Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. SARS-CoV-2 Antibody Detection Assay
2.3. Lymphocyte Subpopulations
2.4. Cytokine Secretion Assay
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
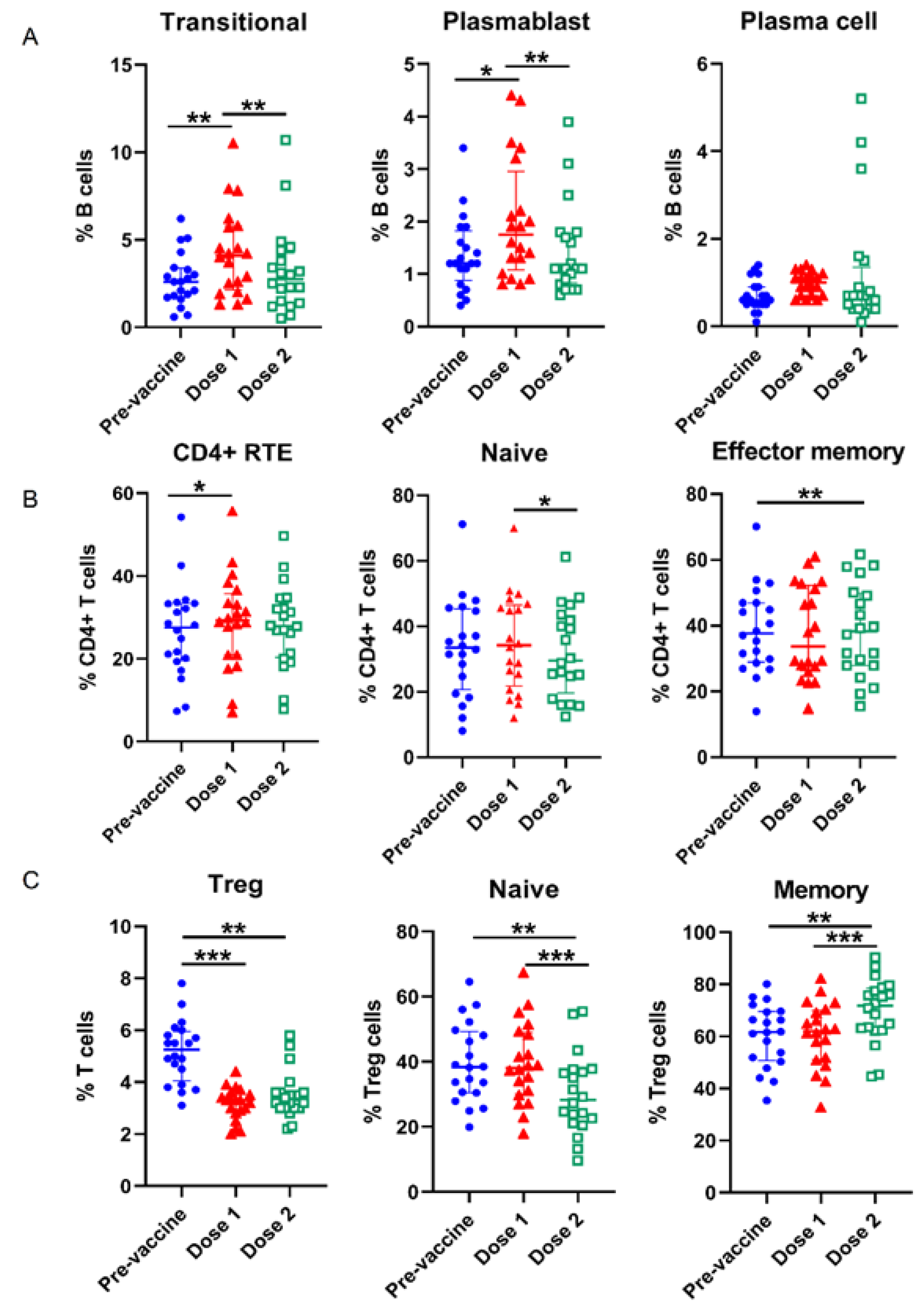
3.2. Alterations of Lymphocyte Subsets in the Course of COVID-19 Vaccination
3.3. Lymphocyte Response to COVID-19 Vaccine Brands
3.4. Cytokines Alterations and Response to COVID-19 Vaccine Brands
3.5. Longitudinal Predictors for Determination of COVID-19 nAbs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Castilletti, C.; Goletti, D.; Sacchi, A.; Bordoni, V.; Mariotti, D.; Notari, S.; Matusali, G.; Meschi, S.; Petrone, L.; et al. Persistent Spike-specific T cell immunity despite antibody reduction after 3 months from SARS-CoV-2 BNT162b2-mRNA vaccine. Sci. Rep. 2022, 12, 6687. [Google Scholar] [CrossRef]
- Hurme, A.; Jalkanen, P.; Heroum, J.; Liedes, O.; Vara, S.; Melin, M.; Terasjarvi, J.; He, Q.; Poysti, S.; Hanninen, A.; et al. Long-Lasting T Cell Responses in BNT162b2 COVID-19 mRNA Vaccinees and COVID-19 Convalescent Patients. Front. Immunol. 2022, 13, 869990. [Google Scholar] [CrossRef] [PubMed]
- Piano Mortari, E.; Russo, C.; Vinci, M.R.; Terreri, S.; Fernandez Salinas, A.; Piccioni, L.; Alteri, C.; Colagrossi, L.; Coltella, L.; Ranno, S.; et al. Highly Specific Memory B Cells Generation after the 2nd Dose of BNT162b2 Vaccine Compensate for the Decline of Serum Antibodies and Absence of Mucosal IgA. Cells 2021, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Terreri, S.; Piano Mortari, E.; Vinci, M.R.; Russo, C.; Alteri, C.; Albano, C.; Colavita, F.; Gramigna, G.; Agrati, C.; Linardos, G.; et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe 2022, 30, 400–408.e4. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Jordan, S.C.; Shin, B.H.; Gadsden, T.M.; Chu, M.; Petrosyan, A.; Le, C.N.; Zabner, R.; Oft, J.; Pedraza, I.; Cheng, S.; et al. T cell immune responses to SARS-CoV-2 and variants of concern (Alpha and Delta) in infected and vaccinated individuals. Cell Mol. Immunol. 2021, 18, 2554–2556. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, M.; Zhu, T.; Zhili, N.; Liu, Z.; Xiang, R.; Zhang, W.; Xu, Y. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. Int. J. Infect. Dis. 2020, 98, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, Y.; Ye, J.; Da, H.; Fang, S.; Chen, L. Dynamic changes of T-lymphocyte subsets and the correlations with 89 patients with coronavirus disease 2019 (COVID-19). Ann. Transl. Med. 2020, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Marjani, M.; Mahmoudi, S.; Mortaz, E.; Mansouri, D. Dynamic Changes of Lymphocyte Subsets in the Course of COVID-19. Int. Arch. Allergy. Immunol. 2021, 182, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020, 5, e137799. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Pape, K.A.; Dileepan, T.; Kabage, A.J.; Kozysa, D.; Batres, R.; Evert, C.; Matson, M.; Lopez, S.; Krueger, P.D.; Graiziger, C.; et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021, 37, 109823. [Google Scholar] [CrossRef]
- Chen, Y.C.; Weng, S.W.; Ding, J.Y.; Lee, C.H.; Ku, C.L.; Huang, W.C.; You, H.L.; Huang, W.T. Clinicopathological Manifestations and Immune Phenotypes in Adult-Onset Immunodeficiency with Anti-interferon-gamma Autoantibodies. J. Clin. Immunol. 2022, 42, 672–683. [Google Scholar] [CrossRef]
- Boldt, A.; Borte, S.; Fricke, S.; Kentouche, K.; Emmrich, F.; Borte, M.; Kahlenberg, F.; Sack, U. Eight-color immunophenotyping of T-, B-, and NK-cell subpopulations for characterization of chronic immunodeficiencies. Cytometry B Clin. Cytom. 2014, 86, 191–206. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Metzler, G.; Kolhatkar, N.S.; Rawlings, D.J. BCR and co-receptor crosstalk facilitate the positive selection of self-reactive transitional B cells. Curr. Opin. Immunol. 2015, 37, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Duah, M.; Li, L.; Shen, J.; Lan, Q.; Pan, B.; Xu, K. Thymus Degeneration and Regeneration. Front. Immunol. 2021, 12, 706244. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Barba-Spaeth, G.; Fernandez, I.; Broketa, M.; Azzaoui, I.; de La Selle, A.; Vandenberghe, A.; Fourati, S.; Roeser, A.; Meola, A.; et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 2021, 54, 2893–2907.e2895. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Chappert, P.; Barba-Spaeth, G.; Roeser, A.; Fourati, S.; Azzaoui, I.; Vandenberghe, A.; Fernandez, I.; Meola, A.; Bouvier-Alias, M.; et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell 2021, 184, 1201–1213.e1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Ben Tanfous, T.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e2133. [Google Scholar] [CrossRef]
- Meckiff, B.J.; Ramirez-Suastegui, C.; Fajardo, V.; Chee, S.J.; Kusnadi, A.; Simon, H.; Eschweiler, S.; Grifoni, A.; Pelosi, E.; Weiskopf, D.; et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell 2020, 183, 1340–1353.e1316. [Google Scholar] [CrossRef]
- Xie, M.M.; Fang, S.; Chen, Q.; Liu, H.; Wan, J.; Dent, A.L. Follicular regulatory T cells inhibit the development of granzyme B-expressing follicular helper T cells. JCI Insight 2019, 4, e128076. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Tabrizi, R.; Lankarani, K.B.; Aria, H.; Vakili, S.; Asadian, F.; Noroozi, S.; Keshavarz, P.; Faramarz, S. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Life Sci. 2020, 258, 118167. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]



| Variable | Vaccination | p Value | ||
|---|---|---|---|---|
| Baseline | After 1st Dose | After 2nd Dose | ||
| White blood cells (×103 cells/μL) | 6.4 (5.2, 7.4) | 6.3 (5.6, 7.0) | 6.3 (5.2, 7.5) | 1.000 |
| Lymphocytes (%) | 31.9 (29.2, 35.2) a | 35.3 (30.5, 43.7) a | 35.0 (28.3, 42.9) | 0.031 |
| T cells (% lymphocytes) | 64.4 (58.3, 72.4) | 63.3 (56.0, 70.7) | 63.5 (56.4, 73.5) | 0.819 |
| α/β T cells (% T cells) | 89.8 (86.6, 93.0) | 88.8 (85.6, 93.4) | 87.4 (82.2, 92.0) | 0.142 |
| γδ T cells (% T cells) | 9.4 (6.2, 12.5) b | 10.0 (5.6, 12.7) | 8.9 (5.3, 12.6) b | 0.047 |
| CD4+ T cells (% T cells) | 33.2 (28.5, 38.7) | 33.0 (26.9, 38.5) | 32.2 (26.7, 38.3) | 0.626 |
| CD45RA+CD62L+CD31+ (% CD4+ cells) | 26.7 (19.5, 33.4) a | 29.0 (20.9, 35.7) a | 27.9 (20.3, 34.0) | 0.029 |
| Naïve (% CD4+ cells) | 33.6 (20.8, 45.4) | 35.1 (21.8, 46.6) c | 32.2 (19.6, 43.2) c | 0.015 |
| CD38+HLA-DR+ (% CD4+ cells) | 4.2 (2.7, 4.6) | 4.8 (3.7, 5.7) c | 4.1 (3.0, 4.8) c | 0.021 |
| TCM (% CD4+ cells) | 16.7 (13.5, 19.1) | 15.6 (12.9, 17.8) | 16.2 (11.4, 18.1) | 0.109 |
| TEM (% CD4+ cells) | 38.8 (28.9, 46.9) b | 39.4 (28.3, 49.9) | 40.9 (31.4, 50.3) b | 0.004 |
| TE (% CD4+ cells) | 6.0 (2.2, 8.3) | 4.8 (2.9, 5.9) | 5.1 (2.9, 6.4) | 0.705 |
| CCR5+ (% CD4+ cells) | 18.5 (12.2, 27.5) | 19.4 (13.5, 26.2) | 19.7 (14.4, 23.2) | 0.387 |
| CCR3+ (% CD4+ cells) | 3.6 (1.2, 3.6) ab | 1.9 (0.8, 2.4) a | 1.7 (0.7, 2.3) b | 0.006 |
| CCR6+ (% CD4+ cells) | 15.7 (12.1, 19.5) | 18.9 (15.1, 22.7) | 19.3 (15.2, 22.1) | 0.127 |
| CD8+ T cells (% T cells) | 25.3 (19.3, 29.9) | 24.7 (20.4, 28.1) | 25.6 (20.6, 30.0) | 0.182 |
| CD45RA+CD62L+CD31+ (% CD8+ cells) | 22.3 (12.7, 27.6) | 25.4 (15.4, 34.0) | 24.5 (16.1, 34.3) | 0.182 |
| Naïve (% CD8+ cells) | 31.4 (15.7, 41.1) | 33.6 (18.5, 50.9) | 31.2 (18.8, 43.1) | 0.064 |
| CD38+HLA-DR+ (% CD8+ cells) | 13.6 (9.0, 15.5) a | 16.2 (10.2, 18.8) ac | 14.3 (10.0, 19.1) c | 0.001 |
| TCM (% CD8+ cells) | 1.7 (1.1, 2.4) a | 1.3 (0.6, 1.6) a | 2.0 (0.8, 1.7) | 0.004 |
| TEM (% CD8+ cells) | 38.8 (28.4, 53.9) | 37.6 (26.3, 52.2) | 38.3 (27.9, 49.9) | 0.462 |
| TE (% CD8+ cells) | 21.7 (12.7, 26) | 21.4 (11.6, 32.3) | 22.2 (10.4, 31.1) | 0.892 |
| TEM CCR5+ (% CD8+ cells) | 26.8 (16.9, 33.3) | 25.5 (16.8, 37.0) c | 27.6 (17.4, 41.1) c | 0.022 |
| TE CCR5+ (% CD8+ cells) | 7.4 (3.3, 9.6) | 8.6 (4.5, 9.9) | 10.7 (4.1, 14.5) | 0.158 |
| Regulatory T (Treg) cells (% T cells) | 5.2 (4.1, 6.0) ab | 3.2 (2.8, 3.6)a | 3.5 (3.0, 3.6) b | <0.001 |
| Naïve (% Treg cells) | 39.7 (30.5, 49.3) b | 39.4 (30.0, 49.0) c | 30.1 (21.5, 37.4) bc | <0.001 |
| Memory (% Treg cells) | 60.4 (50.7, 69.6) b | 60.6 (51.1, 70.0) c | 69.9 (62.6, 78.5) bc | <0.001 |
| HLA-DR+ (% Treg cells) | 14.6 (11.2, 18.6) b | 11.9 (8.3, 15.3) | 11.5 (8.9, 13.7) b | 0.019 |
| B cells (% lymphocytes) | 9.5 (6.6, 12.2) | 10.2 (7.8, 12.5)c | 9.3 (7.4, 11.7) c | 0.032 |
| Transitional (% B cells) | 2.7 (1.7, 3.4) a | 4.3 (2.1, 5.8) ac | 3.3 (1.4, 4.3) c | 0.001 |
| Naïve (% B cells) | 69.3 (58.6, 82.5) | 67.8 (52.7, 79.5) | 69.7 (57.7, 78.7) | 0.308 |
| Non-switched memory (% B cells) | 8.8 (4.9, 10.5) | 7.2 (3.6, 11.2) | 7.7 (3.8, 8.7) | 0.091 |
| Switched memory (% B cells) | 11.6 (6.0, 14.6) | 11.0 (6.8, 16.1) | 11.4 (8.4, 14.5) | 0.316 |
| CD21^lowCD38^low (% B cells) | 5.7 (2.3, 9.0) | 7.3 (4.1, 10.0) | 6.2 (2.7, 9.5) | 0.165 |
| Plasmablast (% B cells) | 1.4 (0.9, 1.8) a | 2.0 (1.1, 3.0) ac | 1.4 (0.8–1.8) c | <0.001 |
| Plasma cell (% B cells) | 0.7 (0.5, 0.9) | 1.0 (0.7, 1.2) | 1.2 (0.4, 1.4) | 0.031 |
| NK cells (% lymphocytes) | 24.9 (19.8, 30.9) | 25.6 (16.0, 33.2) | 25.8 (17.2, 33.3) | 0.729 |
| CD56+ bright (% NK cells) | 4.3 (2.1, 6.0) | 3.8 (1.8, 5.2) | 5.0 (2.4, 6.2) | 0.115 |
| CD56+CD16- (% NK cells) | 3.3 (1.3, 4.5) | 2.9 (1.6, 4.1) c | 4.1 (1.5, 5.4) c | 0.031 |
| CD56+CD16+ (% NK cells) | 94.2 (92.4, 96.2) | 94.8 (93.5, 96.8) | 93.6 (91.6, 96.5) | 0.287 |
| CD94/NKG2D (% NK cells) | 50.3 (38.1, 67.2) | 49.5 (39.2, 65.0) | 50.0 (39.0, 66.5) | 0.767 |
| After First Dose | After Second Dose | |||||
|---|---|---|---|---|---|---|
| ChAdOx1-S (n = 15) | mRNA-1273 (n = 5) | p Value | ChAdOx1-S (n = 13) | mRNA-1273 (n = 7) | p Value | |
| Anti S Ab (U/mL) | 26.9 (1.4, 56.2) | 33.6 (8.4, 329.4) | 0.661 | 937.0 (292.5, 1617.5) | 5568.0 (4716.0, 9182.0) | <0.001 |
| Neutral level (%) | 23.6 (8.9, 61.7) | 75.4 (46.2, 85.7) | 0.026 | 78.3 (51.1, 92.6) | 97.3 (96.7, 97.4) | 0.037 |
| White blood cells (×103 cells/μL) | −0.4 (−0.74, 1.2) | −0.2 (−0.8, 1.5) | 0.612 | 0.3 (−0.9, 0.6) | 0.1 (−0.9, 0.9) | 0.817 |
| Lymphocytes (%) | 4.1 (1.7, 8.8) | −1.2 (−4.8, 5.5) | 0.138 | 6.8 (2.7, 8.9) | −1.8 (−6.1, 5.2) | 0.036 |
| Regulatory T (Treg) cells (% T cells) | −1.9 (−2.4, −1.4) | −2.6 (−3.3, −2.1) | 0.066 | −1.7 (−2.5, −1.5) | −1.6 (−2.4, −0.6) | 0.393 |
| Naïve (% Treg cells) | 2.7 (−0.8, 7.8) | −9.5 (−11.3, −4.9) | 0.002 | −6.4 (−10.0, −1.9) | −13.5 (−18.3, −6.7) | 0.097 |
| Memory (% Treg cells) | −2.7 (−7.8, 0.8) | 9.5 (4.9, 11.3) | 0.002 | 6.4 (1.9, 10.0) | 13.5 (6.7, 18.3) | 0.097 |
| HLA-DR+ (% Treg cells) | −3.0 (−7.9, −1.2) | 0.4 (−6.2, 4.2) | 0.349 | −3.9 (−5.4, −1.4) | −3.6 (−0.3, 0.7) | 0.275 |
| After First Dose | After Second Dose | |||||
|---|---|---|---|---|---|---|
| ChAdOx1-S (n = 10) | mRNA-1273 (n = 5) | p Value | ChAdOx1-S (n = 12) | mRNA-1273 (n = 7) | p Value | |
| CD4+ T cells | ||||||
| TNF-α | 22.0 (16.2, 30.9) | 34.6 (13.8, 41.2) | 0.440 | 6.5 (3.6, 24.2) | 46.5 (30.8, 57.4) | <0.001 |
| IFN-γ | 19.0 (11.9, 27.7) | 27.6 (12.8, 30.9) | 0.440 | 8.6 (4.3, 11.9) | 33.2 (15.9, 43.3) | 0.002 |
| IL-2 | 45.8 (27.8, 61.5) | 47.1 (36.4, 56.6) | 0.953 | 31.4 (21.2, 39.8) | 54.9 (41.6, 65.1) | 0.002 |
| IL-21 | 13.5 (8.6, 15.9) | 14.5 (13.2, 19.0) | 0.206 | 3.2 (2.1, 7.4) | 13.7 (10.8, 19.9) | <0.001 |
| GF-β | 1.6 (0.2, 3.6) | 2.3 (1.2, 5.1) | 0.513 | −0.1 (−0.3, 0.1) | 0 (−0.3, 0.3) | 0.650 |
| IL-17 | 0.3 (−0.1, 3.0) | 3.6 (3.4, 4.1) | 0.206 | 0.8 (−0.3, 1.2) | 4.9 (0.5, 7.8) | 0.068 |
| CD8+ T cells | ||||||
| TNF-α | 18.4 (8.8, 21.8) | 42.6 (12.4, 51.8) | 0.206 | 10.6 (−2.8, 23.2) | 51.7 (36.4, 57.1) | <0.001 |
| IFN-γ | 23.8 (8.3, 40.9) | 64.2 (34.4, 69.3) | 0.040 | 20.45 (9.1, 33.4) | 60.6 (56.0, 68.0) | 0.004 |
| IL-2 | 19.0 (8.8, 24.5) | 18.4 (11.1, 27.4) | 1.000 | 7.3 (0.5, 9.7) | 21.6 (17.0, 28.9) | <0.001 |
| IL-21 | 2.4 (1.3, 4.7) | 3.2 (2.6, 3.8) | 0.513 | 0.5 (0.3, 2.2) | 3.1 (1.7, 3.9) | 0.010 |
| TGF-β | 0.9 (0, 1.0) | 0.2 (0, 1.0) | 0.679 | 0 (−0.1, 0.1) | −0.1 (−0.2, 0) | 0.261 |
| IL-17 | 0.8 (0.6, 1.9) | 1.1 (1.0, 3.7) | 0.055 | 0 (−0.1, 0.1) | 0.8 (0.2, 1.2) | 0.004 |
| NK cells | ||||||
| TNF-α | 17.9 (8.8, 27.1) | 43.4 (2.4, 48.4) | 0.513 | 4.6 (1.9, 10.2) | 24.1 (16.8, 40.2) | 0.028 |
| IFN-γ | 42.9 (13.4, 58.8) | 52.7 (7.7, 57.6) | 1.000 | 1.9 (−2.6, 2.6) | 43.9 (13.9, 59.0) | 0.010 |
| IL-2 | 0.4 (0.1, 0.7) | 0 (0, 5.1) | 0.371 | −0.1 (−0.2, 0) | 0 (0, 0.6) | 0.128 |
| IL-21 | −0.1 (−0.3, 0.1) | 0 (−0.2, 1.7) | 0.310 | 0 (−0.2, 0.1) | −0.1 (−0.3, 0) | 0.142 |
| TGF-β | −0.1 (−0.1, 0.1) | −0.1 (−0.2, 0) | 1.000 | 0 (−0.1, 0) | 0 (−0.2, 2.4) | 0.837 |
| IL-17 | 0 (0, 0.3) | 0 (−0.1, 0) | 0.310 | 0 (−0.1, 0.1) | 0 (0, 0) | 0.711 |
| β-Coefficient Estimate | SE | p | |
|---|---|---|---|
| mRNA-1273 (reference category ChAdOx1-S) | 29.2 | 9.5 | 0.002 |
| Total memory cells (% B cells) | 3.1 | 0.8 | <0.001 |
| Non-switched memory cells (% B cells) | 6.4 | 2.7 | 0.016 |
| Regulatory T (Treg) cells (% T cells) | 4.2 | 3.6 | 0.242 |
| Naïve Treg cells (% Treg cells) | −2.0 | 0.5 | <0.001 |
| Memory Treg cells (% Treg cells) | 2.0 | 0.5 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-T.; Weng, S.-W.; Tzeng, H.-T.; Yen, F.-C.; Chiang, Y.-S.; You, H.-L. Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination. Vaccines 2022, 10, 1550. https://doi.org/10.3390/vaccines10091550
Huang W-T, Weng S-W, Tzeng H-T, Yen F-C, Chiang Y-S, You H-L. Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination. Vaccines. 2022; 10(9):1550. https://doi.org/10.3390/vaccines10091550
Chicago/Turabian StyleHuang, Wan-Ting, Shao-Wen Weng, Hong-Tai Tzeng, Feng-Chun Yen, Yu-Shao Chiang, and Huey-Ling You. 2022. "Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination" Vaccines 10, no. 9: 1550. https://doi.org/10.3390/vaccines10091550
APA StyleHuang, W.-T., Weng, S.-W., Tzeng, H.-T., Yen, F.-C., Chiang, Y.-S., & You, H.-L. (2022). Lymphocyte Subpopulations Associated with Neutralizing Antibody Levels of SARS-CoV-2 for COVID-19 Vaccination. Vaccines, 10(9), 1550. https://doi.org/10.3390/vaccines10091550






