Alopecia Areata Occurring after COVID-19 Vaccination: A Single-Center, Cross-Sectional Study
Abstract: Background
1. Introduction
2. Materials and Methods
Statistical Analysis
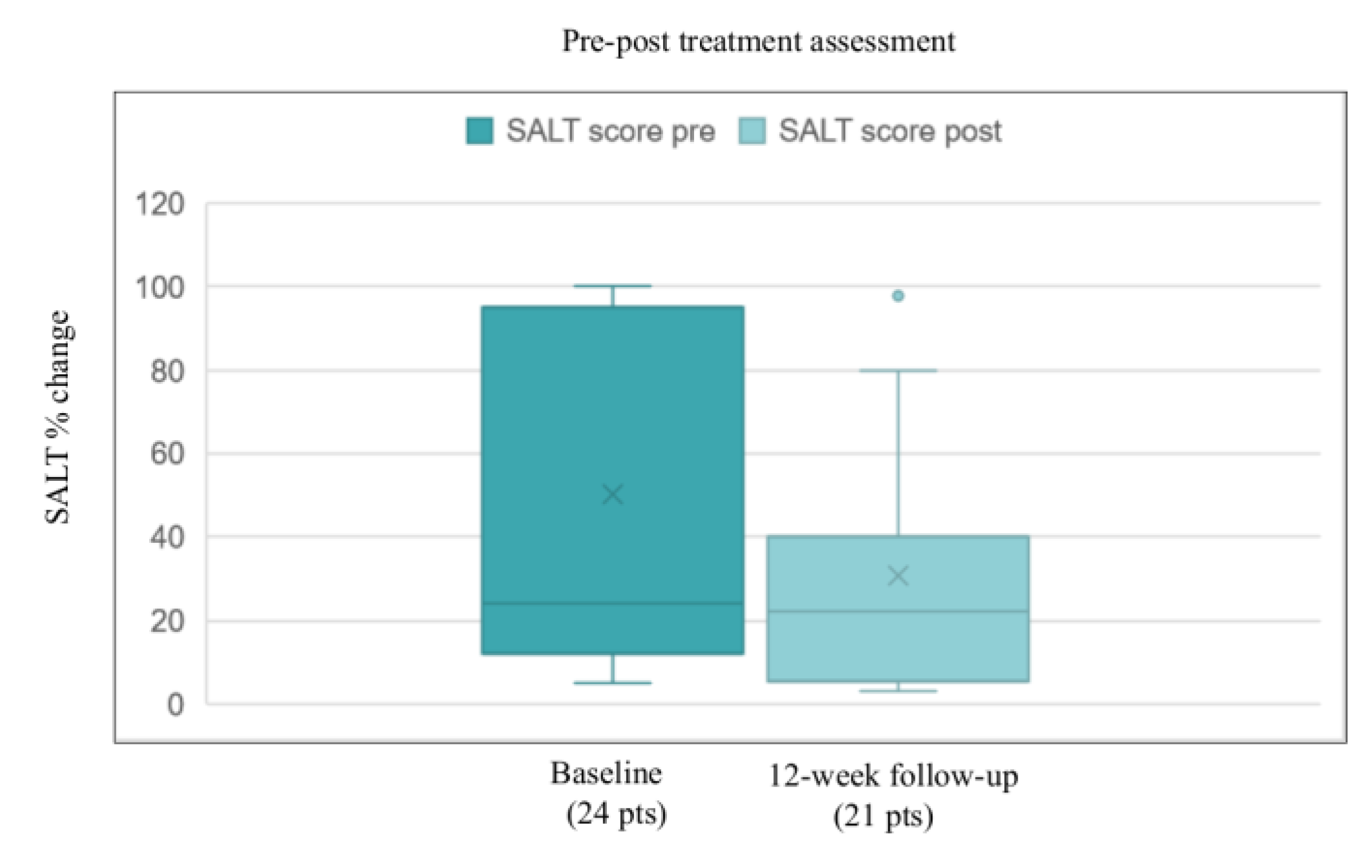
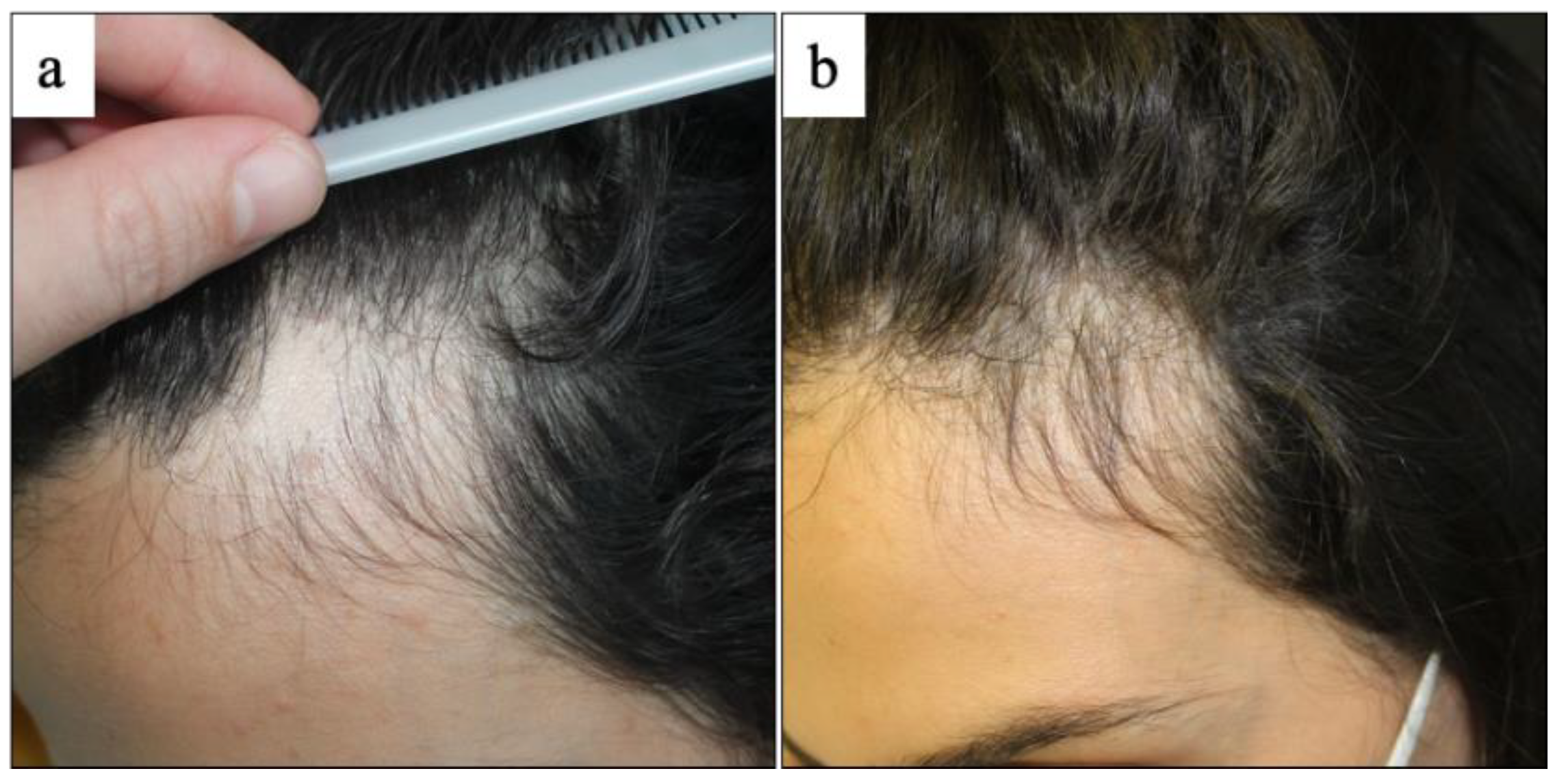
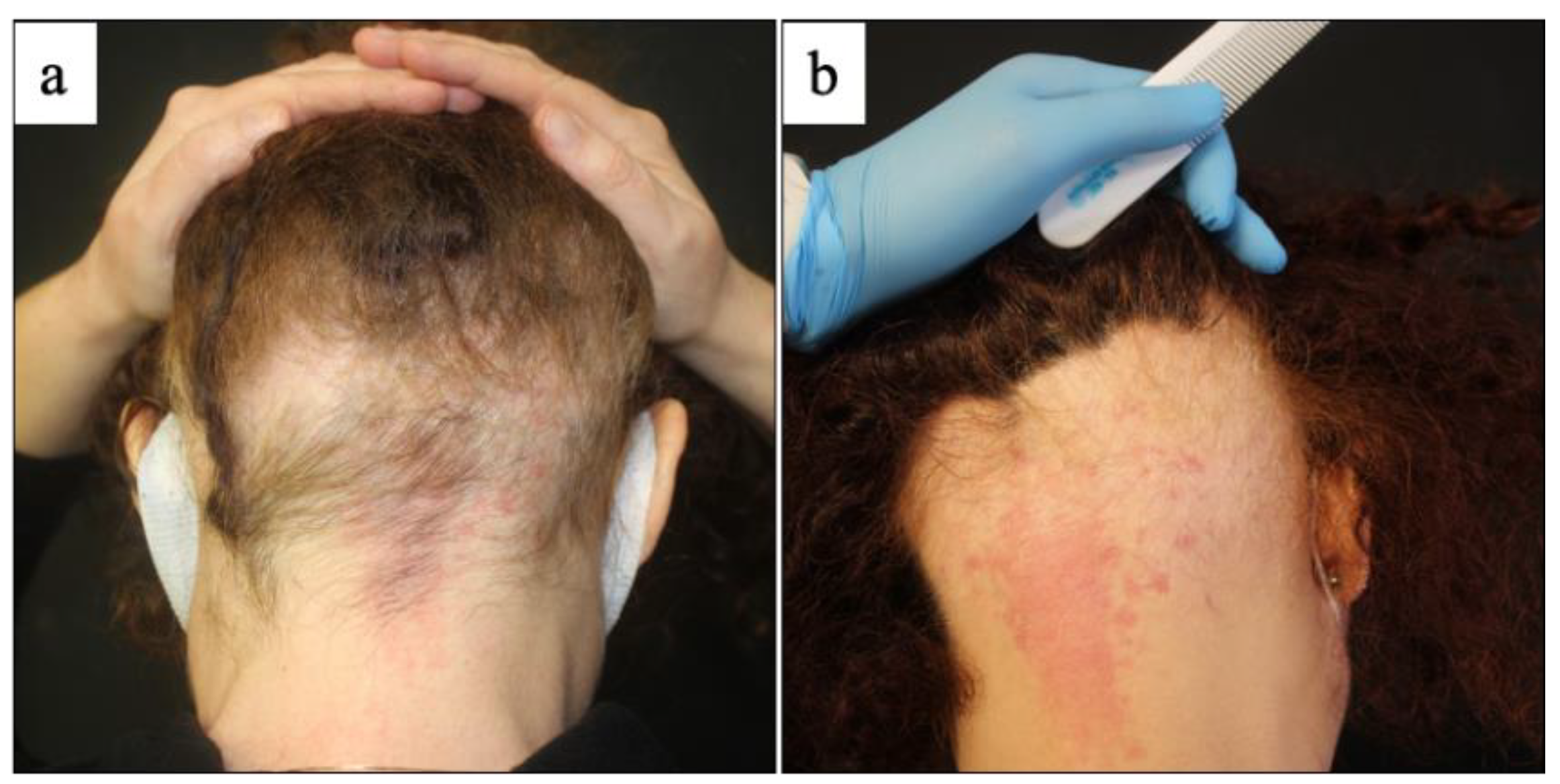
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2022, 86, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Boms, S.; Susok, L.; Dickel, H.; Finis, C.; Abu Rached, N.; Barras, M.; Stücker, M.; Kasakovski, D. Cutaneous findings following COVID-19 vaccination: Review of world literature and own experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Scollan, M.E.; Breneman, A.; Kinariwalla, N.; Soliman, Y.; Youssef, S.; Bordone, L.A.; Gallitano, S.M. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Mastorino, L.; Tonella, L.; Ribero, S.; Quaglino, P. Alopecia areata after COVID-19 vaccination. Clin. Exp. Vaccine Res. 2022, 11, 129–132. [Google Scholar] [CrossRef]
- Rossi, A.; Magri, F.; Michelini, S.; Caro, G.; Di Fraia, M.; Fortuna, M.C.; Pellacani, G.; Carlesimo, M. Recurrence of alopecia areata after Covid-19 vaccination: A report of three cases in Italy. J. Cosmet. Dermatol. 2021, 20, 3753–3757. [Google Scholar] [CrossRef]
- Essam, R.; Ehab, R.; Al-Razzaz, R.; Khater, M.W.; Moustafa, E.A. Alopecia areata after ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca): A potential triggering factor? J. Cosmet. Dermatol. 2021, 20, 3727–3729. [Google Scholar] [CrossRef]
- Su, H.-A.; Juan, C.-K.; Chen, Y.-C. Alopecia areata following ChAdOx1 nCoV-19 vaccination (Oxford/AstraZeneca). J. Formos. Med. Assoc. 2022, in press. [Google Scholar] [CrossRef]
- Lee, M.M.; Bertolani, M.; Pierobon, E.; Feliciani, C.; Satolli, F.; Lotti, T. Alopecia areata following COVID-19 vaccination: Vaccine-induced autoimmunity? Int. J. Dermatol. 2022, 61, 634–635. [Google Scholar] [CrossRef]
- Lo, A.; Mir, A.; Sami, N. Letter in Reply: Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022, 25, 25–26. [Google Scholar] [CrossRef]
- Jensen, A.; Stromme, M.; Moyassari, S.; Chadha, A.S.; Tartaglia, M.C.; Szoeke, C.; Ferretti, M.T. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp. Clin. Trials 2022, 115, 106700. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Lan, C.E. Intractable alopecia areata following the second dose of COVID-19 vaccination: Report of two cases. Dermatol. Ther. 2022, e15689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Talotta, R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin. Immunol. 2021, 224, 108665. [Google Scholar] [CrossRef]
- Richardson, C.T.; Hayden, M.S.; Gilmore, E.S.; Poligone, B. Evaluation of the Relationship between Alopecia Areata and Viral Antigen Exposure. Am. J. Clin. Dermatol. 2018, 19, 119–126. [Google Scholar] [CrossRef]
- Rajabi, F.; Drake, L.A.; Senna, M.; Rezaei, N. Alopecia areata: A review of disease pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Kara Polat, A.; Oguz Topal, I.; Karadag, A.S.; Aksoy, H.; Koku Aksu, A.E.; Ozkur, E.; Ozkok Akbulut, T.; Topaloglu Demir, F.; Engin, B.; Uzuncakmak, T.K.; et al. The impact of COVID-19 in patients with psoriasis: A multicenter study in Istanbul. Dermatol. Ther. 2021, 34, e14691. [Google Scholar] [CrossRef]
- Aram, K.; Patil, A.; Goldust, M.; Rajabi, F. COVID-19 and exacerbation of dermatological diseases: A review of the available literature. Dermatol. Ther. 2021, 34, e15113. [Google Scholar] [CrossRef]
- Nguyen, B.; Tosti, A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int. 2022, 7, 67–77. [Google Scholar] [CrossRef]
- Reinke, S.; Thakur, A.; Gartlan, C.; Bezbradica, J.S.; Milicic, A. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef] [PubMed]
| Clinical Data: No (%) | Count | |
|---|---|---|
| Sample | 24 pts | |
| Sex | Male | 4 (16.7) |
| Female | 20 (83.3) | |
| Age | Mean value 39.1 (14–66 age) | |
| COVID-19 vaccine | BNT162b2 | 15 (62.5) |
| mRNA-1273 | 5 (20.8) | |
| ChAdOx1 nCoV-19 | 4 (16.7) | |
| Onset of AA after vaccine | BNT162b2 | Mean 5.7 (1 week–16 weeks) |
| mRNA-1273 | Mean 6.4 (4 weeks–8 weeks) | |
| ChAdOx1 nCoV-19 | Mean 2.2 weeks (1 week–3 weeks) | |
| Clinical subtype | Patchy AA | 14 (58.3) |
| AAT | 6 (25) | |
| AAU | 4 (16.7) | |
| Treatment regimen | Patchy AA—14 pts (58.3) | Topical steroids—10 pts (41.6) Systemic and topical steroids—1 pt (4.2) Cyclosporin and topical steroids—3 pts (12.5) |
| AAT—6 pts (25) | Oral minoxidil, cyclosporin and topical steroids—3 pts (12.5) Cyclosporin and topical steroids—2 pts (8.4) Systemic and topical steroids—1 pt (4.2) | |
| AAU—4 pts (16.7) | Oral minoxidil, cyclosporin and topical steroids—3 (12.5) Cyclosporin and topical steroids—1 (4.2) | |
| Comorbidities | Autoimmune disease—12 pts (50) | Hashimoto thyroiditis—9 pts (37.5) Celiac disease—2 pts (8.3) Undifferentiated connective tissue disease—1 pt (4.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassone, F.; Cappilli, S.; Antonelli, F.; Zingarelli, R.; Chiricozzi, A.; Peris, K. Alopecia Areata Occurring after COVID-19 Vaccination: A Single-Center, Cross-Sectional Study. Vaccines 2022, 10, 1467. https://doi.org/10.3390/vaccines10091467
Tassone F, Cappilli S, Antonelli F, Zingarelli R, Chiricozzi A, Peris K. Alopecia Areata Occurring after COVID-19 Vaccination: A Single-Center, Cross-Sectional Study. Vaccines. 2022; 10(9):1467. https://doi.org/10.3390/vaccines10091467
Chicago/Turabian StyleTassone, Francesco, Simone Cappilli, Flaminia Antonelli, Ruggiero Zingarelli, Andrea Chiricozzi, and Ketty Peris. 2022. "Alopecia Areata Occurring after COVID-19 Vaccination: A Single-Center, Cross-Sectional Study" Vaccines 10, no. 9: 1467. https://doi.org/10.3390/vaccines10091467
APA StyleTassone, F., Cappilli, S., Antonelli, F., Zingarelli, R., Chiricozzi, A., & Peris, K. (2022). Alopecia Areata Occurring after COVID-19 Vaccination: A Single-Center, Cross-Sectional Study. Vaccines, 10(9), 1467. https://doi.org/10.3390/vaccines10091467







