y.; Wee, J.H.; Kim, J.H.; Choi, H.G. The Effects of Previous Asthma and COPD on the Susceptibility to and Severity of COVID-19: A Nationwide Cohort Study in South Korea. J. Clin. Med. 2021, 10, 4626. [Google Scholar] [CrossRef]
- Kaya, T.; Nalbant, A.; Kiliccioglu, G.K.; Cayir, K.T.; Yaylaci, S.; Varim, C. The prognostic significance of erythrocyte sedimentation rate in COVID-19. Rev. Assoc. Med. Bras. (1992) 2021, 67, 1305–1310. [Google Scholar] [CrossRef]
- Khalid, A.; Ali Jaffar, M.; Khan, T.; Abbas Lail, R.; Ali, S.; Aktas, G.; Waris, A.; Javaid, A.; Ijaz, N.; Muhammad, N. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS-COV-2 infected patients of Pakistan: A retrospective comparative analysis. Hematology 2021, 26, 529–542. [Google Scholar] [CrossRef]
- Khani, M.; Tavana, S.; Tabary, M.; Naseri Kivi, Z.; Khaheshi, I. Prognostic implications of biventricular strain measurement in COVID-19 patients by speckle-tracking echocardiography. Clin. Cardiol. 2021, 44, 1475–1481. [Google Scholar] [CrossRef]
- Kibar Akilli, I.; Bilge, M.; Uslu Guz, A.; Korkusuz, R.; Canbolat Unlu, E.; Kart Yasar, K. Comparison of Pneumonia Severity Indices, qCSI, 4C-Mortality Score and qSOFA in Predicting Mortality in Hospitalized Patients with COVID-19 Pneumonia. J. Pers. Med. 2022, 12, 801. [Google Scholar] [CrossRef]
- Kim, S.H.; Ji, E.; Won, S.H.; Cho, J.; Kim, Y.H.; Ahn, S.; Chang, Y.S. Association of asthma comorbidity with poor prognosis of coronavirus disease 2019. World Allergy Organ J. 2021, 14, 100576. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.M.; Kim, Y.K.; Kim, J.Y.; Lee, Y.M.; Kim, B.O.; Hwangbo, S.; Park, T. Clinical Characteristics and Outcomes of COVID-19 Cohort Patients in Daegu Metropolitan City Outbreak in 2020. J. Korean Med. Sci. 2021, 36, e12. [Google Scholar] [CrossRef] [PubMed]
- Kolivand, P.; Fathi, M.; Kheyrati, L.; Lak, M. Exposure to sulfur mustard increases the risk for mortality in patients with COVID-19 infection: A cohort study. Am. J. Emerg. Med. 2022, 51, 144–149. [Google Scholar] [CrossRef]
- Kong, K.A.; Jung, S.; Yu, M.; Park, J.; Kang, I.S. Association Between Cardiovascular Risk Factors and the Severity of Coronavirus Disease 2019: Nationwide Epidemiological Study in Korea. Front. Cardiovasc. Med. 2021, 8, 732518. [Google Scholar] [CrossRef] [PubMed]
- Kouhpeikar, H.; Khosaravizade Tabasi, H.; Khazir, Z.; Naghipour, A.; Mohammadi Moghadam, H.; Forouzanfar, H.; Abbasifard, M.; Kirichenko, T.V.; Reiner, Z.; Banach, M.; et al. Statin Use in COVID-19 Hospitalized Patients and Outcomes: A Retrospective Study. Front. Cardiovasc. Med. 2022, 9, 820260. [Google Scholar] [CrossRef]
- Kridin, K.; Schonmann, Y.; Tzur Bitan, D.; Damiani, G.; Weinstein, O.; Cohen, A.D. The Burden of Coronavirus Disease 2019 and Its Complications in Patients With Atopic Dermatitis-A Nested Case-Control Study. Dermatitis 2021, 32, S45–S52. [Google Scholar] [CrossRef]
- Kuwahara, M.; Kamigaito, M.; Murakami, H.; Sato, K.; Mambo, N.; Kobayashi, T.; Shirai, K.; Miyawaki, A.; Ohya, M.; Hirata, J.I. Prognostic Factors Associated With Mortality of Patients with COVID-19 Requiring Ventilator Management: A Retrospective Cohort Study. Cureus 2022, 14, e25374. [Google Scholar] [CrossRef]
- Kwok, W.C.; Tam, A.R.; Ho, J.C.M.; Lam, D.C.L.; Tam, T.C.C.; Chan, K.P.F.; Wang, J.K.L.; Ip, M.S.M.; Hung, I.F.N. Asthma, from mild to severe, is an independent prognostic factor for mild to severe Coronavirus disease 2019 (COVID-19). Clin. Respir. J. 2022, 16, 293–300. [Google Scholar] [CrossRef]
- Lee, S.C.; Son, K.J.; Han, C.H.; Jung, J.Y.; Park, S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci. Rep. 2020, 10, 21805. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, G.U.; Moon, Y.R.; Sung, K. Clinical Characteristics and Risk Factors for Fatality and Severity in Patients with Coronavirus Disease in Korea: A Nationwide Population-Based Retrospective Study Using the Korean Health Insurance Review and Assessment Service (HIRA) Database. Int. J. Environ. Res. Public Health 2020, 17, 8559. [Google Scholar] [CrossRef]
- Ma, X.; Li, A.; Jiao, M.; Shi, Q.; An, X.; Feng, Y.; Xing, L.; Liang, H.; Chen, J.; Li, H.; et al. Characteristic of 523 COVID-19 in Henan Province and a Death Prediction Model. Front. Public Health 2020, 8, 475. [Google Scholar] [CrossRef]
- Malundo, A.F.G.; Abad, C.L.R.; Salamat, M.S.S.; Sandejas, J.C.M.; Poblete, J.B.; Planta, J.E.G.; Morales, S.J.L.; Gabunada, R.R.W.; Evasan, A.L.M.; Canal, J.P.A.; et al. Predictors of mortality among inpatients with COVID-19 infection in a tertiary referral center in the Philippines. IJID Reg. 2022, 4, 134–142. [Google Scholar] [CrossRef]
- Moon, H.J.; Kim, K.; Kang, E.K.; Yang, H.-J.; Lee, E. Prediction of COVID-19-related Mortality and 30-Day and 60-Day Survival Probabilities Using a Nomogram. J. Korean Med. Sci. 2021, 36, e248. [Google Scholar] [CrossRef]
- Nakamura, S.; Kanemasa, Y.; Atsuta, Y.; Fujiwara, S.; Tanaka, M.; Fukushima, K.; Kobayashi, T.; Shimoyama, T.; Omuro, Y.; Sekiya, N.; et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: A single-center retrospective observational study in Tokyo, Japan. Int. J. Clin. Oncol. 2021, 26, 485–493. [Google Scholar] [CrossRef]
- Omar, S.M.; Musa, I.R.; Salah, S.E.; Elnur, M.M.; Al-Wutayd, O.; Adam, I. High Mortality Rate in Adult COVID-19 Inpatients in Eastern Sudan: A Retrospective Study. J. Multidiscip. Healthc. 2020, 13, 1887–1893. [Google Scholar] [CrossRef]
- Ong, A.N.; Tan, C.C.; Canete, M.T.; Lim, B.A.; Robles, J. Association Between Metformin Use and Mortality among Patients with Type 2 Diabetes Mellitus Hospitalized for COVID-19 Infection. J. ASEAN Fed. Endocr. Soc. 2021, 36, 133–141. [Google Scholar] [CrossRef]
- Ozger, H.S.; Karakus, R.; Kuscu, E.N.; Bagriacik, U.E.; Oruklu, N.; Yaman, M.; Turkoglu, M.; Erbas, G.; Atak, A.Y.; Senol, E. Serial measurement of cytokines strongly predict COVID-19 outcome. PLoS ONE 2021, 16, e0260623. [Google Scholar] [CrossRef]
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses 2021, 64, 1238–1252. [Google Scholar] [CrossRef]
- Park, B.E.; Lee, J.H.; Park, H.K.; Kim, H.N.; Jang, S.Y.; Bae, M.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Lee, B.Y.; et al. Impact of Cardiovascular Risk Factors and Cardiovascular Diseases on Outcomes in Patients Hospitalized with COVID-19 in Daegu Metropolitan City. J. Korean Med. Sci. 2021, 36, e15. [Google Scholar] [CrossRef]
- Parvin, S.; Islam, M.S.; Majumdar, T.K.; Ahmed, F. Clinicodemographic profile, intensive care unit utilization and mortality rate among COVID-19 patients admitted during the second wave in Bangladesh. IJID Reg. 2022, 2, 55–59. [Google Scholar] [CrossRef]
- Patgiri, P.R.; Rajendran, V.; Ahmed, A.B. Clinico-Epidemiological Profiles of COVID-19 Elderly Patients in Guwahati City, Assam, India: A Cross-Sectional Study. Cureus 2022, 14, e24043. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Gautam, P.L.; Sharma, S.; Kumar, J.; Gupta, A.; Sharma, M.; Khehra, A.S.; Paul, B.S.; Mohan, B. Analysis of trimodal pattern of mortality among hospitalized COVID-19 patients- Lessons from tertiary care hospital. J. Anaesthesiol. Clin. Pharmacol. 2022, 38 (Suppl. 1), S107–S114. [Google Scholar] [PubMed]
- Pramudita, A.; Rosidah, S.; Yudia, N.; Simatupang, J.; Sigit, W.P.; Novariani, R.; Myriarda, P.; Siswanto, B.B. Cardiometabolic Morbidity and Other Prognostic Factors for Mortality in Adult Hospitalized COVID-19 Patients in North Jakarta, Indonesia. Glob Heart 2022, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Puah, S.H.; Cove, M.E.; Phua, J.; Kansal, A.; Venkatachalam, J.; Ho, V.K.; Sewa, D.W.; Gokhale, R.S.; Liew, M.F.; Ho, B.C.H.; et al. Association between lung compliance phenotypes and mortality in COVID-19 patients with acute respiratory distress syndrome. Ann. Acad. Med. Singap. 2021, 50, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, P.; Amniati, S.; Farahmandrad, R.; Faiz, S.H.R.; Hedayati Emami, S.; Habibi, A. Clinical Characteristics of Critically Ill Patients Infected with COVID-19 in Rasoul Akram Hospital in Iran: A Single Center Study. Anesth Pain Med. 2020, 10, e107211. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Ranjan, A.; Ameet, H.; Pandey, S. Clinical and Laboratory Predictors of Mortality in COVID-19 Infection: A Retrospective Observational Study in a Tertiary Care Hospital of Eastern India. Cureus 2021, 13, e17660. [Google Scholar] [CrossRef]
- Rehman, S.; Rehman, N.; Mumtaz, A.; Jiang, J. Association of Mortality-Related Risk Factors in Patients with COVID-19: A Retrospective Cohort Study. Healthcare 2021, 9, 1468. [Google Scholar] [CrossRef]
- Ro, S.; Nishimura, N.; Imai, R.; Tomishima, Y.; So, C.; Murakami, M.; Okafuji, K.; Kitamura, A.; Jinta, T.; Tamura, T. Identification of patients with COVID-19 who are optimal for methylprednisolone pulse therapy. Multidiscip. Respir. Med. 2021, 16, 781. [Google Scholar] [CrossRef]
- Rohani-Rasaf, M.; Mirjalili, K.; Vatannejad, A.; Teimouri, M. Are lipid ratios and triglyceride-glucose index associated with critical care outcomes in COVID-19 patients? PLoS ONE 2022, 17, e0272000. [Google Scholar] [CrossRef]
- Safari, M.; Faradmal, J.; Bashirian, S.; Soltanian, A.R.; Khazaei, S.; Roshanaei, G. Identifying the Risk Factors for Mortality in Patients with Cancer and COVID-19 in Hamadan, the West of Iran. J. Gastrointest. Cancer 2021, 53, 614–622. [Google Scholar] [CrossRef]
- Saha, A.; Ahsan, M.M.; Quader, T.U.; Shohan, M.U.S.; Naher, S.; Dutta, P.; Akash, A.S.; Mehedi, H.M.H.; Chowdhury, A.A.U.; Karim, H.; et al. Characteristics, management and outcomes of critically ill COVID-19 patients admitted to ICU in hospitals in Bangladesh: A retrospective study. J. Prev. Med. Hyg. 2021, 62, E33–E45. [Google Scholar]
- Satici, C.; Demirkol, M.A.; Sargin Altunok, E.; Gursoy, B.; Alkan, M.; Kamat, S.; Demirok, B.; Surmeli, C.D.; Calik, M.; Cavus, Z.; et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int. J. Infect. Dis. 2020, 98, 84–89. [Google Scholar] [CrossRef]
- Satici, M.O.; Islam, M.M.; Satici, C.; Uygun, C.N.; Ademoglu, E.; Altunok, I.; Aksel, G.; Eroglu, S.E. The role of a noninvasive index ‘Spo2/ Fio2’ in predicting mortality among patients with COVID-19 pneumonia. Am. J. Emerg. Med. 2022, 57, 54–59. [Google Scholar] [CrossRef]
- Sehgal, T.; Gupta, N.; Kohli, S.; Khurana, A.; Dass, J.; Diwan, S.; Mahendran, A.J.; Khan, M.; Aggarwal, M.; Subramanian, A. A Prospective Study of Specialized Coagulation Parameters in Admitted COVID-19 Patients and Their Correlation With Acute Respiratory Distress Syndrome and Outcome. Cureus 2021, 13, e17463. [Google Scholar] [CrossRef]
- Sener, M.U.; Cicek, T.; Ozturk, A. Highlights of clinical and laboratory parameters among severe COVID-19 patients treated with tocilizumab: A retrospective observational study. Sao Paulo Med. J. 2022, 140, 627–635. [Google Scholar] [CrossRef]
- Serin, I.; Sari, N.D.; Dogu, M.H.; Acikel, S.D.; Babur, G.; Ulusoy, A.; Onar, M.I.; Gokce, E.C.; Altunok, O.; Yaylaci Mert, F.; et al. A new parameter in COVID-19 pandemic: Initial lactate dehydrogenase (LDH)/Lymphocyte ratio for diagnosis and mortality. J. Infect. Public Health 2020, 13, 1664–1670. [Google Scholar] [CrossRef]
- Shah, M.M.; Abbas, S.; Khan, J.Z.; Iftikhar, M.; Jamal, A.; Zeb Khan, J.; Ullah, S. Psychological and Clinical Predictors of COVID-19 Severity and Outcomes. Cureus 2021, 13, e19458. [Google Scholar] [CrossRef]
- Shesha, N.; Melebari, S.; Alghamdi, S.; Refaat, B.; Naffadi, H.; Alquthami, K. Associations of Clinical Factors and Blood Groups With the Severity of COVID-19 Infection in Makkah City, Saudi Arabia. Front. Cell Infect. Microbiol. 2022, 12, 870096. [Google Scholar] [CrossRef]
- Shin, E.; Jin, J.; Park, S.Y.; Yoo, Y.S.; Lee, J.H.; An, J.; Song, W.J.; Kwon, H.S.; Cho, Y.S.; Moon, H.B.; et al. Impact of asthma, chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap on the prognosis of coronavirus disease 2019. Asia Pac. Allergy 2022, 12, e21. [Google Scholar] [CrossRef]
- Statsenko, Y.; Al Zahmi, F.; Habuza, T.; Gorkom, K.N.; Zaki, N. Prediction of COVID-19 severity using laboratory findings on admission: Informative values, thresholds, ML model performance. BMJ Open 2021, 11, e044500. [Google Scholar] [CrossRef]
- Tanaka, C.; Tagami, T.; Nakayama, F.; Kudo, S.; Takehara, A.; Fukuda, R.; Kaneko, J.; Ishiki, Y.; Sato, S.; Shibata, A.; et al. Association between mortality and age among mechanically ventilated COVID-19 patients: A Japanese nationwide COVID-19 database study. Ann. Intensive Care 2021, 11, 171. [Google Scholar] [CrossRef]
- Trabulus, S.; Karaca, C.; Balkan, I.I.; Dincer, M.T.; Murt, A.; Ozcan, S.G.; Karaali, R.; Mete, B.; Bakir, A.; Kuskucu, M.A.; et al. Kidney function on admission predicts in-hospital mortality in COVID-19. PLoS ONE 2020, 15, e0238680. [Google Scholar] [CrossRef] [PubMed]
- Ucan, E.S.; Ozgen Alpaydin, A.; Ozuygur, S.S.; Ercan, S.; Unal, B.; Sayiner, A.A.; Ergan, B.; Gokmen, N.; Savran, Y.; Kilinc, O.; et al. Pneumonia severity indices predict prognosis in coronavirus disease-2019. Respir. Med. Res. 2021, 79, 100826. [Google Scholar] [CrossRef] [PubMed]
- Zarei, J.; Jamshidnezhad, A.; Haddadzadeh Shoushtari, M.; Mohammad Hadianfard, A.; Cheraghi, M.; Sheikhtaheri, A. Machine Learning Models to Predict In-Hospital Mortality among Inpatients with COVID-19: Underestimation and Overestimation Bias Analysis in Subgroup Populations. J. Healthc. Eng. 2022, 2022, 1644910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Cao, Y.Y.; Tan, G.; Dong, X.; Wang, B.C.; Lin, J.; Yan, Y.Q.; Liu, G.H.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2021, 76, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, C.; Hu, Y.; Lv, W.; Ai, T.; Xia, L. Chest CT imaging features and severity scores as biomarkers for prognostic prediction in patients with COVID-19. Ann. Transl. Med. 2020, 8, 1449. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F., Jr.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Kumar, K.; Hinks, T.S.C.; Singanayagam, A. Treatment of COVID-19-exacerbated asthma: Should systemic corticosteroids be used? Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L1244–L1247. [Google Scholar] [CrossRef]
- Brough, H.A.; Kalayci, O.; Sediva, A.; Untersmayr, E.; Munblit, D.; Rodriguez Del Rio, P.; Vazquez-Ortiz, M.; Arasi, S.; Alvaro-Lozano, M.; Tsabouri, S.; et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics—The 2020 COVID-19 pandemic: A statement from the EAACI-section on pediatrics. Pediatr. Allergy Immunol. 2020, 31, 442–448. [Google Scholar] [CrossRef]
- Johnston, S.L. Asthma and COVID-19: Is asthma a risk factor for severe outcomes? Allergy 2020, 75, 1543–1545. [Google Scholar] [CrossRef]
- Cakebread, J.A.; Xu, Y.; Grainge, C.; Kehagia, V.; Howarth, P.H.; Holgate, S.T.; Davies, D.E. Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J. Allergy Clin. Immunol. 2011, 127, 1148–1154.e9. [Google Scholar] [CrossRef]
- Zhu, J.; Message, S.D.; Mallia, P.; Kebadze, T.; Contoli, M.; Ward, C.K.; Barnathan, E.S.; Mascelli, M.A.; Kon, O.M.; Papi, A.; et al. Bronchial mucosal IFN-alpha/beta and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J. Allergy Clin. Immunol. 2019, 143, 114–125.e4. [Google Scholar] [CrossRef]
- Konopka, K.E.; Wilson, A.; Myers, J.L. Postmortem Lung Findings in a Patient With Asthma and Coronavirus Disease 2019. Chest 2020, 158, e99–e101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).