The Waxing, Waning, and Predictors of Humoral Responses to Vector-Based SARS-CoV-2 Vaccine in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
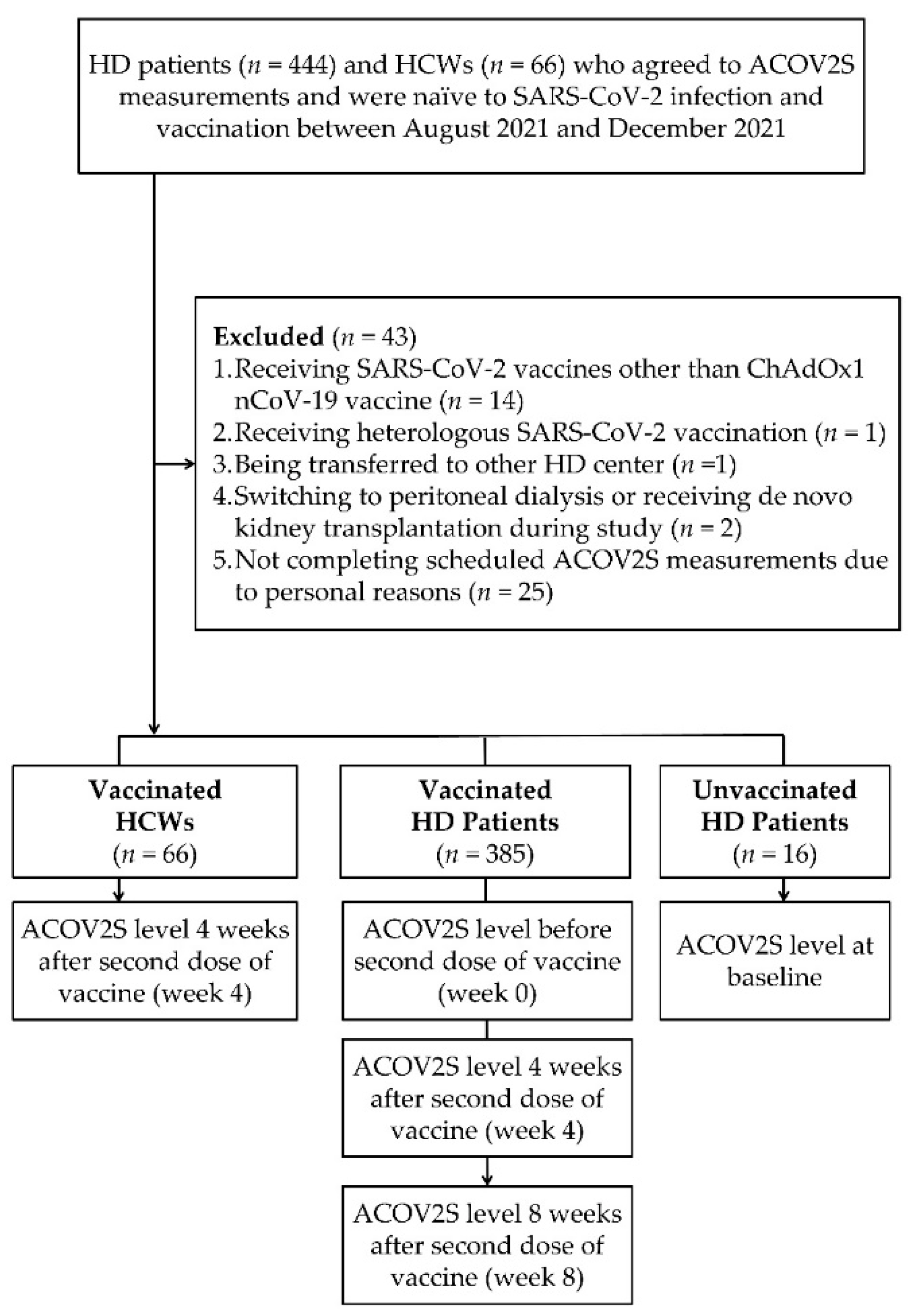
2.1. Enrollment of Study Cohort
2.2. Vaccination Schedule and Measurement of Humoral Response after Vaccination
2.3. Collection of Patient Characteristics and Adverse Events after Vaccination
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Enrolled Patients
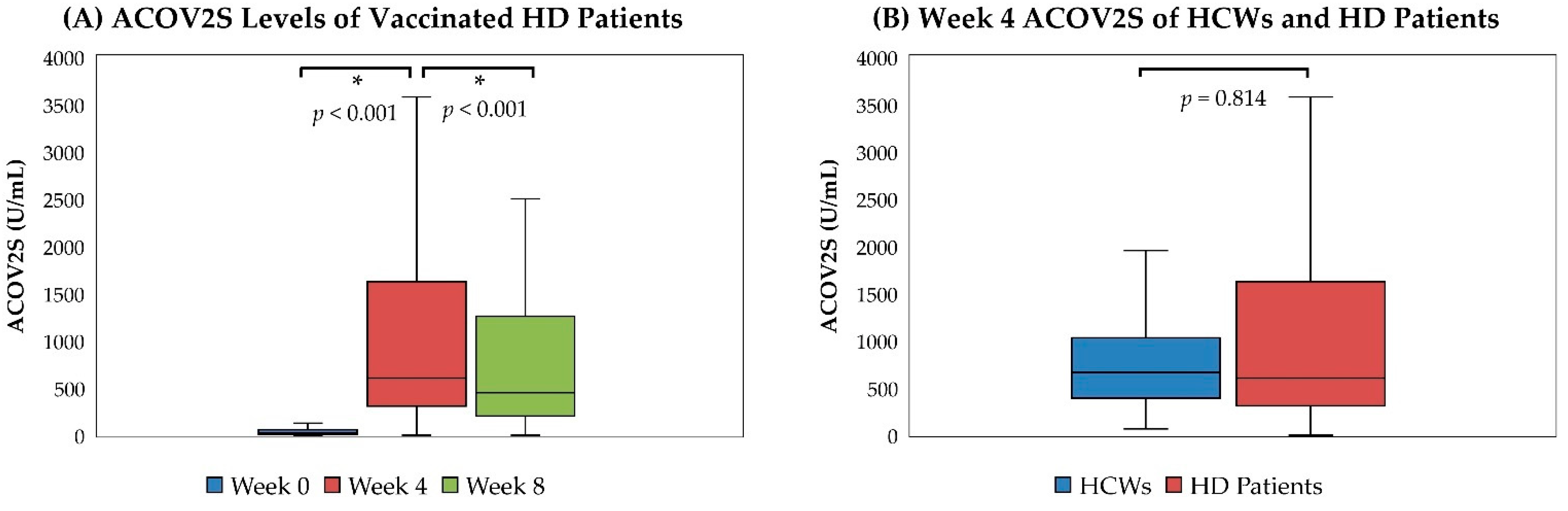
3.2. Humoral Responses of HD Patients and HCWs after Vaccination
3.3. The Adverse Events after Vaccination and Their Associations with Humoral Responses
3.4. Independent Predictors of Humoral Responses and Antibody Waning after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weekly Epidemiological Update on COVID-19. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-may-2022 (accessed on 7 November 2021).
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef] [PubMed]
- El Karoui, K.; De Vriese, A.S. COVID-19 in dialysis: Clinical impact, immune response, prevention, and treatment. Kidney Int. 2022, 101, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Miskulin, D.C.; Manley, H.J.; Stewart, C.; Ladik, V.; Hosford, J.; Lacson, E.C.; Johnson, D.S.; et al. COVID-19 Among US Dialysis Patients: Risk Factors and Outcomes From a National Dialysis Provider. Am. J. Kidney Dis. 2021, 77, 748–756.e741. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, J.E.; Tong, D.C.; Cobb, J.; Rahbari-Oskoui, F.F.; Hosein, D.; Caberto, S.C.; Lea, J.P.; Franch, H.A. Epidemiology of COVID-19 Infection in Hospitalized End-Stage Kidney Disease Patients in a Predominantly African-American Population. Am. J. Nephrol. 2021, 52, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Caplin, B.; Ashby, D.; McCafferty, K.; Hull, R.; Asgari, E.; Ford, M.L.; Cole, N.; Antonelou, M.; Blakey, S.A.; Srinivasa, V.; et al. Risk of COVID-19 Disease, Dialysis Unit Attributes, and Infection Control Strategy among London In-Center Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2021, 16, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Davari, M.; Gnanasampanthan, S.; Roth, N.; Young, G.; Rajakariar, R.; Cove-Smith, A.; Yaqoob, M.M.; Cutino-Moguel, T.; Mahalingasivam, V.; et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol. Dial. Transplant. 2021, 36, 1292–1297. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sibbel, S.; McKeon, K.; Luo, J.; Wendt, K.; Walker, A.G.; Kelley, T.; Lazar, R.; Zywno, M.L.; Connaire, J.J.; Tentori, F.; et al. Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2022, 33, 49–57. [Google Scholar] [CrossRef]
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med. J. 2022, 98, 389–394. [Google Scholar] [CrossRef]
- Taiwan Society of Nephrology. Kidney Disease in Taiwan 2020 Annual Report; Taiwan Society of Nephrology: Taipei, Taiwan, 2020. [Google Scholar]
- Li, P.K.; Chow, K.M.; Van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Jim, B.; Santos, J.; Spath, F.; Cijiang He, J. Biomarkers of diabetic nephropathy, the present and the future. Curr. Diabetes Rev. 2012, 8, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease Control (CDC) and Central Epidemic Command Center (CECC). CECC Reminds Public to follow Its Recommendations for People Receiving Their Second Dose of AstraZeneca or Moderna COVID-19 Vaccine (23 July 2021). Available online: https://www.cdc.gov.tw/En/Bulletin/Detail/GCbQJM2LJoGHepli88kVuw?typeid=158 (accessed on 23 July 2021).
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef]
- Jochum, S.; Kirste, I.; Hortsch, S.; Grunert, V.P.; Legault, H.; Eichenlaub, U.; Kashlan, B.; Pajon, R. Clinical Utility of Elecsys Anti-SARS-CoV-2 S Assay in COVID-19 Vaccination: An Exploratory Analysis of the mRNA-1273 Phase 1 Trial. Front. Immunol. 2021, 12, 798117. [Google Scholar] [CrossRef]
- Rubio-Acero, R.; Castelletti, N.; Fingerle, V.; Olbrich, L.; Bakuli, A.; Wölfel, R.; Girl, P.; Müller, K.; Jochum, S.; Strobl, M.; et al. In Search of the SARS-CoV-2 Protection Correlate: Head-to-Head Comparison of Two Quantitative S1 Assays in Pre-characterized Oligo-/Asymptomatic Patients. Infect. Dis. Ther. 2021, 10, 1505–1518. [Google Scholar] [CrossRef]
- Roche Diagnostics GmbH. Elecsys® Anti-SARS-CoV-2-S, Instructions for Use; Roche Diagnostics GmbH: Basel, Switzerland, 2021. [Google Scholar]
- Hubbard, A.E.; Ahern, J.; Fleischer, N.L.; Van der Laan, M.; Lippman, S.A.; Jewell, N.; Bruckner, T.; Satariano, W.A. To GEE or not to GEE: Comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010, 21, 467–474. [Google Scholar] [CrossRef]
- Lang, T. Documenting research in scientific articles: Guidelines for authors: 3. Reporting multivariate analyses. Chest 2007, 131, 628–632. [Google Scholar] [CrossRef]
- Carr, E.J.; Kronbichler, A.; Graham-Brown, M.; Abra, G.; Argyropoulos, C.; Harper, L.; Lerma, E.V.; Suri, R.S.; Topf, J.; Willicombe, M.; et al. Review of Early Immune Response to SARS-CoV-2 Vaccination Among Patients With CKD. Kidney Int. Rep. 2021, 6, 2292–2304. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; A.Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A.; et al. Antibody Response to COVID-19 vaccination in Patients Receiving Dialysis. J. Am. Soc. Nephrol. 2021, 32, 2435–2438. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.D.M.; Marcos, M.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.L.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.; Abe, K.T.; Naimark, D.; Oliver, M.J.; Perl, J.; Leis, J.A.; Bolotin, S.; Tran, V.; Mullin, S.I.; Shadowitz, E.; et al. Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis. JAMA Netw. Open 2021, 4, e2123622. [Google Scholar] [CrossRef]
- Simon, B.; Rubey, H.; Treipl, A.; Gromann, M.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Paal, M.; Arend, F.M.; Lau, T.; Hasmann, S.; Soreth-Rieke, D.; Sorodoc-Otto, J.; Beuthien, W.; Krappe, J.; Toepfer, M.; von Gersdorff, G.; et al. Antibody response to mRNA SARS-CoV-2 vaccines in haemodialysis patients. Clin. Kidney J. 2021, 14, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Ionita, C.; Marcelli, D.; Nita, C.; Anton, C.; Berca, S.; Vacar, S.; Schiller, O.; Gheorghiu, C.; Barth, C. Comparison of antibody response to two different mRNA COVID-19 vaccines in patients on hemodialysis. J. Nephrol. 2022, 35, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Wu, M.; Harvey, R.; Wall, E.C.; Kelly, G.; Hussain, S.; Howell, M.; Kassiotis, G.; Swanton, C.; Gandhi, S.; et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 2021, 398, 1038–1041. [Google Scholar] [CrossRef]
- Billany, R.E.; Selvaskandan, H.; Adenwalla, S.F.; Hull, K.L.; March, D.S.; Burton, J.O.; Bishop, N.C.; Carr, E.J.; Beale, R.; Tang, J.W.; et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: A call to arms. Kidney Int. 2021, 99, 1492–1494. [Google Scholar] [CrossRef]
- Hsu, C.M.; Weiner, D.E.; Manley, H.J.; Aweh, G.N.; Ladik, V.; Frament, J.; Miskulin, D.; Argyropoulos, C.; Abreo, K.; Chin, A.; et al. Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months. Clin. J. Am. Soc. Nephrol. 2022, 17, 403–413. [Google Scholar] [CrossRef]
- Polewska, K.; Tylicki, P.; Biedunkiewicz, B.; Rucińska, A.; Szydłowska, A.; Kubanek, A.; Rosenberg, I.; Rodak, S.; Ślizień, W.; Renke, M.; et al. Safety and Tolerability of the BNT162b2 mRNA COVID-19 Vaccine in Dialyzed Patients. COViNEPH Project. Medicina 2021, 57, 732. [Google Scholar] [CrossRef]
- Beilhack, G.; Monteforte, R.; Frommlet, F.; Gaggl, M.; Strassl, R.; Vychytil, A. Antibody Response and Safety After mRNA-1273 SARS-CoV-2 Vaccination in Peritoneal Dialysis Patients—The Vienna Cohort. Front. Immunol. 2021, 12, 780594. [Google Scholar] [CrossRef]
- Sanders, J.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.L.; Lin, Z.J.; Bi, Z.F.; Qiu, L.X.; Hu, F.F.; Liu, X.H.; Lin, B.Z.; Su, Y.Y.; Pan, H.R.; Zhang, T.Y.; et al. Inflammation-related adverse reactions following vaccination potentially indicate a stronger immune response. Emerg. Microbes Infect. 2021, 10, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, T.; Bourdenx, J.P.; Martinez, C.; Mucha, S.; Martin-Dupont, P.; Perier, V.; Pommereau, A. Humoral response after SARS-CoV-2 mRNA vaccines in dialysis patients: Integrating anti-SARS-CoV-2 Spike-Protein-RBD antibody monitoring to manage dialysis centers in pandemic times. PLoS ONE 2021, 16, e0257646. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2021, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Aksu, K.; Demir, Ş.; Topel, M.; Yeşilkaya, S.; Ateş, H.; Koca Kalkan, İ.; Öncül, A.; Çuhadar Erçelebi, D.; Türkyılmaz, S. COVID-19 in patients with severe asthma using biological agents. Tuberk Toraks 2021, 69, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Krawczyk, A.; Brochhagen, L.; van de Sand, L.; Sorge-Hädicke, B.; Tyczynski, B.; Witzke, O.; et al. Decline of Humoral Responses 6 Months after Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2022, 10, 327. [Google Scholar] [CrossRef]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after BNT162b2 mRNA COVID-19 vaccination in patients on haemodialysis depends on immune status. Clin. Kidney J. 2021, 14, 2266–2267. [Google Scholar] [CrossRef]
- Espi, M.; Charmetant, X.; Barba, T.; Mathieu, C.; Pelletier, C.; Koppe, L.; Chalencon, E.; Kalbacher, E.; Mathias, V.; Ovize, A.; et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022, 101, 390–402. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Zhang, M.; Hou, J.; Wang, H.; Jiang, Y.; Wang, H.; Gao, P. The shifted balance between circulating follicular regulatory T cells and follicular helper T cells in patients with ulcerative colitis. Clin. Sci. 2017, 131, 2933–2945. [Google Scholar] [CrossRef]
- Shan, Y.; Qi, C.; Liu, Y.; Gao, H.; Zhao, D.; Jiang, Y. Increased frequency of peripheral blood follicular helper T cells and elevated serum IL-21 levels in patients with knee osteoarthritis. Mol. Med. Rep. 2017, 15, 1095–1102. [Google Scholar] [CrossRef]
- Ghamar Talepoor, A.; Khosropanah, S.; Doroudchi, M. Functional subsets of circulating follicular helper T cells in patients with atherosclerosis. Physiol. Rep. 2020, 8, e14637. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Wuescher, L.M.; Worth, R.G. Platelets: Essential components of the immune system. Curr. Trends Immunol. 2015, 16, 65–78. [Google Scholar]
- Li, T.; Yang, Y.; Li, Y.; Wang, Z.; Ma, F.; Luo, R.; Xu, X.; Zhou, G.; Wang, J.; Niu, J.; et al. Platelets mediate inflammatory monocyte activation by SARS-CoV-2 spike protein. J. Clin. Investig. 2022, 132, e150101. [Google Scholar] [CrossRef]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate with SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Shah, R.; Haddad, N.; Vachharajani, T.J.; Asif, A.; Agarwal, A. Thrombocytopenia in ESRD patients: Epidemiology, mechanisms and interventional nephrology perspective. Semin. Dial. 2014, 27, 618–625. [Google Scholar] [CrossRef]
- Hamza, E.; Metzinger, L.; Metzinger-Le Meuth, V. Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review. Cells 2020, 9, 39. [Google Scholar] [CrossRef]
- Boccardo, P.; Remuzzi, G.; Galbusera, M. Platelet dysfunction in renal failure. Semin. Thromb. Hemost. 2004, 30, 579–589. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chan, M.J.; Su, Y.J.; Fu, J.F.; Wang, I.K.; Chen, C.Y.; Weng, C.H.; Huang, W.H.; Hsu, C.W.; Yen, T.H. Bone Marrow Hypocellularity in Patients with End-Stage Kidney Disease. Healthcare 2021, 9, 1452. [Google Scholar] [CrossRef]
- COVID-19 Dashboard–World Map. Available online: https://coronavirus.jhu.edu/region/taiwan (accessed on 13 July 2022).
- Prevention and Control of COVID-19 in Taiwan. Available online: https://www.cdc.gov.tw/En/Category/Page/0vq8rsAob_9HCi5GQ5jH1Q (accessed on 13 July 2022).
- Chen, S.C. Taiwan’s experience in fighting COVID-19. Nat. Immunol. 2021, 22, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Irsara, C.; Egger, A.E.; Prokop, W.; Nairz, M.; Loacker, L.; Sahanic, S.; Pizzini, A.; Sonnweber, T.; Holzer, B.; Mayer, W.; et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin. Chem. Lab. Med. 2021, 59, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Korth, J.; Jahn, M.; Kribben, A. COVID-19 vaccination in patients receiving dialysis. Nat. Rev. Nephrol. 2021, 17, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Morgan, C.; Kerschmann, R.; Beyer, P.; Dittrich, M.; et al. SARS-CoV-2 Vaccine Antibody Response and Breakthrough Infection in Patients Receiving Dialysis. Ann. Intern. Med. 2022, 175, 371–378. [Google Scholar] [CrossRef]

| Demographic Profiles | |
| Age (year), median (IQR) | 64 (55–70) |
| Female, n (%) | 185 (48.05) |
| BMI (kg/m2), median (IQR) | 22.10 (19.50–24.70) |
| Dry Body Weight (kg), median (IQR) | 58.00 (51.00–66.60) |
| HD Vintage (year), median (IQR) | 5.62 (2.56–14.31) |
| Urea Reduction Ratio, median (IQR) | 0.74 (0.69–0.78) |
| Kt/V, median (IQR) | 1.63 (1.39–1.86) |
| Diabetes, n (%) | 137 (35.58) |
| Hypertension, n (%) | 201 (52.21) |
| Chronic Hepatitis B, n (%) | 55 (14.29) |
| Chronic Hepatitis C, n (%) | 31 (8.05) |
| SLE, n (%) | 13 (3.38) |
| Transplantation History #, n (%) | 15 (3.90) |
| Immunosuppressant, n (%) | 40 (10.39) |
| Clinical Profiles | |
| Hemoglobin (g/L), median (IQR) | 108.00 (101.00–114.00) |
| Leukocyte (109/L), median (IQR) | 6.10 (5.10–7.30) |
| Platelet (109/L), median (IQR) | 180.00 (147.00–222.50) |
| BUN (mmol/L), median (IQR) | 22.85 (19.28–27.13) |
| SCr (μmol/L), median (IQR) | 935.27 (811.07–1086.88) |
| Alb (g/L), median (IQR) | 40.40 (38.40–42.05) |
| ALT (μkat/L), median (IQR) | 0.20 (0.15–0.28) |
| Total Bilirubin (μmol/L), median (IQR) | 5.13 (3.42–6.84) |
| Total Cholesterol (mmol/L), median (IQR) | 4.07 (3.52–4.69) |
| Triglyceride (mmol/L), median (IQR) | 1.30 (0.88–2.07) |
| Ferritin (μg/L), median (IQR) | 308.10 (179.05–462.25) |
| Transferrin Saturation (%), median (IQR) | 28.37 (22.25–35.90) |
| Potassium (mmol/L), median (IQR) | 4.80 (4.30–5.20) |
| Total Calcium (mmol/L), median (IQR) | 2.38 (2.25–2.53) |
| Phosphorus (mmol/L), median (IQR) | 1.62 (1.36–1.89) |
| Intact-PTH (ng/L), median (IQR) | 254.90 (73.35–550.00) |
| CRP (mg/L), median (IQR) | 3.01 (1.24–6.56) |
| ACOV2S Levels before and after Second Dose of Vaccination | ||
|---|---|---|
| ACOV2S (U/mL), Median (IQR) | Seroconversion (≥0.8 U/mL), n (%) | |
| Week 0 | 23.10 (7.30–56.60) | 356 (92.47) |
| Week 4 | 602.00 (307.50–1623.00) | 381 (98.96) |
| Week 8 | 449.00 (203.00–1258.00) | 381 (98.96) |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95%CI) | SE | p-Value | |
| Week 0 ACOV2S (Log U/mL) | Reference | Reference | |||
| Week 4 ACOV2S (Log U/mL) | 1.5539 (1.4791–1.6286) | <0.001 * | 1.5539 (1.4791–1.6286) | 0.0381 | <0.001 ** |
| Week 8 ACOV2S (Log U/mL) | 1.3982 (1.3248–1.4716) | <0.001 * | 1.3982 (1.3248–1.4716) | 0.0374 | <0.001 ** |
| Age (year) | −0.0142 (−0.0089–−0.0195) | <0.001 * | −0.0134 (−0.0071–−0.0197) | 0.0032 | <0.001 ** |
| Female | 0.0506 (−0.0708–0.1720) | 0.414 | 0.0893 (−0.0452–0.2238) | 0.0686 | 0.193 |
| BMI (kg/m2) | −0.0007 (−0.0139–0.0125) | 0.917 | −0.0045 (−0.0173–0.0083) | 0.0065 | 0.491 |
| Diabetes | −0.0808 (−0.2098–0.0482) | 0.220 | −0.0631 (−0.1966–0.0704) | 0.0681 | 0.354 |
| HD vintage (year) | 0.0025 (−0.0058–0.0107) | 0.557 | 0.0028 (−0.0057–0.0113) | 0.0043 | 0.518 |
| Urea Reduction Ratio | 0.2302 (−0.7168–1.1771) | 0.634 | |||
| Kt/V | 0.1032 (−0.0607–0.2670) | 0.217 | |||
| Dry Body Weight (kg) | 0.0008 (−0.0032–0.0047) | 0.703 | |||
| Hypertension | −0.1019 (−0.2229–0.0192) | 0.099 # | −0.0633 (−0.1758–0.0492) | 0.0574 | 0.270 |
| Chronic Hepatitis B | 0.1590 (−0.0001–0.3182) | 0.050 # | 0.0894 (−0.0695–0.2483) | 0.0811 | 0.270 |
| Chronic Hepatitis C | 0.0679 (−0.1622–0.2980) | 0.563 | |||
| SLE | −0.1829 (−0.5521–0.1862) | 0.331 | |||
| Transplantation History | −0.1983 (−0.5873–0.1907) | 0.318 | |||
| Immunosuppressant | −0.2034 (−0.4246–0.0179) | 0.072 # | −0.2546 (−0.0451–−0.4640) | 0.1069 | 0.017 ** |
| Hemoglobin (g/L) | −0.0039 (−0.0089–0.0011) | 0.125 | |||
| Leukocyte (109/L) | 0.0298 (−0.0031–0.0628) | 0.076 # | 0.0042 (−0.0325–0.0410) | 0.0187 | 0.821 |
| Platelet (109/L) | 0.0018 (0.0009–0.0028) | <0.001 * | 0.0007 (−0.0004–0.0018) | 0.0006 | 0.206 |
| BUN (mmol/L) | 0.0108 (0.0010–0.0206) | 0.031 * | 0.0083 (−0.0043–0.0209) | 0.0064 | 0.195 |
| SCr (μmol/L) | 0.0005 (0.0002–0.0008) | 0.001 * | 0.0001 (−0.0003–0.0006) | 0.0002 | 0.552 |
| Alb (g/L) | 0.0080 (−0.0130–0.0289) | 0.455 | |||
| ALT (μkat/L) | −0.2010 (−0.4511–0.0491) | 0.115 | |||
| Total Bilirubin (μmol/L) | −0.0092 (−0.0250–0.0065) | 0.251 | |||
| Total Cholesterol (mmol/L) | 0.0520 (−0.0080–0.1119) | 0.089 # | 0.0025 (−0.0577–0.0627) | 0.0307 | 0.935 |
| Triglyceride (mmol/L) | 0.0253 (−0.0107–0.0612) | 0.168 | |||
| Ferritin (10 μg/L) | −0.0004 (−0.0012–0.0005) | 0.391 | |||
| Transferrin Saturation (%) | −0.0012 (−0.0073–0.0049) | 0.704 | |||
| Potassium (mmol/L) | −0.0186 (−0.1236–0.0864) | 0.728 | |||
| Total Calcium (mmol/L) | −0.0363 (−0.3543–0.2816) | 0.823 | |||
| Phosphorus (mmol/L) | 0.1274 (−0.0095–0.2644) | 0.068 # | −0.0712 (−0.2489–0.1065) | 0.0907 | 0.432 |
| Intact-PTH (10 ng/L) | 0.0010 (−0.0001–0.0019) | 0.078 # | 0.0002 (−0.0008–0.0012) | 0.0005 | 0.694 |
| CRP (mg/L) | 0.0081 (0.0014–0.0148) | 0.018 * | 0.0082 (0.0014–0.0149) | 0.0035 | 0.018 ** |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | SE | p-Value | |
| Week 4 ACOV2S (Log U/mL) | Reference | Reference | |||
| Week 8 ACOV2S (Log U/mL) | −0.1558 (−0.1401–−0.1715) | <0.001 * | −0.1558 (−0.1401–−0.1715) | 0.0080 | <0.001 ** |
| Age (year) | −0.0122 (−0.0060–−0.0183) | <0.001 * | −0.0067 (−0.0140–0.0005) | 0.0037 | 0.070 |
| Female | −0.0421 (−0.1813–0.0970) | 0.553 | −0.0160 (−0.1730–0.1410) | 0.0801 | 0.841 |
| BMI (kg/m2) | 0.0001 (−0.0147–0.0149) | 0.992 | −0.0082 (−0.0225–0.0062) | 0.0073 | 0.264 |
| Diabetes | −0.0569 (−0.2091–0.0953) | 0.464 | −0.0500 (−0.2079–0.1079) | 0.0806 | 0.535 |
| HD vintage (year) | −0.0015 (−0.0108–0.0079) | 0.760 | −0.0012 (−0.0110–0.0086) | 0.0050 | 0.810 |
| Urea Reduction Ratio | −0.0556 (-1.1037–0.9924) | 0.917 | |||
| Kt/V | 0.0324 (−0.1486–0.2133) | 0.726 | |||
| Dry Body Weight (kg) | 0.0019 (−0.0024–0.0061) | 0.390 | |||
| Hypertension | −0.0600 (−0.1989–0.0790) | 0.397 | |||
| Chronic Hepatitis B | 0.1584 (−0.0020–0.3187) | 0.053 # | 0.0986 (−0.0544–0.2515) | 0.0780 | 0.206 |
| Chronic Hepatitis C | 0.1453 (−0.1046–0.3953) | 0.254 | |||
| SLE | −0.1843 (−0.5396–0.1709) | 0.309 | |||
| Transplantation History | −0.1812 (−0.6343–0.2718) | 0.433 | |||
| Immunosuppressant | −0.1759 (−0.4132–0.0613) | 0.146 | |||
| Hemoglobin (g/L) | −0.0048 (−0.0103–0.0007) | 0.085 # | −0.0057 (−0.0114–0.0000) | 0.0029 | 0.051 |
| Leukocyte (109/L) | 0.0257 (−0.0098–0.0613) | 0.156 | |||
| Platelet (109/L) | 0.0021 (0.0010–0.0031) | <0.001 * | 0.0014 (0.0002–0.0025) | 0.0006 | 0.017 ** |
| BUN (mmol/L) | 0.0133 (0.0018–0.0249) | 0.024 * | 0.0047 (−0.0100–0.0194) | 0.0075 | 0.530 |
| SCr (μmol/L) | 0.0005 (0.0002–0.0009) | 0.005 * | 0.0004 (−0.0002–0.0009) | 0.0003 | 0.211 |
| Alb (g/L) | −0.0069 (−0.0290–0.0152) | 0.542 | |||
| ALT (μkat/L) | −0.0356 (−0.2974–0.2262) | 0.790 | |||
| Total Bilirubin (μmol/L) | −0.0072 (−0.0243–0.0099) | 0.408 | |||
| Total Cholesterol (mmol/L) | 0.0149 (−0.0554–0.0852) | 0.678 | |||
| Triglyceride (mmol/L) | 0.0491 (0.0063–0.0919) | 0.024 * | 0.0266 (−0.0147–0.0680) | 0.0211 | 0.207 |
| Ferritin (10 μg/L) | −0.0002 (−0.0011–0.0006) | 0.603 | |||
| Transferrin Saturation (%) | −0.0012 (−0.0074–0.0051) | 0.713 | |||
| Potassium (mmol/L) | −0.0200 (−0.1397–0.0996) | 0.743 | |||
| Total Calcium (mmol/L) | −0.0099 (−0.3637–0.3439) | 0.956 | |||
| Phosphorus (mmol/L) | 0.1967 (0.0465–0.3469) | 0.010 * | −0.0085 (−0.2140–0.1971) | 0.1049 | 0.936 |
| Intact-PTH (10 ng/L) | 0.0014 (0.0002–0.0025) | 0.019 * | 0.0007 (−0.0004–0.0019) | 0.0006 | 0.229 |
| CRP (mg/L) | 0.0154 (0.0080–0.0228) | <0.001 * | 0.0137 (0.0059–0.0215) | 0.0040 | 0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.-M.; Tsai, K.-F.; Kuo, W.-H.; Wu, C.-H.; Yu, C.-I.; You, H.-L.; Lee, C.-T. The Waxing, Waning, and Predictors of Humoral Responses to Vector-Based SARS-CoV-2 Vaccine in Hemodialysis Patients. Vaccines 2022, 10, 1537. https://doi.org/10.3390/vaccines10091537
Fu C-M, Tsai K-F, Kuo W-H, Wu C-H, Yu C-I, You H-L, Lee C-T. The Waxing, Waning, and Predictors of Humoral Responses to Vector-Based SARS-CoV-2 Vaccine in Hemodialysis Patients. Vaccines. 2022; 10(9):1537. https://doi.org/10.3390/vaccines10091537
Chicago/Turabian StyleFu, Chung-Ming, Kai-Fan Tsai, Wei-Hung Kuo, Chien-Hsing Wu, Ching-I Yu, Huey-Ling You, and Chien-Te Lee. 2022. "The Waxing, Waning, and Predictors of Humoral Responses to Vector-Based SARS-CoV-2 Vaccine in Hemodialysis Patients" Vaccines 10, no. 9: 1537. https://doi.org/10.3390/vaccines10091537
APA StyleFu, C.-M., Tsai, K.-F., Kuo, W.-H., Wu, C.-H., Yu, C.-I., You, H.-L., & Lee, C.-T. (2022). The Waxing, Waning, and Predictors of Humoral Responses to Vector-Based SARS-CoV-2 Vaccine in Hemodialysis Patients. Vaccines, 10(9), 1537. https://doi.org/10.3390/vaccines10091537







