Efficacy and Safety of COVID-19 Vaccination in Older Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Comprehensive Data Analysis
3. Results
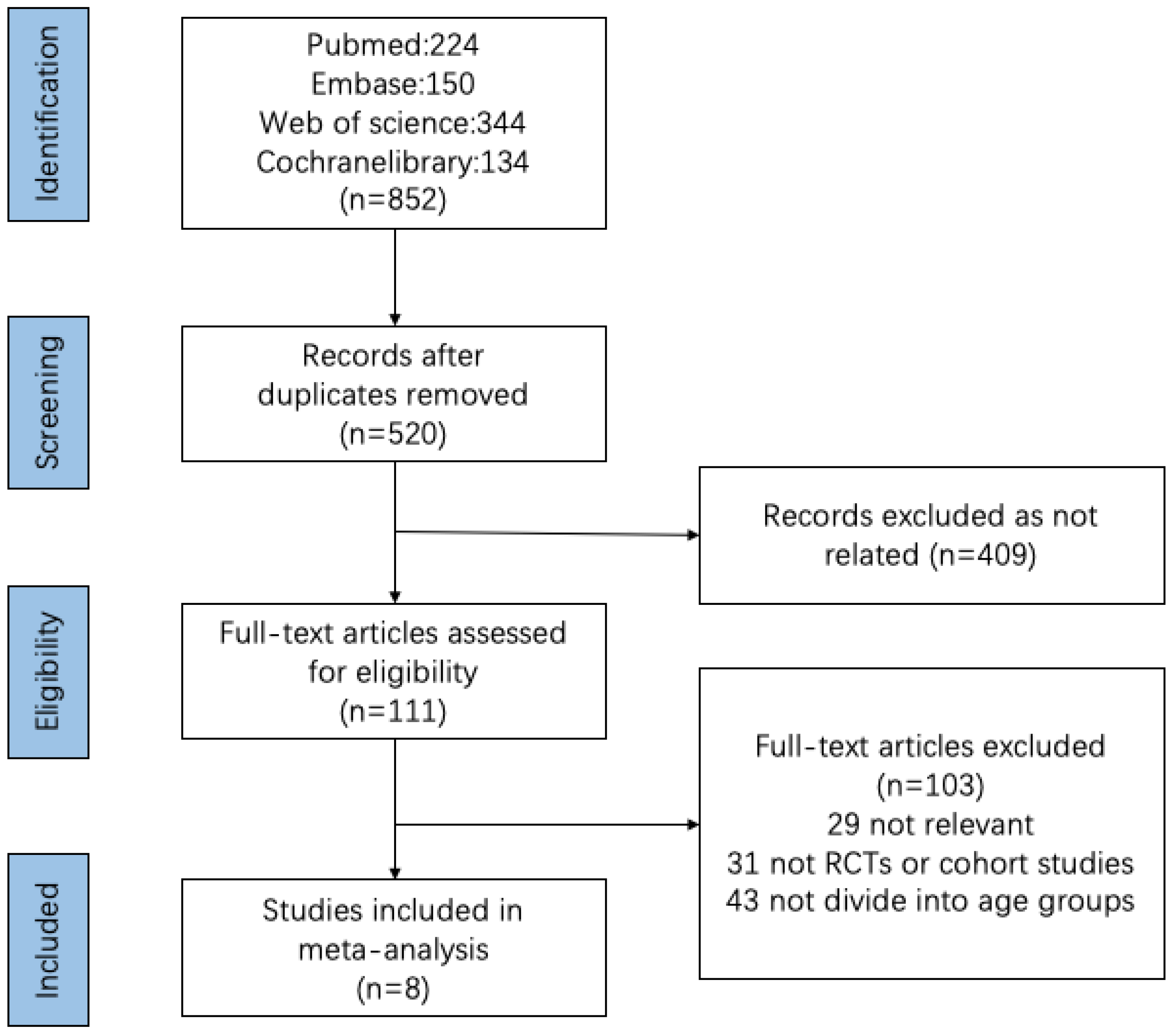
3.1. Search Results
3.2. Characteristics of the Study
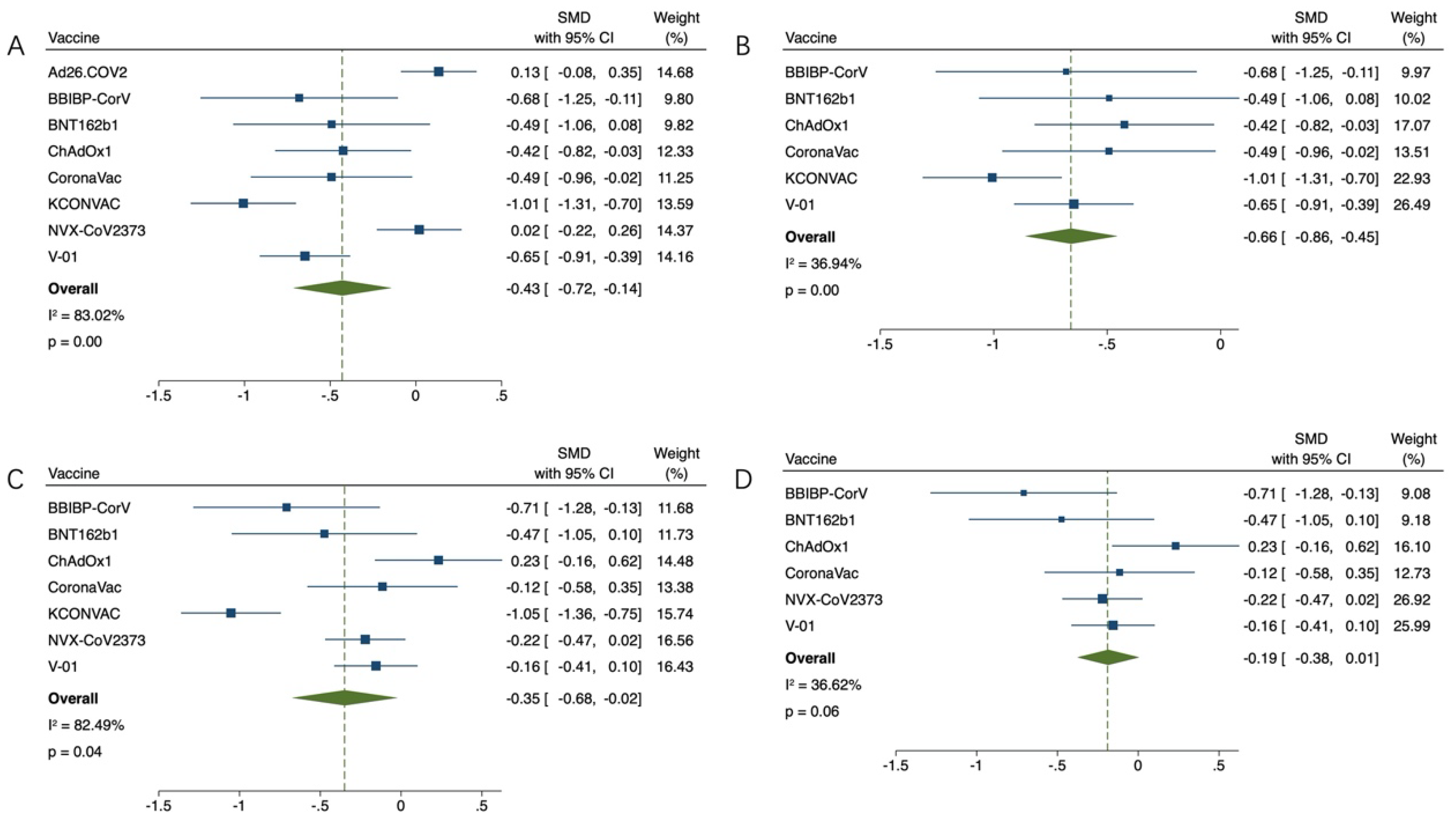
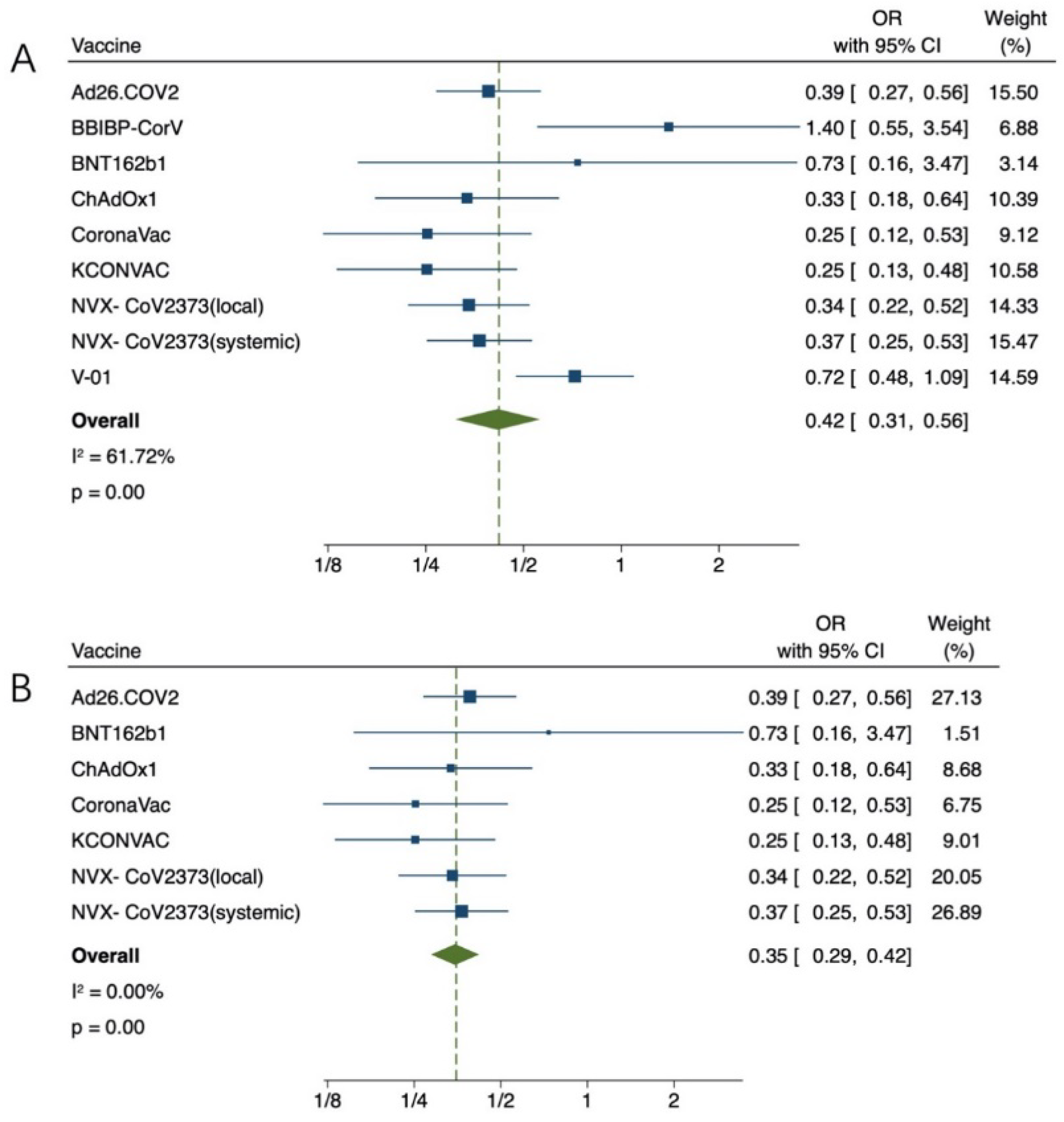
3.3. Effectiveness of COVID-19 Vaccination for Older Adults
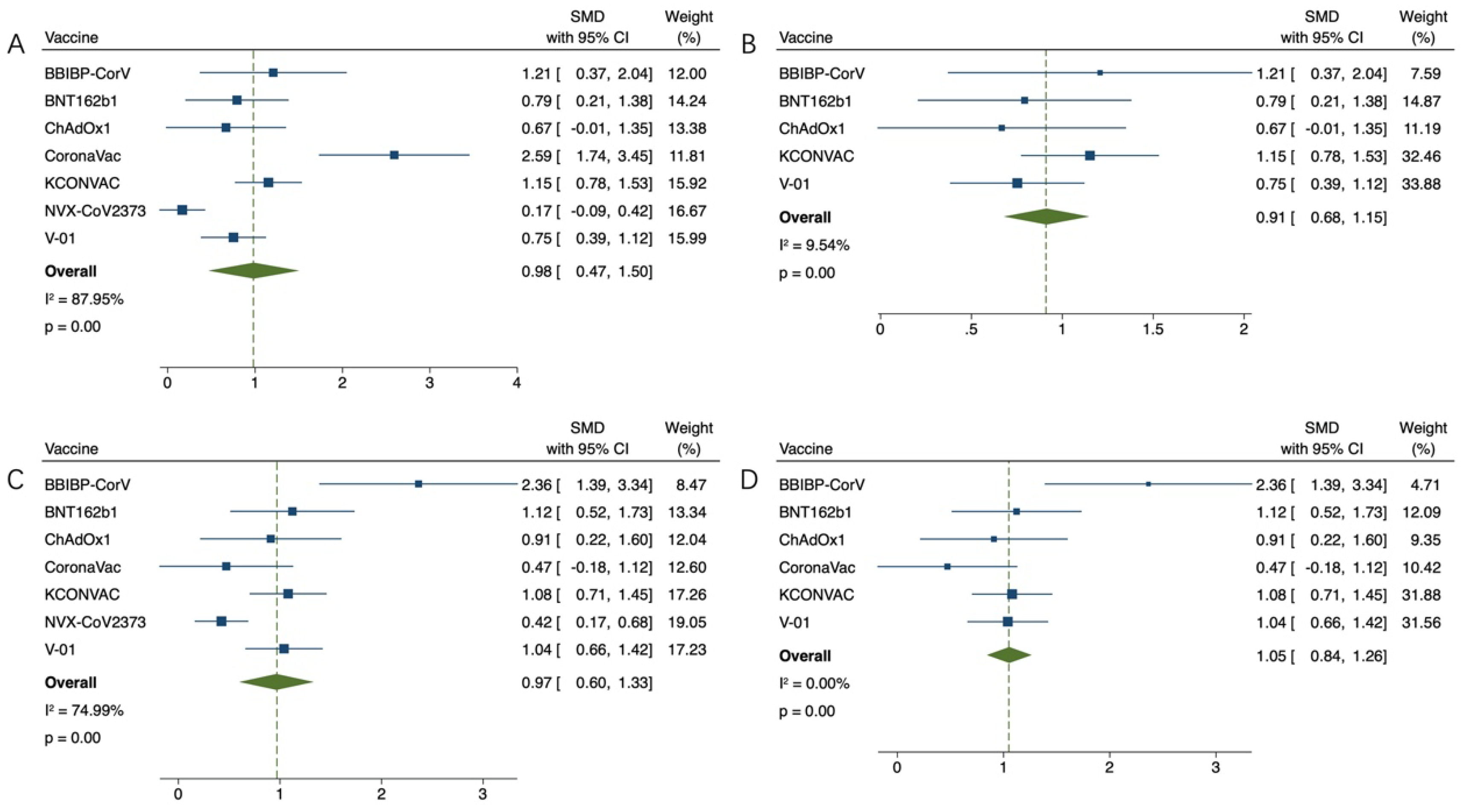
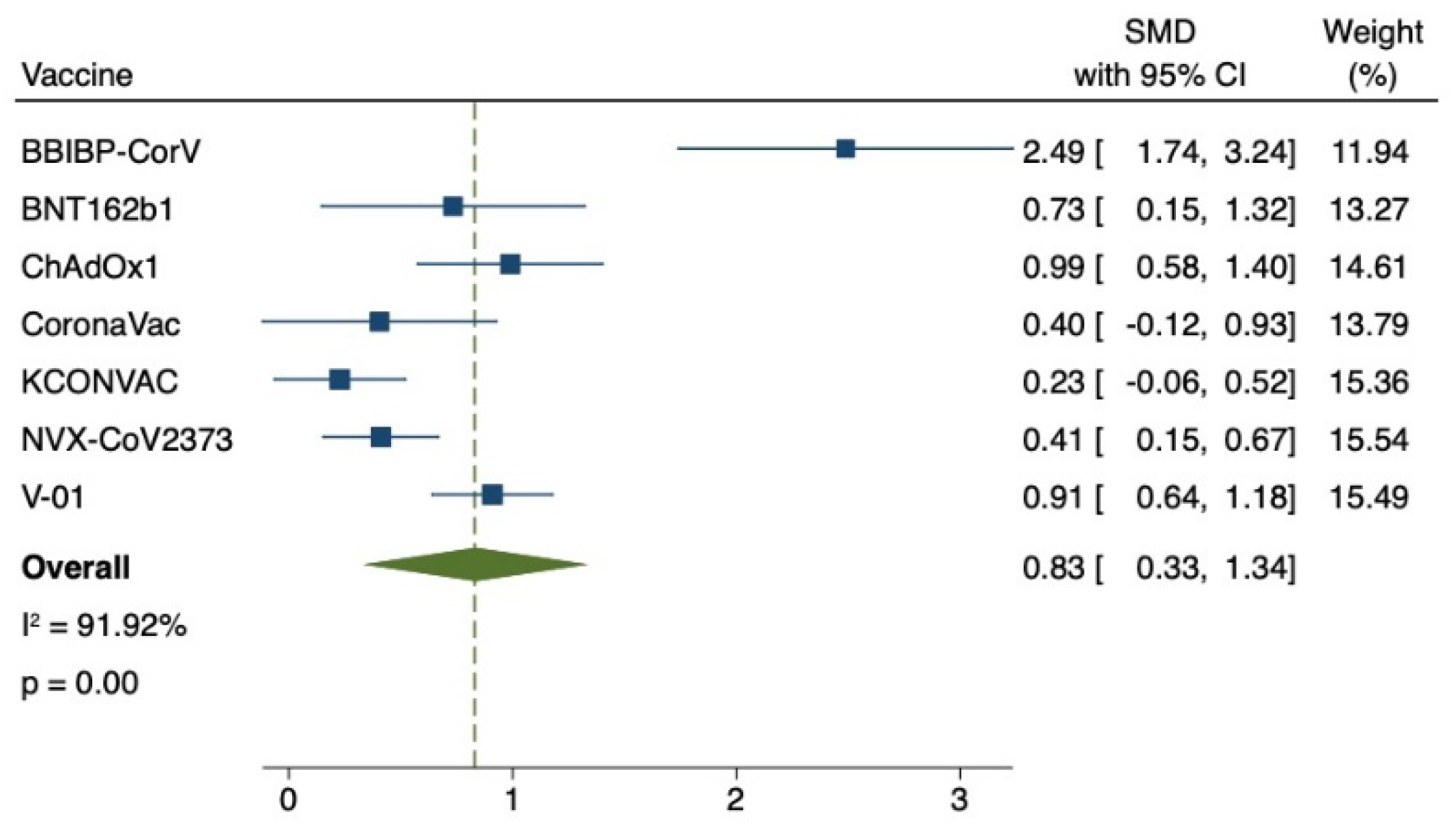
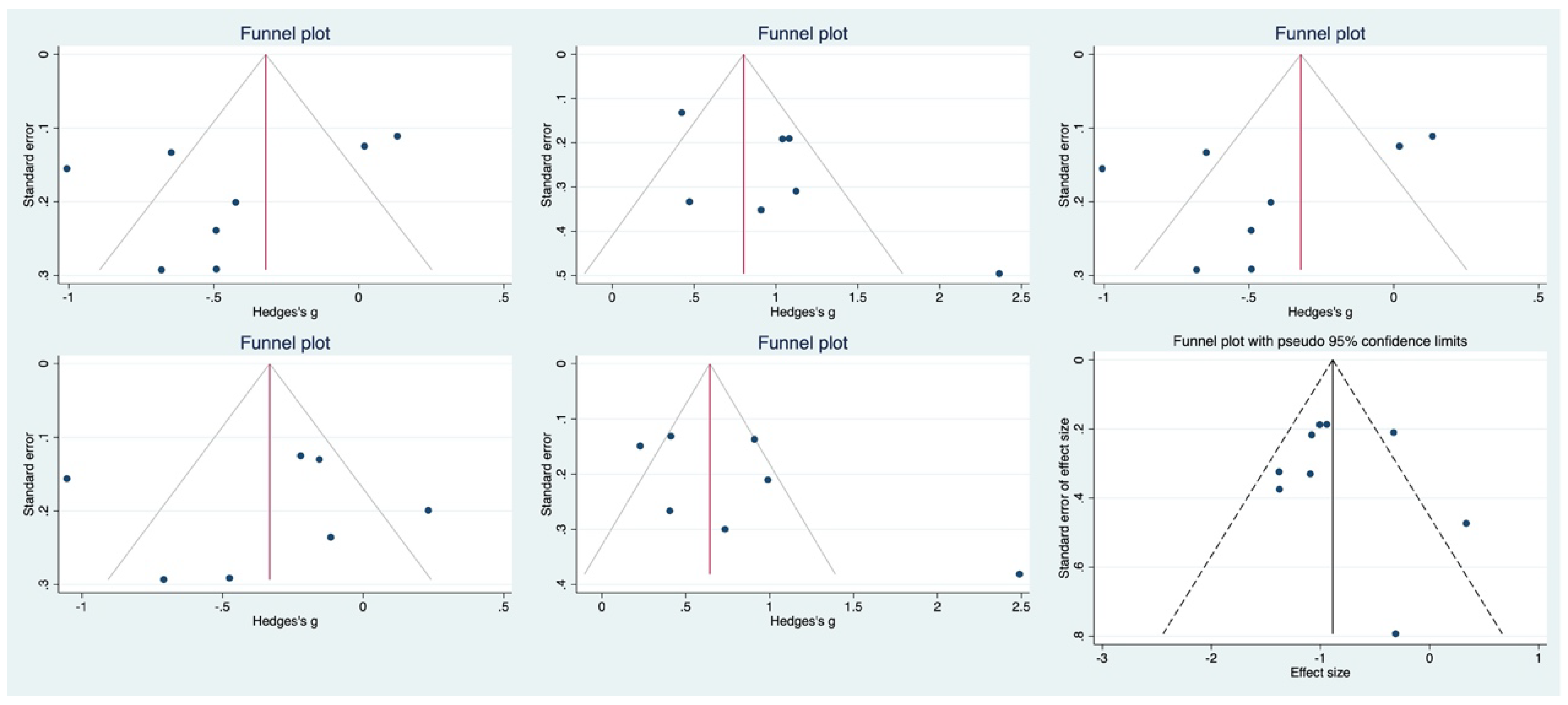
3.4. Safety of COVID-19 Vaccination for Older Adults
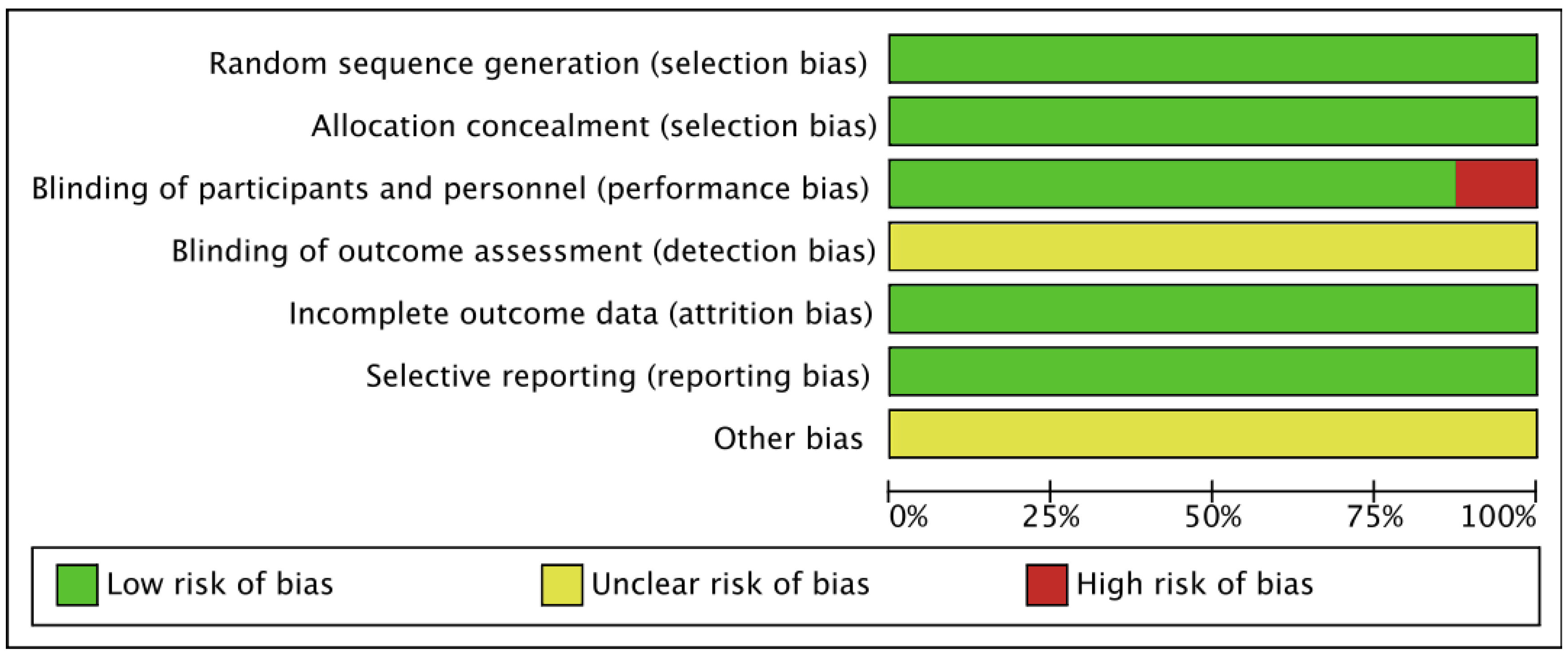
3.5. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Jebril, N. World Health Organization declared a pandemic public health menace: A systematic review of the “COVID-19”, up to 26th March 2020. SSRN Electron. J. 2020, 24, 9160–9166. [Google Scholar]
- Qin, C.; Liu, F.; Yen, T.-C.; Lan, X. 18F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur. J. Nucl. Med. 2020, 47, 1281–1286. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Dos Santos, G.R.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2020, 590, 140–145. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA J. Am. Med. Assoc. 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Sadarangani, M.; Abu Raya, B.; Conway, J.M.; Iyaniwura, S.A.; Falcao, R.C.; Colijn, C.; Coombs, D.; Gantt, S. Importance of COVID-19 vaccine efficacy in older age groups. Vaccine 2021, 39, 2020–2023. [Google Scholar] [CrossRef]
- Veronese, N.; Barbagallo, M. Specific approaches to patients affected by dementia and COVID-19 in nursing homes: The role of the geriatrician. Ageing Res. Rev. 2021, 69, 101373. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: A systematic review and meta-analysis of observational studies. BMJ Open 2021, 11, e052777. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 535–545. [Google Scholar] [CrossRef]
- Aliberti, M.J.R.; Szlejf, C.; Avelino-Silva, V.I.; Suemoto, C.K.; Apolinario, D.; Dias, M.B.; Garcez, F.B.; Trindade, C.B.; Amaral, J.R.D.G.; de Melo, L.R.; et al. COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J. Am. Geriatr. Soc. 2021, 69, 1116–1127. [Google Scholar] [CrossRef]
- Apea, V.J.; Wan, Y.I.; Dhairyawan, R.; APuthucheary, Z.; Pearse, R.M.; Orkin, C.M.; Prowle, J.R. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: An observational cohort study. BMJ Open 2021, 11, e042140. [Google Scholar] [CrossRef]
- Bielza, R.; Sanz, J.; Zambrana, F.; Arias, E.; Malmierca, E.; Portillo, L.; Thuissard, I.J.; Lung, A.; Neira, M.; Moral, M.; et al. Clinical Characteristics, Frailty, and Mortality of Residents With COVID-19 in Nursing Homes of a Region of Madrid. J. Am. Med. Dir. Assoc. 2020, 22, 245–252.e2. [Google Scholar] [CrossRef]
- Colin, F. The COVID-19 Pandemic, Biogerontology, and the Ageing of Humanity. J. Gerontol. Ser. A 2021, 76, e92–e96. [Google Scholar]
- Yancy, C.W. COVID-19 and African Americans. JAMA J. Am. Med. Assoc. 2020, 323, 1891–1892. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef]
- Heath, P.T.; Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021; online ahead of print. [Google Scholar]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA J. Am. Med. Assoc. 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020, 21, 181–192. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2021, 22, 483–495. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Chan, K.-H.; Hung, I.F.-N. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. [Google Scholar] [CrossRef]
- Shu, Y.J.; He, J.F.; Pei, R.J.; He, P.; Huang, Z.H.; Chen, S.M.; Ou, Z.Q.; Deng, J.L.; Zeng, P.Y.; Zhou, J.; et al. Immunogenicity and safety of a recombinant fusion protein vaccine (V-01) against coronavirus disease 2019 in healthy adults: A randomized, double-blind, placebo-controlled, phase II trial. Chin. Med. J. 2021, 134, 1967–1976. [Google Scholar] [CrossRef]
- Formica, N.; Mallory, R.; Albert, G.; Robinson, M.; Plested, J.S.; Cho, I.; Robertson, A.; Dubovsky, F.; Glenn, G.M. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: A phase 2 randomized placebo-controlled trial. PLoS Med. 2021, 18, e1003769. [Google Scholar] [CrossRef]
- Seyahi, E.; Bakhdiyarli, G.; Oztas, M.; Kuskucu, M.A.; Tok, Y.; Sut, N.; Ozcifci, G.; Ozcaglayan, A.; Balkan, I.I.; Saltoglu, N.; et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: A controlled study among hospital workers and elderly. Rheumatol. Int. 2021, 41, 1429–1440. [Google Scholar] [CrossRef]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2020, 11, 571416. [Google Scholar] [CrossRef]
- Haq, K.; McElhaney, J.E. Immunosenescence: Influenza vaccination and the elderly. Curr. Opin. Immunol. 2014, 29, 38–42. [Google Scholar] [CrossRef]
- Howard, W.A.; Gibson, K.L.; Dunn-Walters, D.K. Antibody Quality in Old Age. Rejuvenation Res. 2006, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Dersimonian, R.; Nan, L. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, B.; Li, G.; Chang, X.; Liu, Y.; Chu, K.; Hu, J.; Deng, Y.; Zhu, D.; Wu, J.; et al. Immunogenicity and Safety of a Three-Dose Regimen of a SARS-CoV-2 Inactivated Vaccine in Adults: A Randomized, Double-blind, Placebo-controlled Phase 2 Trial. J. Infect. Dis. 2021, 225, 1701–1709. [Google Scholar] [CrossRef]
- Li, J.; Hui, A.; Zhang, X.; Yang, Y.; Tang, R.; Ye, H.; Ji, R.; Lin, M.; Zhu, Z.; Türeci, Ö.; et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: A randomized, placebo-controlled, double-blind phase 1 study. Nat. Med. 2021, 27, 1062–1070. [Google Scholar] [CrossRef]
- Wibawa, T. COVID-19 vaccine research and development: Ethical issues. Trop. Med. Int. Health 2021, 26, 14–19. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020, 27, 225–228. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2017, 105, 4–9. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Shay, D.K.; Gee, J.; Su, J.R.; Myers, T.R.; Marquez, P.; Liu, R.; Zhang, B.; Licata, C.; Clark, T.A.; Shimabukuro, T.T. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine-United States, March–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 680–684. [Google Scholar] [PubMed]
- Al-Maqbali, J.S.; Al Rasbi, S.; Kashoub, M.S.; Al Hinaai, A.M.; Farhan, H.; Al Rawahi, B.; Al Alawi, A.M. A 59-Year-Old Woman with Extensive Deep Vein Thrombosis and Pulmonary Thromboembolism 7 Days Following a First Dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Am. J. Case Rep. 2021, 22, e932946. [Google Scholar] [CrossRef] [PubMed]
- Alarmanazi, F.; Bangash, B.A.; Lahoti, L.; Farabi, B. Acute Extensive Deep Vein Thrombosis After Heterogeneous Administration of Moderna mRNA Booster Vaccine: A Case Report. Cureus 2022, 14, e25779. [Google Scholar] [CrossRef] [PubMed]
- Shazley, O.; Alshazley, M. A COVID-Positive 52-Year-Old Man Presented With Venous Thromboembolism and Disseminated Intravascular Coagulation Following Johnson & Johnson Vaccination: A Case-Study. Cureus 2021, 13, e16383. [Google Scholar] [PubMed]
- Alberto, G.; Julian, S.; Dominic, W. COVID-19 vaccine: Vaccinate the young to protect the old? J. Law Biosci. 2020, 7, lsaa050. [Google Scholar]
- Seiffert, P.; Konka, A.; Kasperczyk, J.; Kawa, J.; Lejawa, M.; Maślanka-Seiffert, B.; Zembala-John, J.; Bugdol, M.; Romanik, M.; Bułdak, R.; et al. Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in older residents of a long-term care facility: Relation with age, frailty and prior infection status. Biogerontology 2022, 23, 53–64. [Google Scholar] [CrossRef]
- Tian, D.; Song, Y.; Zhang, M.; Pan, Y.; Ge, Z.; Zhang, Y.; Ren, X.; Wen, J.; Xu, Y.; Guo, H.; et al. Genomic, immunological, and clinical analysis of COVID-19 vaccine breakthrough infections in Beijing, China. J. Med. Virol. 2022, 94, 2237–2249. [Google Scholar] [CrossRef]
- Medeiros, G.X.; Sasahara, G.L.; Magawa, J.Y.; Nunes, J.P.S.; Bruno, F.R.; Kuramoto, A.C.; Almeida, R.R.; Ferreira, M.A.; Scagion, G.P.; Candido, É.D.; et al. Reduced T Cell and Antibody Responses to Inactivated Coronavirus Vaccine Among Individuals Above 55 Years Old. Front. Immunol. 2022, 13, 812126. [Google Scholar] [CrossRef]
- Goldstein, J.R.; Cassidy, T.; Wachter, K.W. Vaccinating the oldest against COVID-19 saves both the most lives and most years of life. Proc. Natl. Acad. Sci. USA 2021, 118, e2026322118. [Google Scholar] [CrossRef]
- Bell, M.R.; Kutzler, M.A. An old problem with new solutions: Strategies to improve vaccine efficacy in the elderly. Adv. Drug Deliv. Rev. 2022, 183, 114175. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Robertson, C.A.; Talbot, H.K.; Landolfi, V.; Dunning, A.J.; Greenberg, D.P. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine 2015, 33, 4988–4993. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Shimabukuro, T.T.; Martin, D.B.; Zuber, P.L.; Weibel, D.M.; Sturkenboom, M. Enhancing vaccine safety capacity globally: A lifecycle perspective. Vaccine 2015, 33 (Suppl. S4), D46–D54. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Auricchio, A.; Vecchiet, J.; Falasca, K. Improving BNT162b2 mRNA vaccine tolerability without efficacy loss by Pidotimod supplementation. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022023. [Google Scholar] [CrossRef] [PubMed]
| Study | Vaccine | Participants (Young/Old) | Controls (Young/Old) | Age Range (Young/Old) | Country |
|---|---|---|---|---|---|
| J. Sadoff et al. | Ad26.COV2.S | 162/161 | 82/81 | 18–55/≥65 | Belgium and United States |
| Shengli Xia et al. | BBIBP-CorV | 72/72 | 24/24 | 18–59/≥60 | China |
| Jingxin Li et al. | BNT162b1 | 48/48 | 24/24 | 18–55/65–85 | China |
| Maheshi N Ramasamy et al. | ChAdOx1 | 50/96 | 49/20 | 18–55/≥70 | United Kingdom |
| Gang Zeng et al. | CoronaVac | 430/256 | 110/47 | 18–59/≥60 | China |
| Jiankai Liu et al. | KCONVAC | 200/200 | 50/50 | 18–59/≥60 | China |
| Neil Formica et al. | NVX-CoV2373 | 561/467 | 139/116 | 18–59/≥60 | United States, Australia |
| Ya-Jun Shu et al. | V-01 | 360/360 | 80/80 | 18–59/≥60 | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, L.; Tian, T.; Li, W.; Pan, Y.; Wang, Y. Efficacy and Safety of COVID-19 Vaccination in Older Adults: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 33. https://doi.org/10.3390/vaccines11010033
Zhang L, Jiang L, Tian T, Li W, Pan Y, Wang Y. Efficacy and Safety of COVID-19 Vaccination in Older Adults: A Systematic Review and Meta-Analysis. Vaccines. 2023; 11(1):33. https://doi.org/10.3390/vaccines11010033
Chicago/Turabian StyleZhang, Lei, Lihong Jiang, Tian Tian, Wenjing Li, Yonghui Pan, and Yongchen Wang. 2023. "Efficacy and Safety of COVID-19 Vaccination in Older Adults: A Systematic Review and Meta-Analysis" Vaccines 11, no. 1: 33. https://doi.org/10.3390/vaccines11010033
APA StyleZhang, L., Jiang, L., Tian, T., Li, W., Pan, Y., & Wang, Y. (2023). Efficacy and Safety of COVID-19 Vaccination in Older Adults: A Systematic Review and Meta-Analysis. Vaccines, 11(1), 33. https://doi.org/10.3390/vaccines11010033





