Molecular Crosstalk between the Immunological Mechanism of the Tumor Microenvironment and Epithelial–Mesenchymal Transition in Oral Cancer
Abstract
1. Introduction
2. Epithelial–Mesenchymal Transition in Oral Cancer
3. The Role of Inflammatory Proteins in Influencing EMT in Oral Cancer
3.1. Role of Transforming Growth Factor β (TGF-β) in Oral Cancer
3.2. The Connection between the Tumor Necrosis Factor—Alpha (TNF-α), Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-8 (IL-8), Monocyte Chemoattractant Protein-1(MCP-1/CCL2), Macrophage, and Oral Cancer
4. EMT-Induced Tumor Hypoxia and Oral Cancer
5. Alteration of Cytoskeleton Involved in EMT Acts as a Diagnostic Marker for Oral Cancer Therapy
6. Transcription Factors Induced by EMT
6.1. Family—Twist
6.2. Family—Snail
6.3. ΔNp63
6.4. Family—ZEB
6.5. Other Transcriptional Factors
7. Transcription Factors Inhibited by EMT
8. Receptors Induced by EMT during Oral Cancer
8.1. Tyrosine Kinase Receptor and EMT during Oral Cancer
8.1.1. Fibroblast Growth Factor Receptor (FGFR) and EMT during Oral Cancer
8.1.2. Epidermal Growth Factor Receptor (EGFR) and EMT during Oral Cancer
8.1.3. Ephrin (Ephs) Receptor and EMT during Oral Cancer
8.1.4. Other Tyrosine Kinase Receptors and EMT during Oral Cancer
8.2. G-Protein Coupled Receptor and EMT during Oral Cancer
8.2.1. C-X-C Chemokine Receptors and EMT
8.2.2. Histamine Receptors and EMT
9. Other Signaling Events Induced by EMT during Oral Cancer
9.1. PI3 Kinase/mTOR/Akt Signaling and EMT with Oral Cancer
9.2. Wnt Signaling in Oral Cancer and EMT
9.3. Matrix Signaling and EMT in Oral Cancer
9.4. Notch Signaling Pathway and EMT in Oral Cancer
9.5. Hedgehog Signaling and EMT in Oral Cancer
| S.No. | Receptors | Genes Involved | Outcome | References |
|---|---|---|---|---|
| 1. |
Elevated Integrin/EGFR-ERK/MAPK
| Increased ZEB1/2 Decreased ZO-1, E-cadherin, β–catenin Increased Ephs - Augmented level of vimentin, Snail, and matrix metalloproteinases Increased H1R | EMT induction EMT induction Cell morphology transformation Increased EMT Increased tumor size, recurrence, lymph invasion, and metastasis Increased tumorigenesis EMT induction Migration of cells and invasion Elevated OSCC generation | [65] [70] [71,72] [3] [75] [77] |
| 2. | 2.1. PI3 kinase/mTOR/Akt signaling TGF-β activates PI3/Akt signaling | Mutation in Akt, PI3, PTEN, and RAS | Increased proliferation, invasiveness, anti-apoptosis, and growth | [78,79] |
| 3. | 3.1. Wnt signaling Decreased Wnt antagonist, WIF1 | Increased LEF1 and β-catenin | EMT induction and increased oral cancer progression | [82,83] |
| 4. | 4.1. Matrix signaling Increased MMP1, MMP7, MMP9, and MMP2, and collagen type 1 | Elevated ERK/MEK level | Degradation of the extracellular matrix and basement membrane | [85] |
| 5. | 5.1. Notch signaling | Increased Snail and reduced expression of E-cadherin | Increased tumor metastasis | [87,88] |
| 6. | 6.1. Hedgehog signaling SHH enhancement | - | Increased tumorigenesis via EMT induction | [89,90] |
| 7. | 7.1. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) | Decreased PTEN | Cancer invasion via EMT induction | [6] |
9.6. Phosphatase and Tensin Homolog Deleted on Chromosome 10 (PTEN) and EMT in Oral Cancer
9.7. Neuropilin-1 (NRP1) and EMT in Oral Cancer
9.8. Pituitary Tumor-Transforming Gene 1 (PTTG1) and EMT in Oral Cancer
9.9. Transforming Growth Factor-β 1-Activated Kinase 1 Binding Protein 2 (TAB2) and EMT in Oral Cancer
9.10. Engulfment and Cell Motility (ELMO) Proteinsand EMT in Oral Cancer
10. MicroRNAs and EMT in Oral Cancer
11. Oral Cancer—EMT Regulation via Microenvironment
11.1. Oral Cancer and Fibroblasts
11.2. Integrins
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar]
- Almangush, A.; Mäkitie, A.A.; Triantafyllou, A.; de Bree, R.; Strojan, P.; Rinaldo, A.; Hernandez-Prera, J.C.; Suárez, C.; Kowalski, L.P.; Ferlito, A. Staging and grading of oral squamous cell carcinoma: An update. Oral Oncol. 2020, 107, 104799. [Google Scholar] [PubMed]
- Ling, Z.; Cheng, B.; Tao, X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int. J. Cancer 2021, 148, 1548–1561. [Google Scholar]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [PubMed]
- Jayanthi, P.; Varun, B.; Selvaraj, J. Epithelial–mesenchymal transition in oral squamous cell carcinoma: An insight into molecular mechanisms and clinical implications. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 189. [Google Scholar] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [PubMed]
- Gloushankova, N.; Zhitnyak, I.; Rubtsova, S. Role of epithelial-mesenchymal transition in tumor progression. Biochemistry (Moscow) 2018, 83, 1469–1476. [Google Scholar] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar]
- Wu, F.; Weigel, K.J.; Zhou, H.; Wang, X.-J. Paradoxical roles of TGF-β signaling in suppressing and promoting squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2018, 50, 98–105. [Google Scholar]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and extracellular TGF-β signaling in cancer: Some recent topics. Front. Med. 2018, 12, 387–411. [Google Scholar] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [PubMed]
- Titidej, A.; Eshghyar, N.; Jolehar, M. Prognostic New Marker (Bone Morphogenetic Protein 7) in Squamous Cell Carcinoma. J. Contemp. Dent. Pract. 2018, 19, 675–679. [Google Scholar]
- Meng, W.; Xia, Q.; Wu, L.; Chen, S.; He, X.; Zhang, L.; Gao, Q.; Zhou, H. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer 2011, 11, 88. [Google Scholar]
- Cirillo, N.; Hassona, Y.; Celentano, A.; Lim, K.; Manchella, S.; Parkinson, E.; Prime, S. Cancer-associated fibroblasts regulate keratinocyte cell–cell adhesion via TGF-β-dependent pathways in genotype-specific oral cancer. Carcinogenesis 2017, 38, 76–85. [Google Scholar] [CrossRef]
- Chiba, T.; Ishisaki, A.; Kyakumoto, S.; Shibata, T.; Yamada, H.; Kamo, M. Transforming growth factor-β1 suppresses bone morphogenetic protein-2-induced mesenchymal-epithelial transition in HSC-4 human oral squamous cell carcinoma cells via Smad1/5/9 pathway suppression. Oncol. Rep. 2017, 37, 713–720. [Google Scholar] [PubMed]
- Bertazza, L.; Mocellin, S. The dual role of tumor necrosis factor (TNF) in cancer biology. Curr. Med. Chem. 2010, 17, 3337–3352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-W.; Zhou, J.-P.; Bi, Y.-L.; Wang, J.-Y.; Yu, R.; Deng, C.; Wang, W.-K.; Li, X.-Z.; Huang, R.; Zhang, J. The role of MAPK signaling pathway in formation of EMT in oral squamous carcinoma cells induced by TNF-α. Mol. Biol. Rep. 2019, 46, 3149–3156. [Google Scholar]
- Zheng, Z.; Luan, X.; Zha, J.; Li, Z.; Wu, L.; Yan, Y.; Wang, H.; Hou, D.; Huang, L.; Huang, F. TNF-α inhibits the migration of oral squamous cancer cells mediated by miR-765-EMP3-p66Shc axis. Cell. Signal. 2017, 34, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFα Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar]
- Chattopadhyay, I.; Ambati, R.; Gundamaraju, R. Exploring the crosstalk between inflammation and epithelial-mesenchymal transition in cancer. Mediat. Inflamm. 2021, 2021, 9918379. [Google Scholar]
- Lopez-Labady, J.; Bologna-Molina, R.; Villarroel-Dorrego, M. Expression of Interleukin-1ß and Interleukin-8 in Oral Potentially Malignant Disorders and Carcinomas. Front. Oral Health 2021, 2, 649406. [Google Scholar] [PubMed]
- Kumari, N.; Dwarakanath, B.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar]
- Wu, D.; Cheng, J.; Sun, G.; Wu, S.; Li, M.; Gao, Z.; Zhai, S.; Li, P.; Su, D.; Wang, X. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget 2016, 7, 36539. [Google Scholar] [PubMed]
- Dineshkumar, T.; Ashwini, B.K.; Rameshkumar, A.; Rajashree, P.; Ramya, R.; Rajkumar, K. Salivary and serum interleukin-6 levels in oral premalignant disorders and squamous cell carcinoma: Diagnostic value and clinicopathologic correlations. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 4899. [Google Scholar]
- Zhang, R.-L.; Peng, L.-X.; Yang, J.-P.; Zheng, L.-S.; Xie, P.; Wang, M.-Y.; Huang, B.-J.; Zhao, H.-R.; Bao, Y.-X.; Qian, C.-N. IL-8 suppresses E-cadherin expression in nasopharyngeal carcinoma cells by enhancing E-cadherin promoter DNA methylation. Int. J. Oncol. 2016, 48, 207–214. [Google Scholar]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697. [Google Scholar] [PubMed]
- Ling, Z.; Yang, X.; Chen, X.; Xia, J.; Cheng, B.; Tao, X. CCL2 promotes cell migration by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-T.; Chen, H.-R.; Lin, C.-H.; Lee, J.-W.; Lee, C.-C. Monocyte chemotactic protein 1 (MCP-1) modulates pro-survival signaling to promote progression of head and neck squamous cell carcinoma. PLoS ONE 2014, 9, e88952. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, M.N.M.R.; Cherubini, K.; Salum, F.G.; De Figueiredo, M.A.Z. Role of tumour-associated macrophages in oral squamous cells carcinoma progression: An update on current knowledge. Diagn. Pathol. 2017, 12, 32. [Google Scholar] [PubMed]
- Udeabor, S.E.; Adisa, A.O.; Orlowska, A.; Sader, R.A.; Ghanaati, S. Tumor-associated macrophages, angiogenesis, and tumor cell migration in oral squamous cell carcinoma. Ann. Afr. Med. 2017, 16, 181–185. [Google Scholar] [PubMed]
- Lothaire, P.; de Azambuja, E.; Dequanter, D.; Lalami, Y.; Sotiriou, C.; Andry, G.; Castro, G., Jr.; Awada, A. Molecular markers of head and neck squamous cell carcinoma: Promising signs in need of prospective evaluation. Head Neck J. Sci. Spec. Head Neck 2006, 28, 256–269. [Google Scholar] [CrossRef]
- Eckert, A.; Kappler, M.; Schubert, J.; Taubert, H. Correlation of expression of hypoxia-related proteins with prognosis in oral squamous cell carcinoma patients. Oral Maxillofac. Surg. 2012, 16, 189–196. [Google Scholar] [CrossRef]
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Wickenhauser, C.; Salins, P.C.; Kappler, M.; Bukur, J.; Seliger, B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J. Transl. Med. 2016, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Prahl, L.S.; Odde, D.J. Modeling cell migration mechanics. Biomech. Oncol. 2018, 1092, 159–187. [Google Scholar]
- Sawant, S.; Vaidya, M.; Chaukar, D.; Alam, H.; Dmello, C.; Gangadaran, P.; Kannan, S.; Kane, S.; Dange, P.; Dey, N. Clinical significance of aberrant vimentin expression in oral premalignant lesions and carcinomas. Oral Dis. 2014, 20, 453–465. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, D.; Xu, Q.; Gao, Z.; Tang, D. Expression of E-cadherin and vimentin in oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3150. [Google Scholar] [PubMed]
- Dmello, C.; Sawant, S.; Chaudhari, P.R.; Dongre, H.; Ahire, C.; D’Souza, Z.C.; Charles, S.E.; Rane, P.; Costea, D.E.; Chaukar, D. Aberrant expression of vimentin predisposes oral premalignant lesion derived cells towards transformation. Exp. Mol. Pathol. 2018, 105, 243–251. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.-D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Li, C.-H.; Nie, M.-H. Expression of cytokeratin 19 in the development and progression of oral squamous cell carcinoma. Shanghai Kou Qiang Yi Xue = Shanghai J. Stomatol. 2016, 25, 600–603. [Google Scholar]
- Ernst, J.; Ikenberg, K.; Apel, B.; Schumann, D.M.; Huber, G.; Studer, G.; Rordorf, T.; Riesterer, O.; Rössle, M.; Korol, D. Expression of CK19 is an independent predictor of negative outcome for patients with squamous cell carcinoma of the tongue. Oncotarget 2016, 7, 76151. [Google Scholar] [CrossRef]
- Matsuhira, A.; Noguchi, S.; Sato, K.; Tanaka, Y.; Yamamoto, G.; Mishima, K.; Katakura, A. Cytokeratin 13, cytokeratin 17, Ki-67 and p53 expression in upper layers of epithelial dysplasia surrounding tongue squamous cell carcinoma. Bull. Tokyo Dent. Coll. 2015, 56, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Yagyuu, T.; Obayashi, C.; Ueyama, Y.; Takano, M.; Tanaka, Y.; Kawaguchi, M.; Takeda, M.; Kasai, T.; Kirita, T. Multivariate analyses of Ki-67, cytokeratin 13 and cytokeratin 17 in diagnosis and prognosis of oral precancerous lesions. J. Oral Pathol. Med. 2015, 44, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Nienstedt, J.C.; Gröbe, A.; Clauditz, T.; Simon, R.; Muenscher, A.; Knecht, R.; Sauter, G.; Moebius, C.; Blessmann, M.; Heiland, M.; et al. High-level β III-tubulin overexpres-sion occurs in most head and neck cancers but is unrelated to clinical outcome. J. Oral Pathol. Med. 2017, 46, 986–990. [Google Scholar]
- Saba, N.F.; Magliocca, K.R.; Kim, S.; Muller, S.; Chen, Z.; Owonikoko, T.K.; Sarlis, N.J.; Eggers, C.; Phelan, V.; Grist, W.J.; et al. Acetylated tubulin (AT) as a prognostic marker in squamous cell carcinoma of the head and neck. Head Neck Pathol. 2014, 8, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Raffat, M.A.; Hadi, N.I.; Hosein, M.; Mirza, S.; Ikram, S.; Akram, Z. S100 proteins in oral squamous cell carcinoma. Clin. Chim. Acta 2018, 480, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, H.; Song, Y. Expression of twist and relation with epithelial-Mesenchymal transition in oral squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi = West China J. Stomatol. 2015, 33, 534–538. [Google Scholar]
- Zhou, Y.; Zhang, H.; Zhuo, X.; Liu, Y.; Zhang, G.; Tan, Y. Over-expression of TWIST, an epithelial-mesenchymal transition inducer, predicts poor survival in patients with oral carcinoma. Int. J. Clin. Exp. Med. 2015, 8, 9239. [Google Scholar] [PubMed]
- Seyedmajidi, M.; Seifi, S.; Moslemi, D.; Mozaffari, S.-F.; Gholinia, H.; Zolfaghari, Z. Immunohistochemical expression of TWIST in oral squamous cell carcinoma and its correlation with clinicopathologic factors. J. Cancer Res. Ther. 2018, 14, 964. [Google Scholar] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; P Zhou, B. The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Ota, I.; Masui, T.; Kurihara, M.; Yook, J.-I.; Mikami, S.; Kimura, T.; Shimada, K.; Konishi, N.; Yane, K.; Yamanaka, T. Snail-induced EMT promotes cancer stem cell-like properties in head and neck cancer cells. Oncol. Rep. 2016, 35, 261–266. [Google Scholar] [CrossRef]
- Mollo, M.R.; Cirillo, L.; Russo, C.; Antonini, D.; Missero, C. Functional and mechanistic insights into the pathogenesis of P63-associated disorders. J. Investig. Dermatol. Symp. Proc. 2018, 19, S98–S100. [Google Scholar] [CrossRef]
- Saghravanian, N.; Anvari, K.; Ghazi, N.; Memar, B.; Shahsavari, M.; Aghaee, M.A. Expression of p63 and CD44 in oral squamous cell carcinoma and correlation with clinicopathological parameters. Arch. Oral Biol. 2017, 82, 160–165. [Google Scholar] [CrossRef]
- Patel, S.B.; Manjunatha, B.S.; Shah, V.; Soni, N.; Sutariya, R. Immunohistochemical evaluation of p63 and cyclin D1 in oral squamous cell carcinoma and leukoplakia. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kawano, S.; Matsubara, R.; Kiyosue, T.; Hirano, M.; Jinno, T.; Maruse, Y.; Toyoshima, T.; Kitamura, R.; Tanaka, H. Possible involvement of ΔNp63 downregulation in the invasion and metastasis of oral squamous cell carcinoma via induction of a mesenchymal phenotype. Clin. Exp. Metastasis 2014, 31, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.; Vandamme, N.; Van Vlierberghe, P.; Berx, G. EMT transcription factors in cancer development re-evaluated: Beyond EMT and MET. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2017, 1868, 584–591. [Google Scholar] [CrossRef]
- Yao, X.; Sun, S.; Zhou, X.; Zhang, Q.; Guo, W.; Zhang, L. Clinicopathological significance of ZEB-1 and E-cadherin proteins in patients with oral cavity squamous cell carcinoma. OncoTargets Ther. 2017, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Yeom, S.; Kwak, J.; Ahn, H.-J.; Jang, K.L. Hepatitis B virus X protein induces epithelial–mesenchymal transition by repressing E-cadherin expression via upregulation of E12/E47. J. Gen. Virol. 2016, 97, 134–143. [Google Scholar]
- Naini, F.B.; Shakib, P.A.; Abdollahi, A.; Hodjat, M.; Mohammadpour, H.; Khoozestani, N.K. Relative expression of OCT4, SOX2 and NANOG in oral squamous cell carcinoma versus adjacent non-tumor tissue. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 1649. [Google Scholar] [CrossRef]
- Chung, V.Y.; Tan, T.Z.; Tan, M.; Wong, M.K.; Kuay, K.T.; Yang, Z.; Ye, J.; Muller, J.; Koh, C.M.; Guccione, E. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci. Rep. 2016, 6, 19943. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.M.; Talebian, V.; Jolly, M.K.; Jia, D.; Gromala, M.; Levine, H.; McConkey, B.J. The GRHL2/ZEB feedback loop—A key axis in the regulation of EMT in breast cancer. J. Cell. Biochem. 2017, 118, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.; Breeze, A.L. Structure, activation and dysregulation of fibroblast growth factor receptor kinases: Perspectives for clinical targeting. Biochem. Soc. Trans. 2018, 46, 1753–1770. [Google Scholar] [CrossRef]
- Mariz, B.A.; Soares, C.D.; de Carvalho, M.G.; Jorge-Júnior, J. FGF-2 and FGFR-1 might be independent prognostic factors in oral tongue squamous cell carcinoma. Histopathology 2019, 74, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Osada, A.H.; Endo, K.; Kimura, Y.; Sakamoto, K.; Nakamura, R.; Sakamoto, K.; Ueki, K.; Yoshizawa, K.; Miyazawa, K.; Saitoh, M. Addiction of mesenchymal phenotypes on the FGF/FGFR axis in oral squamous cell carcinoma cells. PLoS ONE 2019, 14, e0217451. [Google Scholar] [CrossRef] [PubMed]
- Cantor, A.J.; Shah, N.H.; Kuriyan, J. Deep mutational analysis reveals functional trade-offs in the sequences of EGFR autophosphorylation sites. Proc. Natl. Acad. Sci. USA 2018, 115, E7303–E7312. [Google Scholar] [CrossRef]
- Mitchell, R.A.; Luwor, R.B.; Burgess, A.W. Epidermal growth factor receptor: Structure-function informing the design of anticancer therapeutics. Exp. Cell Res. 2018, 371, 1–19. [Google Scholar] [CrossRef]
- Sheng, W.; Chen, C.; Dong, M.; Wang, G.; Zhou, J.; Song, H.; Li, Y.; Zhang, J.; Ding, S. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017, 8, e3147. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.; Vairaktaris, E.; Agaimy, A.; Bechtold, M.; Gorecki, P.; Neukam, F.W.; Nkenke, E. The relevance of EGFR overexpression for the prediction of the malignant transformation of oral leukoplakia. Oncol. Rep. 2013, 30, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.-i.; Okamoto, K.; Calderwood, S.K.; Kozaki, K.-i. Anti-EGFR antibody cetuximab is secreted by oral squamous cell carcinoma and alters EGF-driven mesenchymal transition. Biochem. Biophys. Res. Commun. 2018, 503, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Kou, C.-T.J.; Kandpal, R.P. Differential expression patterns of Eph receptors and ephrin ligands in human cancers. BioMed Res. Int. 2018, 2018, 7390104. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Oikawa, M.; Kouketsu, A.; Takahashi, T.; Kumamoto, H. Immunohistochemical assessment of Eph/ephrin expression in oral squamous cell carcinoma and precursor lesions. Odontology 2020, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [PubMed]
- Duan, Y.; Zhang, S.; Wang, L.; Zhou, X.; He, Q.; Liu, S.; Yue, K.; Wang, X. Targeted silencing of CXCR4 inhibits epithelial-mesenchymal transition in oral squamous cell carcinoma. Oncol. Lett. 2016, 12, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.-B.; João-Baptista-Macuco Janini, L.-M.; Paranaíba, R.; Lozano-Burgos, C.; Olivero, P.; González-Arriagada, W.-A. Prognostic value of immunoexpression of CCR4, CCR5, CCR7 and CXCR4 in squamous cell carcinoma of tongue and floor of the mouth. Med. Oral Patol. Oral Y Cir. Bucal 2019, 24, e354. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Almahmoudi, R.; Listyarifah, D.; Siponen, M.; Maaninka, K.; Al-Samadi, A.; Salo, T.; Eklund, K.K. Histamine H4 receptor signalling in tongue cancer and its potential role in oral carcinogenesis-a short report. Cell. Oncol. 2017, 40, 621–630. [Google Scholar] [CrossRef]
- Grimm, M.; Krimmel, M.; Alexander, D.; Munz, A.; Kluba, S.; Keutel, C.; Hoffmann, J.; Polligkeit, J.; Reinert, S.; Hoefert, S. Prognostic value of histamine H1 receptor expression in oral squamous cell carcinoma. Clin. Oral Investig. 2013, 17, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, J.; Paeng, J.; Kim, M.; Hong, S.; Lee, J.; Hong, S. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J. Clin. Pathol. 2005, 58, 1199–1205. [Google Scholar] [CrossRef]
- Cohen, Y.; Goldenberg-Cohen, N.; Shalmon, B.; Shani, T.; Oren, S.; Amariglio, N.; Dratviman-Storobinsky, O.; Shnaiderman-Shapiro, A.; Yahalom, R.; Kaplan, I. Mutational analysis of PTEN/PIK3CA/AKT pathway in oral squamous cell carcinoma. Oral Oncol. 2011, 47, 946–950. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, L.; Mashrah, M.; Zhu, Y.; Liu, J.; Yang, X.; He, Z.; Wang, L.; Xiang, T.; Yao, Z. Deregulation of secreted frizzled-related proteins is associated with aberrant β-catenin activation in the carcinogenesis of oral submucous fibrosis. OncoTargets Ther. 2015, 8, 2923. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.S. Wnt Signaling in Oral Cancer Initiating Cells. Ph.D. Thesis, Harvard School of Dental Medicine, Boston, MA, USA, 2017. [Google Scholar]
- Marimuthu, M.; Andiappan, M.; Wahab, A.; Muthusekhar, M.; Balakrishnan, A.; Shanmugam, S. Canonical Wnt pathway gene expression and their clinical correlation in oral squamous cell carcinoma. Indian J. Dent. Res. 2018, 29, 291. [Google Scholar] [PubMed]
- Goteri, G.; Capretti, R.; Rubini, C.; Vinella, A.; Fumarulo, R.; Bianchi, F.; Mastrangelo, F.; Porfiri, E.; Mariggiò, M. Beta-catenin gene analysis in oral squamous cell carcinoma. Int. J. Immunopathol. Pharmacol. 2005, 18, 33–38. [Google Scholar]
- De Vicente, J.C.; Lequerica-Fernández, P.; Santamaria, J.; Fresno, M.F. Expression of MMP-7 and MT1-MMP in oral squamous cell carcinoma as predictive indicator for tumor invasion and prognosis. J. Oral Pathol. Med. 2007, 36, 415–424. [Google Scholar]
- Hayashido, Y.; Kitano, H.; Sakaue, T.; Fujii, T.; Suematsu, M.; Sakurai, S.; Okamoto, T. Overexpression of integrin αv facilitates proliferation and invasion of oral squamous cell carcinoma cells via MEK/ERK signaling pathway that is activated by interaction of integrin αvβ8 with type I collagen. Int. J. Oncol. 2014, 45, 1875–1882. [Google Scholar]
- Sakamoto, K. Notch signaling in oral squamous neoplasia. Pathol. Int. 2016, 66, 609–617. [Google Scholar] [PubMed]
- Zhang, J.; Zheng, G.; Zhou, L.; Li, P.; Yun, M.; Shi, Q.; Wang, T.; Wu, X. Notch signalling induces epithelial-mesenchymal transition to promote metastasis in oral squamous cell carcinoma. Int. J. Mol. Med. 2018, 42, 2276–2284. [Google Scholar] [PubMed]
- Yoshida, R.; Ito, T.; Abdo Hassan, W.; Nakayama, H. Notch1 in oral squamous cell carcinoma. Histol. Histopathol. 2017, 32, 315–323. [Google Scholar]
- Cannonier, S.A.; Gonzales, C.B.; Ely, K.; Guelcher, S.A.; Sterling, J.A. Hedgehog and TGFβ signaling converge on Gli2 to control bony invasion and bone destruction in oral squamous cell carcinoma. Oncotarget 2016, 7, 76062. [Google Scholar] [PubMed]
- Chen, G.; Yan, M.; Li, R.R.; Chen, W.T. Sonic hedgehog signalling activation contributes to ALCAM over-expression and poor clinical outcome in patients with oral squamous cell carcinoma. Chin. J. Dent. Res. 2018, 21, 31–40. [Google Scholar]
- Song, Q.; Wang, B.; Liu, M.; Ren, Z.; Fu, Y.; Zhang, P.; Yang, M. MTA1 promotes the invasion and migration of oral squamous carcinoma by inducing epithelial–mesenchymal transition via the hedgehog signaling pathway. Exp. Cell Res. 2019, 382, 111450. [Google Scholar] [PubMed]
- Chu, W.; Song, X.; Yang, X.; Ma, L.; Zhu, J.; He, M.; Wang, Z.; Wu, Y. Neuropilin-1 promotes epithelial-to-mesenchymal transition by stimulating nuclear factor-kappa B and is associated with poor prognosis in human oral squamous cell carcinoma. PLoS ONE 2014, 9, e101931. [Google Scholar]
- Zhang, E.; Liu, S.; Xu, Z.; Huang, S.; Tan, X.; Sun, C.; Lu, L. Pituitary tumor-transforming gene 1 (PTTG1) is overexpressed in oral squamous cell carcinoma (OSCC) and promotes migration, invasion and epithelial–mesenchymal transition (EMT) in SCC15 cells. Tumor Biol. 2014, 35, 8801–8811. [Google Scholar]
- Liu, H.; Zhang, H.; Fan, H.; Tang, S.; Weng, J. TAB2 Promotes the Biological Functions of Head and Neck Squamous Cell Carcinoma Cells via EMT and PI3K Pathway. Dis. Markers 2022, 2022, 1217918. [Google Scholar] [PubMed]
- McCauley, H.A.; Chevrier, V.; Birnbaum, D.; Guasch, G. De-repression of the RAC activator ELMO1 in cancer stem cells drives progression of TGFβ-deficient squamous cell carcinoma from transition zones. eLife 2017, 6, e22914. [Google Scholar] [PubMed]
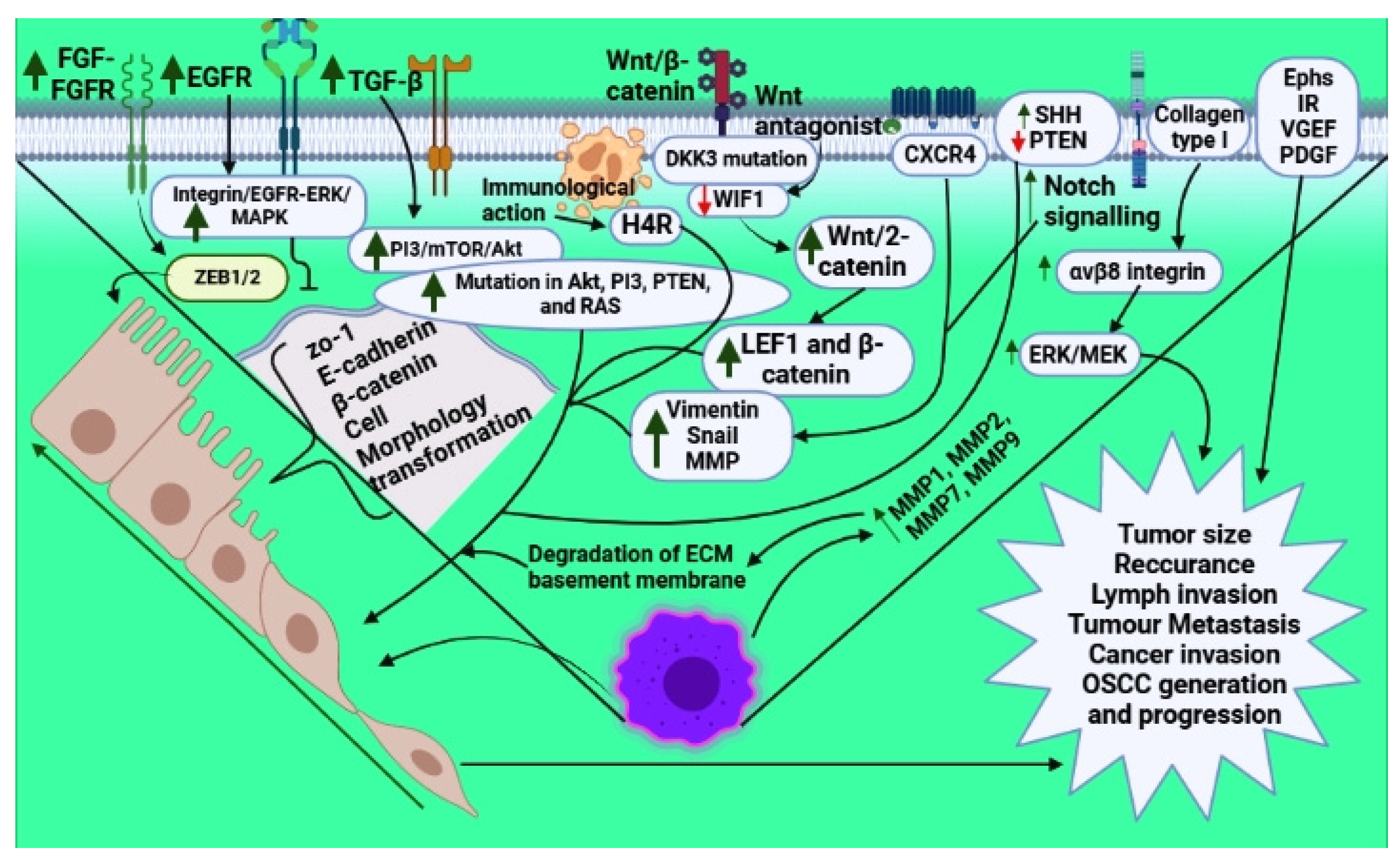
| S.No. | Inflammatory Proteins | Factors/Genes Involved | Outcome | References |
|---|---|---|---|---|
| 1. |
| Decreases E-cadherin and increases vimentin Increases MMPs Decreases Snail; N-cadherin and CK9 | EMT induction EMT induction EMT induction; increases tumor differentiation, lymph node metastasis | [13,14,15,16] |
| 2. |
| Increases MAPK level | EMT augmentation Increased mesenchymal marker Decreased epithelial marker | [17,18,19,20] |
| 3. |
| Increased NF-kB; AP-1; IL-8; GROα, IL-6 | EMT induction | [22] |
| 4. |
| Activation of JAK/STAT3 | EMT induction | [24,25] |
| 5. |
| Increased p38 and MAPK kinase | EMT activation E-cadherin epigenetic silencing | [26] |
| 6. |
| EMT activation | [29] | |
| 7. |
| Decreased ZO-1 and E-cadherin (epithelial markers) Increased vimentin and N-cadherin (mesenchymal markers) | Increased EMT Increased tumor metastasis | [30,31] |
| S.No. | Transcription Factor | Genes Involved | Outcome | References |
|---|---|---|---|---|
| 1. | Family—Twist Increased Twist 1 and Twist 2 | Decreased E-cadherin and increased N-cadherin | Increased EMT level Increased metastasis of cancer | [48,49,50] |
| 2. | Family—Snail Increased Snail | Decreased E-cadherin, occludins, and claudins | Decreased epithelial markers EMT activation | [51] |
| 3. | ΔNp63 Augmented ΔNp63 | - | Increased dysplasia Increased transformation of malignancy | [54] |
| 4. | Family—ZEB Increased ZEB2 or ZEB1 | Decreased E-cadherin and increased N-cadherin; MMPs; vimentin; ZEB | EMT induction | [57,58] |
| 5. | Other transcriptional factors Increased E12/E47 Increased SOX2 | Decreased E-cadherin and SOX2 | Increased OSCC progression | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renu, K.; Vinayagam, S.; Veeraraghavan, V.P.; Mukherjee, A.G.; Wanjari, U.R.; Prabakaran, D.S.; Ganesan, R.; Dey, A.; Vellingiri, B.; Kandasamy, S.; et al. Molecular Crosstalk between the Immunological Mechanism of the Tumor Microenvironment and Epithelial–Mesenchymal Transition in Oral Cancer. Vaccines 2022, 10, 1490. https://doi.org/10.3390/vaccines10091490
Renu K, Vinayagam S, Veeraraghavan VP, Mukherjee AG, Wanjari UR, Prabakaran DS, Ganesan R, Dey A, Vellingiri B, Kandasamy S, et al. Molecular Crosstalk between the Immunological Mechanism of the Tumor Microenvironment and Epithelial–Mesenchymal Transition in Oral Cancer. Vaccines. 2022; 10(9):1490. https://doi.org/10.3390/vaccines10091490
Chicago/Turabian StyleRenu, Kaviyarasi, Sathishkumar Vinayagam, Vishnu Priya Veeraraghavan, Anirban Goutam Mukherjee, Uddesh Ramesh Wanjari, D. S. Prabakaran, Raja Ganesan, Abhijit Dey, Balachandar Vellingiri, Sabariswaran Kandasamy, and et al. 2022. "Molecular Crosstalk between the Immunological Mechanism of the Tumor Microenvironment and Epithelial–Mesenchymal Transition in Oral Cancer" Vaccines 10, no. 9: 1490. https://doi.org/10.3390/vaccines10091490
APA StyleRenu, K., Vinayagam, S., Veeraraghavan, V. P., Mukherjee, A. G., Wanjari, U. R., Prabakaran, D. S., Ganesan, R., Dey, A., Vellingiri, B., Kandasamy, S., Ramanathan, G., Doss C, G. P., George, A., & Gopalakrishnan, A. V. (2022). Molecular Crosstalk between the Immunological Mechanism of the Tumor Microenvironment and Epithelial–Mesenchymal Transition in Oral Cancer. Vaccines, 10(9), 1490. https://doi.org/10.3390/vaccines10091490










