Determination of Conformational and Functional Stability of Potential Plague Vaccine Candidate in Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Materials
2.3. Methods
2.3.1. LcrV Formulations
2.3.2. Rheology Measurements
2.3.3. UV–Visible and Fluorescence Spectroscopy
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Particle Size Distribution and Zeta Analysis
2.3.6. Polyacrylamide Gel Electrophoresis (PAGE)
2.3.7. Circular Dichroism
2.3.8. Transmission Electron Microscopy
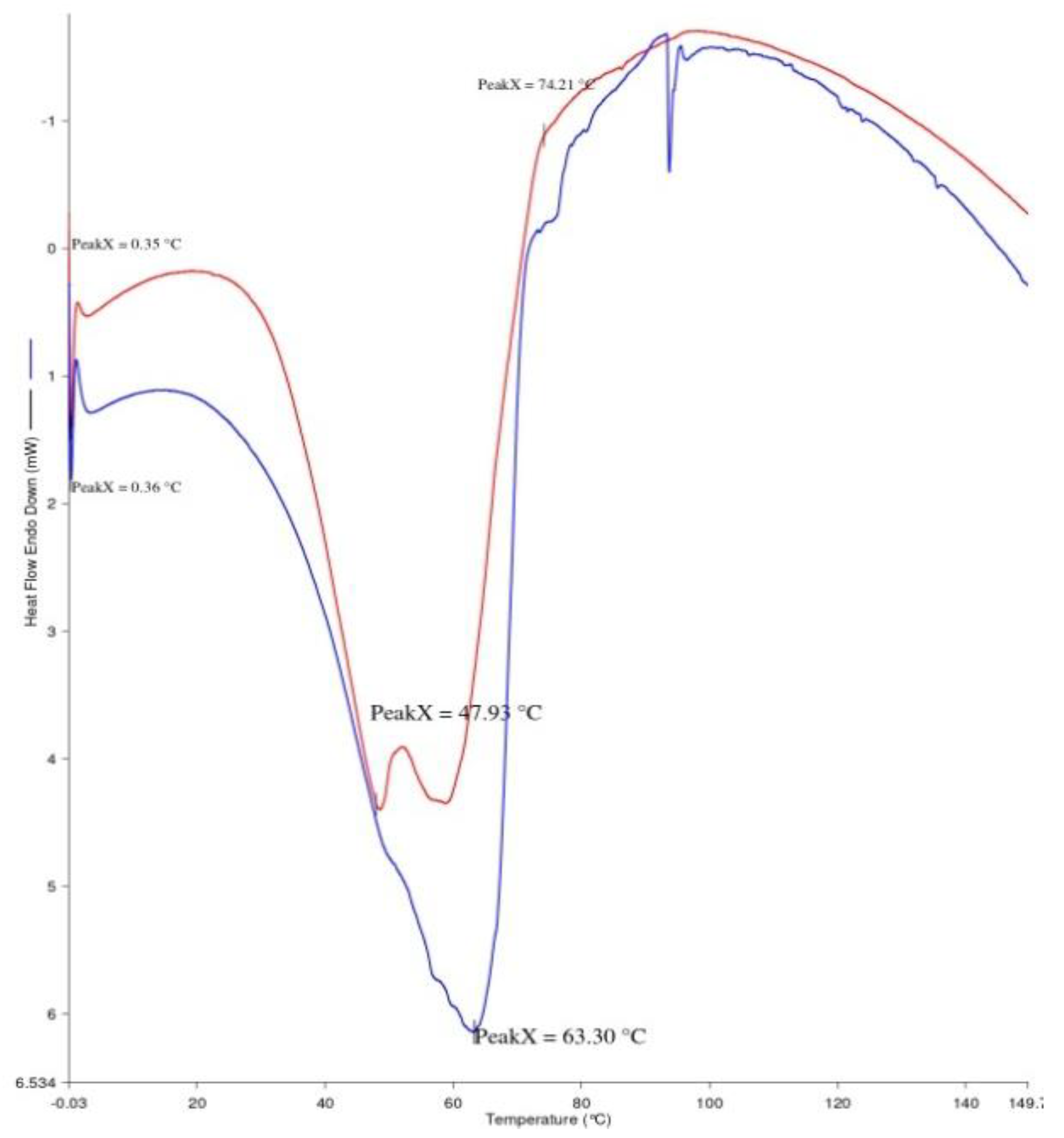
2.3.9. Differential Scanning Calorimetry
2.3.10. Immunization of Mice
2.3.11. IgG Antibody Immune Response
2.3.12. Statistical Analysis
3. Results and Discussion
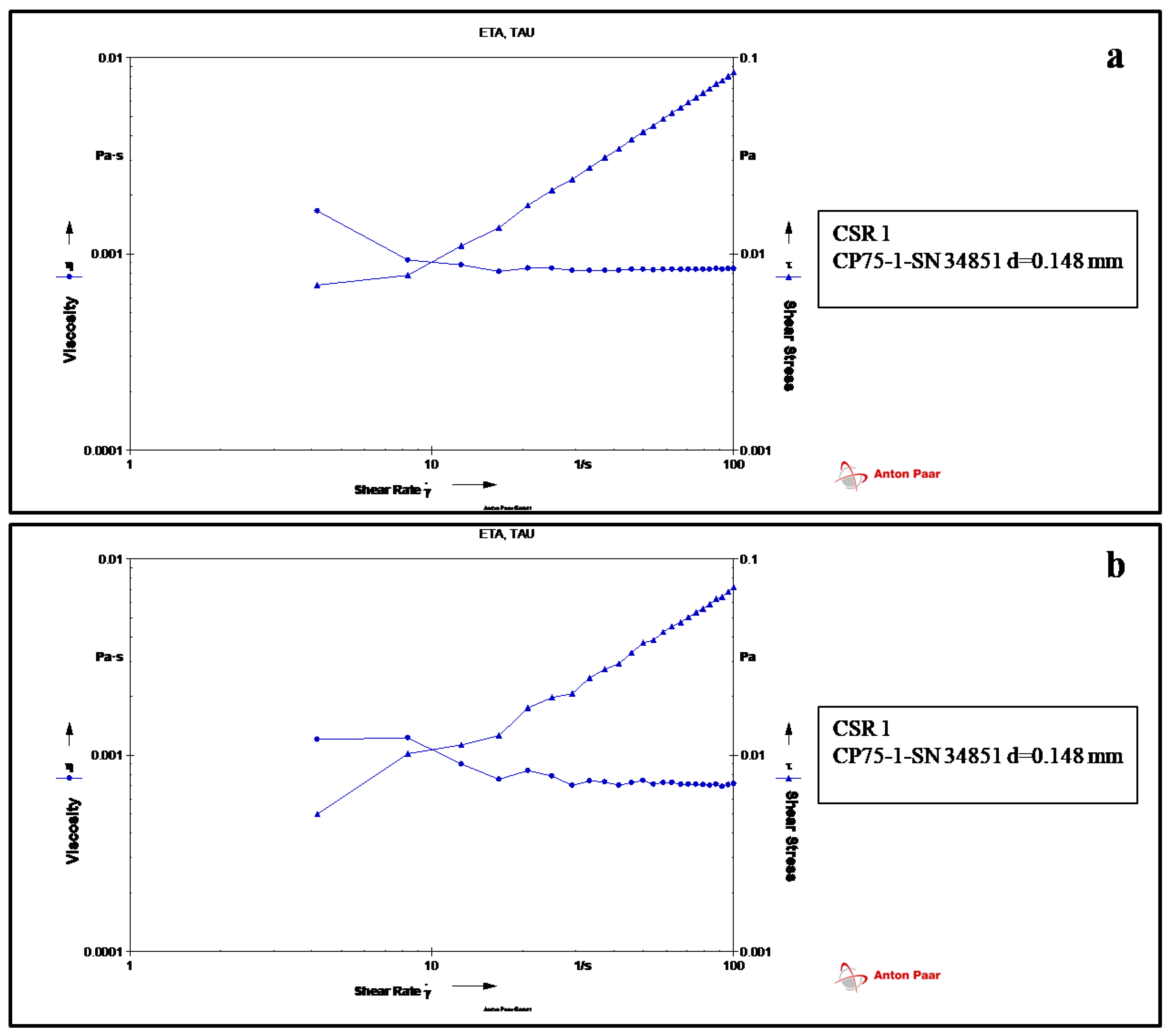
3.1. Rheology
3.2. UV-Visible Spectroscopy
3.3. Fluorescence Spectroscopy
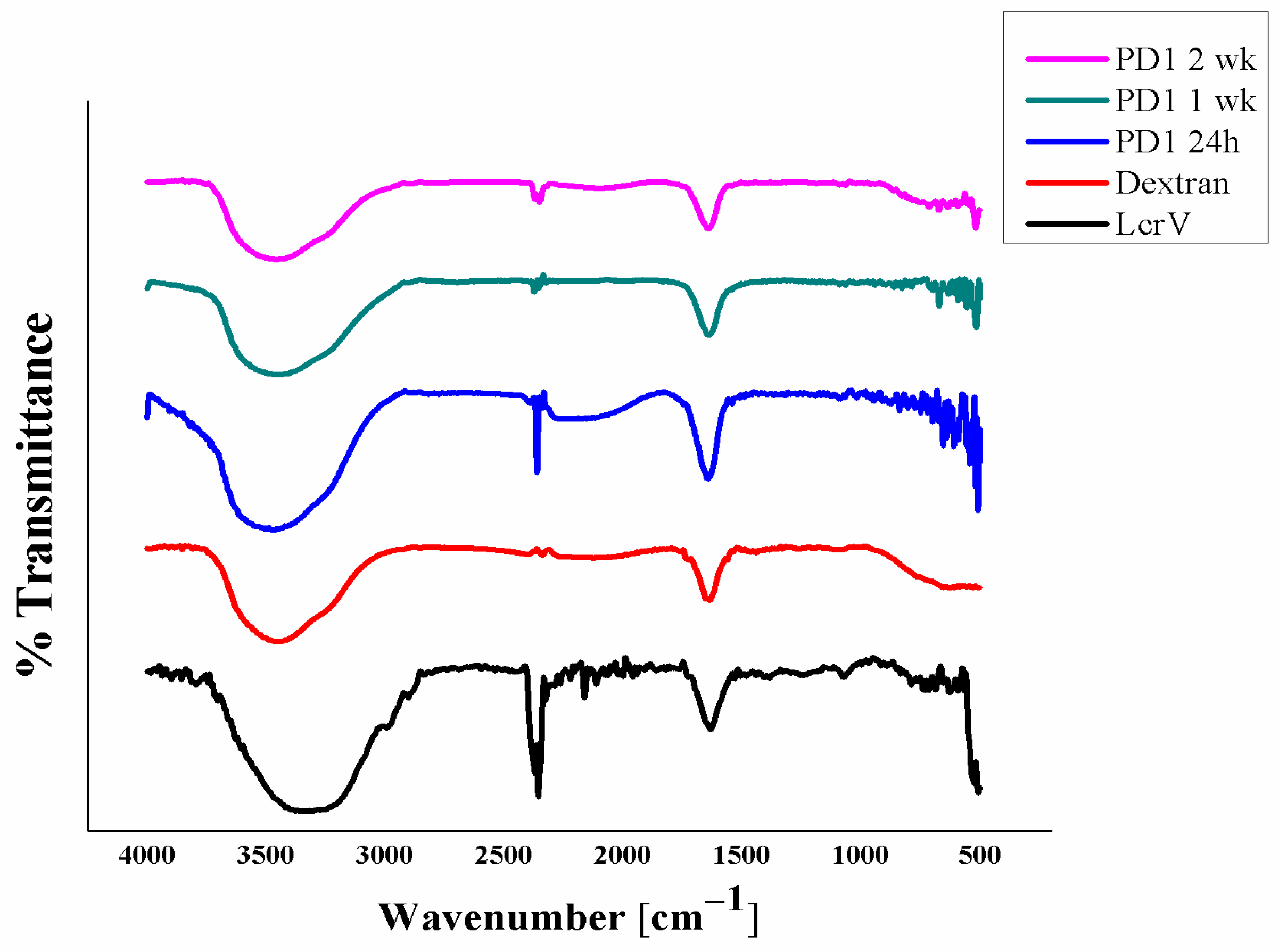
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Particle Size Distribution Analysis
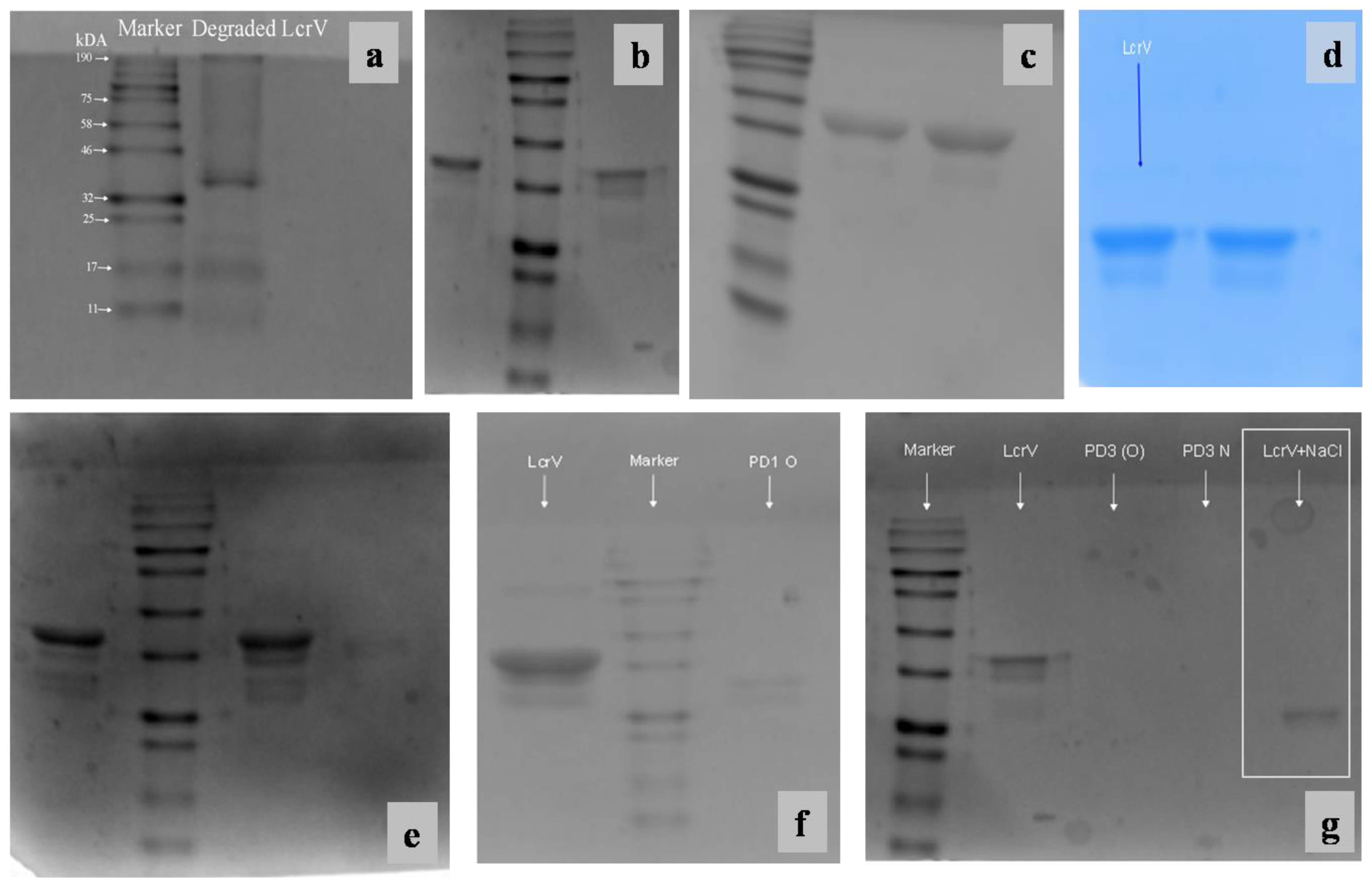
3.6. Gel Electrophoresis
3.7. Circular Dichroism
3.8. Transmission Electron Microscopy
3.9. Differential Scanning Calorimetry
3.10. IgG Antibody Response
3.11. Mechanism of Protein Stabilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Naim, A. 162 Dominant forces in protein folding. J. Biomol. Struct. Dyn. 2013, 31, 106. [Google Scholar] [CrossRef]
- Konrad, W. Mistakes in Storage May Alter Medication. 2011. The New York Times. Available online: https://www.nytimes.com/2011/08/16/health/16consumer.html (accessed on 18 February 2022).
- Batra, L.; Verma, S.K.; Nagar, D.P.; Saxena, N.; Pathak, P.; Pant, S.C.; Tuteja, U. HSP70 Domain II of Mycobacterium tuberculosis Modulates Immune Response and Protective Potential of F1 and LcrV Antigens of Yersinia pestis in a Mouse Model. PLoS Negl. Trop. Dis. 2014, 8, e3322. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Plague. Available online: https://www.who.int/news-room/fact-sheets/detail/plague (accessed on 18 February 2022).
- Dos Santos Grácio, A.J.; Grácio, M.A.A. Plague: A Millenary Infectious Disease Reemerging in the XXI Century. BioMed Res. Int. 2017, 2017, 5696542. [Google Scholar] [CrossRef]
- CDC, Centers for Disease Control and Prevention. 2018. Available online: https://emergency.cdc.gov/agent/agentlist-category.asp (accessed on 18 February 2022).
- Biosafety Levels for Biological Agents—Stanford Environmental Health & Safety. Available online: https://ehs.stanford.edu/reference/biosafety-levels-biological-agents (accessed on 18 February 2022).
- Drancourt, M.; Raoult, D. Yersinia pestis and the three plague pandemics. Lancet Infect. Dis. 2014, 14, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.G.; Anderson, G.W., Jr.; Mauro, J.M.; Welkos, S.L.; Andrews, G.P.; Adamovicz, J.; Friedlander, A.M. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 1998, 16, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J. Therapeutic protein aggregation: Mechanisms, design, and control. Trends Biotechnol. 2014, 32, 372–380. [Google Scholar] [CrossRef]
- Ragoonanan, V.; Aksan, A. Protein Stabilization. Transfus. Med. Hemother. 2007, 34, 246–252. [Google Scholar] [CrossRef]
- Al-Tahami, K.; Singh, J. Smart Polymer Based Delivery Systems for Peptides and Proteins. Recent Pat. Drug Deliv. Formul. 2007, 1, 65–71. [Google Scholar] [CrossRef]
- Burckbuchler, V.; Mekhloufi, G.; Giteau, A.P.; Grossiord, J.L.; Huille, S.; Agnely, F. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur. J. Pharm. Biopharm. 2010, 76, 351–356. [Google Scholar] [CrossRef]
- Garidel, P.; Hegyi, M.; Bassarab, S.; Weichel, M. A rapid, sensitive and economical assessment of monoclonal antibody conformational stability by intrinsic tryptophan fluorescence spectroscopy. Biotechnol. J. 2008, 3, 1201–1211. [Google Scholar] [CrossRef]
- Antosiewicz, J.M.; Shugar, D. UV–Vis spectroscopy of tyrosine side-groups in studies of protein structure. Part 2: Selected applications. Biophys. Rev. 2016, 8, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.; Li, C.; Eremina, N.; Goormaghtigh, E.; Barth, A. Simultaneous Fitting of Absorption Spectra and Their Second Derivatives for an Improved Analysis of Protein Infrared Spectra. Molecules 2015, 20, 12599–12622. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.S.; Kalonia, D.S. Viscosity Analysis of Dual Variable Domain Immunoglobulin Protein Solutions: Role of Size, Electroviscous Effect and Protein-Protein Interactions. Pharm. Res. 2015, 33, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.J. Quantitative two-dimensional gel electrophoresis: From proteins to proteomes. Biochem. Soc. Trans. 1997, 25, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Koristka, S.; Bartsch, H.; Bachmann, M. Native Polyacrylamide Gels. Methods Mol. Biol. 2012, 49–53. [Google Scholar] [CrossRef]
- Benjwal, S. Monitoring protein aggregation during thermal unfolding in circular dichroism experiments. Protein Sci. 2006, 15, 635–639. [Google Scholar] [CrossRef]
- Stevenson, H.P.; Makhov, A.M.; Calero, M.; Edwards, A.L.; Zeldin, O.B.; Mathews, I.I.; Lin, G.; Barnes, C.O.; Santamaria, H.; Ross, T.M.; et al. Use of transmission electron microscopy to identify nanocrystals of challenging protein targets. Proc. Natl. Acad. Sci. USA 2014, 111, 8470–8475. [Google Scholar] [CrossRef]
- Vaskoska, R.; Vénien, A.; Ha, M.; White, J.D.; Unnithan, R.R.; Astruc, T.; Warner, R.D. Thermal denaturation of proteins in the muscle fibre and connective tissue from bovine muscles composed of type I (masseter) or type II (cutaneous trunci) fibres: DSC and FTIR microspectroscopy study. Food Chem. 2021, 343, 128544. [Google Scholar] [CrossRef]
- Morgan, S.h.M.; Diab, M.A.; El-Sonbati, A.Z. Synthesis, spectroscopic, thermal properties, Calf thymus DNA binding and quantum chemical studies of M(II) complexes. Appl. Organomet. Chem. 2018, 32, e4281. [Google Scholar] [CrossRef]
- Jiang, J.; Abramavicius, D.; Bulheller, B.M.; Hirst, J.D.; Mukamel, S. Ultraviolet Spectroscopy of Protein Backbone Transitions in Aqueous Solution: Combined QM and MM Simulations. J. Phys. Chem. B 2010, 114, 8270–8277. [Google Scholar] [CrossRef]
- Pignataro, M.F.; Herrera, M.G.; Dodero, V.I. Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods. Molecules 2020, 25, 4854. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Satish, L.; Das, D.P.; Sahoo, H.; Ghosh, M.K. Construing the interactions between MnO2 nanoparticle and bovine serum albumin: Insight into the structure and stability of a protein–nanoparticle complex. New J. Chem. 2017, 41, 8130–8139. [Google Scholar] [CrossRef]
- Caroline, G.; Eric, F.; Bohn, Y.-S.T.; Sylvie, E.; Attree, I. Oligomerization of PcrV and LcrV, Protective Antigens of Pseudomonas aeruginosa and Yersinia pestis. J. Biol. Chem. 2008, 283, 23940–23949. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Etemad SGh Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluid. Nanofluid 2014, 18, 1023–1030. [Google Scholar] [CrossRef]
- Clarke, S. Development of Hierarchical Magnetic Nanocomposite Materials for Biomedical Applications. Ph.D. Dissertation, Dublin City University, Dublin, Ireland, 2013. [Google Scholar]
- Hirsjarvi, S.; Peltonen, L.; Hirvonen, J. Surface pressure measurements in particle interaction and stability studies of poly(lactic acid) nanoparticles. Int. J. Pharm. 2008, 348, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Srivastava, A.; Vasaikar, S.V.; Mukherjee, G.; Mishra, P.; Kundu, B. Selective Interception of Gelsolin Amyloidogenic Stretch Results in Conformationally Distinct Aggregates with Reduced Toxicity. ACS Chem. Neurosci. 2014, 5, 982–992. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Angioletti-Uberti, S.; Dzubiella, J.; Wischke, C.; Neffe, A.T.; Lendlein, A.; Ballauff, M.; Haag, R. Protein Interactions with Polymer Coatings and Biomaterials. Angew. Chem. Int. Ed. 2014, 53, 8004–8031. [Google Scholar] [CrossRef]
- Malhotra, A.; Coupland, J.N. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocoll. 2004, 18, 101–108. [Google Scholar] [CrossRef]
- Tatkiewicz, W.; Elizondo, E.; Moreno, E.; Díez-Gil, C.; Ventosa, N.; Veciana, J.; Ratera, I. Methods for Characterization of Protein Aggregates. Methods Mol. Biol. 2015, 1258, 387–401. [Google Scholar] [CrossRef]
- Durowoju, I.B.; Bhandal, K.S.; Hu, J.; Carpick, B.; Kirkitadze, M. Differential Scanning Calorimetry—A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J. Vis. Exp. 2017, 2017, 55262. [Google Scholar] [CrossRef]
- Ohtake, S.; Kita, Y.; Arakawa, T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv. Drug Deliv. Rev. 2011, 63, 1053–1073. [Google Scholar] [CrossRef] [PubMed]
- Derewenda, U.; Mateja, A.; Devedjiev, Y.; Routzahn, K.M.; Evdokimov, A.G.; Derewenda, Z.S.; Waugh, D.S. The Structure of Yersinia pestis V-Antigen, an Essential Virulence Factor and Mediator of Immunity against Plague. Structure 2004, 12, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.A.; Nilles, M.L. Structure-Function Analysis of the C-Terminal Domain of LcrV from Yersinia pestis. J. Bacteriol. 2007, 189, 6734–6739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Espina, M.; Ausar, S.F.; Middaugh, C.R.; Baxter, M.A.; Picking, W.D.; Picking, W.L. Conformational stability and differential structural analysis of LcrV, PcrV, BipD, and SipD from type III secretion systems. Protein Sci. 2007, 16, 704–714. [Google Scholar] [CrossRef]
- Piehler, J.; Brecht, A.; Hehl, K.; Gauglitz, G. Protein interactions in covalently attached dextran layers. Colloids Surf. B Biointerfaces 1999, 13, 325–336. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Wu, L.-J.; Chen, J.; Liang, Y. Effects of macromolecular crowding on the structural stability of human α-lactalbumin. Acta Biochim. Biophys. Sin. 2012, 44, 703–711. [Google Scholar] [CrossRef]
- Shahid, S.; Hasan, I.; Ahmad, F.; Hassan, M.I.; Islam, A. Carbohydrate-Based Macromolecular Crowding-Induced Stabilization of Proteins: Towards Understanding the Significance of the Size of the Crowder. Biomolecules 2019, 9, 477. [Google Scholar] [CrossRef]
- Spencer, D.S.; Xu, K.; Logan, T.M.; Zhou, H.-X. Effects of pH, Salt, and Macromolecular Crowding on the Stability of FK506-binding Protein: An Integrated Experimental and Theoretical Study. J. Mol. Biol. 2005, 351, 219–232. [Google Scholar] [CrossRef]
- Batra, J.; Xu, K.; Zhou, H.-X. Nonadditive effects of mixed crowding on protein stability. Proteins 2009, 77, 133–138. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Smith, T.; Hicks, R.H.; Doekhie, A.; Koumanov, F.; Wells, S.A.; Edler, K.J.; van den Elsen, J.; Holman, G.D.; Marchbank, K.J.; et al. Thermal stability, storage and release of proteins with tailored fit in silica. Sci. Rep. 2017, 7, 46568. [Google Scholar] [CrossRef]
- Verma, S.K.; Batra, L.; Tuteja, U. A Recombinant Trivalent Fusion Protein F1–LcrV–HSP70(II) Augments Humoral and Cellular Immune Responses and Imparts Full Protection against Yersinia pestis. Front. Microbiol. 2016, 7, 1053. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Narayan, B.; Kumar, S.; Verma, S.K. Vaccine Potential of a Recombinant Bivalent Fusion Protein LcrV-HSP70 Against Plague and Yersiniosis. Front. Immunol. 2020, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Crofts, F.; Devitt, A.; Griffiths, H.R.; Kastner, E.; Nadella, V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2016, 99, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Timasheff, S.N. Control of Protein Stability and Reactions by Weakly Interacting Cosolvents: The Simplicity of the Complicated. Adv. Protein Chem. 1998, 51, 355–432. [Google Scholar] [CrossRef]
- Timasheff, S.N. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA 2002, 99, 9721–9726. [Google Scholar] [CrossRef]
- Dextran, National Center for Biotechnology Information. PubChem Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dextran (accessed on 18 February 2022).
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2016, 45, D313–D319. [Google Scholar] [CrossRef]
- Allison, S.D.; Manning, M.C.; Randolph, T.W.; Middleton, K.; Davis, A.; Carpenter, J.F. Optimization of storage stability of lyophilized actin using combinations of disaccharides and dextran. J. Pharm. Sci. 2000, 89, 199. [Google Scholar] [CrossRef]
- Udema, I.I.; Onigbindec, A.O. Activity Coefficient of Solution Components and Salts as Special Osmolyte from Kirkwood-Buff Theoretical Perspective. Asian J. Res. Biochem. 2019, 4, 1–20. [Google Scholar] [CrossRef]
- Athirathinam, K.; Nandakumar, S.; Kandasamy, R. Biopolymers and Osmolytes—A Focus towards the Prospects of Stability and Adjuvanticity of Vaccines. Macromol. Res. 2022, 30, 599–608. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athirathinam, K.; Nandakumar, S.; Verma, S.K.; Kandasamy, R. Determination of Conformational and Functional Stability of Potential Plague Vaccine Candidate in Formulation. Vaccines 2023, 11, 27. https://doi.org/10.3390/vaccines11010027
Athirathinam K, Nandakumar S, Verma SK, Kandasamy R. Determination of Conformational and Functional Stability of Potential Plague Vaccine Candidate in Formulation. Vaccines. 2023; 11(1):27. https://doi.org/10.3390/vaccines11010027
Chicago/Turabian StyleAthirathinam, Krubha, Selvasudha Nandakumar, Shailendra Kumar Verma, and Ruckmani Kandasamy. 2023. "Determination of Conformational and Functional Stability of Potential Plague Vaccine Candidate in Formulation" Vaccines 11, no. 1: 27. https://doi.org/10.3390/vaccines11010027
APA StyleAthirathinam, K., Nandakumar, S., Verma, S. K., & Kandasamy, R. (2023). Determination of Conformational and Functional Stability of Potential Plague Vaccine Candidate in Formulation. Vaccines, 11(1), 27. https://doi.org/10.3390/vaccines11010027







